Abstract
Background: Darier disease is an autosomal dominant hereditary skin disease that is susceptible to secondary bacterial or fungal infections, but rarely to human papillomavirus (HPV) infections. Multiple or extensive warts from HPV remain a therapeutic challenge, but local hyperthermia is effective. We treated a patient with Darier disease who had superimposed warts in the genital and neck regions.
Materials and methods: The patient was treated with tolerable local hyperthermia with infrared light from a halogen lamp (surface temperature, 40°C) to a single target lesion on the genitalia (30 min daily) for 3 consecutive days.
Results: Within 2 weeks, the target lesion cleared and synchronous regression of untreated lesions on the neck was observed.
Conclusions: In Darier disease, local hyperthermia treatment of HPV warts in 1 region was effective in treating multiple lesions, including lesions at a remote site, possibly by promoting an immune response against HPV.
Introduction
Human papillomavirus (HPV) infection of the skin causes proliferative lesions known as verruca vulgaris or warts. Conventional treatment of warts usually consists of repeated topical application of immunomodulatory and cytotoxic agents, cryotherapy, laser ablation, and surgery. These treatments may be complicated by pain, limited efficacy, long healing time, secondary bacterial infection, or scarring. Extensive or large warty lesions are a major therapeutic challenge to dermatologists.
Elevated body temperature is a defensive mechanism by which the human body may fight against microbial infection and neoplasms Citation[1]. Artificial systemic or local hyperthermia has been used to treat cancers and infectious diseases, with varied efficacy Citation[2]. Warts may be effectively treated with local hyperthermia Citation[3–10]. Heat sources for treatment of warts have included the neodymium-doped yttrium aluminium garnet (Nd:YAG) laser Citation[3], Citation[6], hot water bath Citation[10], radiofrequency Citation[5], infrared radiation Citation[4], Citation[9], and a self-applied heat patch Citation[7]. Different protocols for treatment of warts have included different temperature (40°C to 50°C), treatment duration (30 s to 30 min), and timing (successive or intermittent treatment with intervals from several days to weeks) Citation[3–10].
Infrared radiation may be absorbed by normal human skin. Absorbance percentage may vary with wavelength and skin level, including epidermis and dermis (absorbance at 1000 nm: epidermis, 35% and dermis, 48%; absorbance at 1400 nm: epidermis, 72% and dermis, 20%), with the remaining infrared radiation absorbed by subcutaneous fat Citation[8]. More heat may be absorbed in an epidermal wart lesion than normal skin because of the hypertrophic character of the epidermis in HPV-infected skin. The amount of heat absorption by the tissue is quantified by the thermal dose (D), which is a function of temperature (T), duration of treatment (t), and a factor R, according to the equation D = t × RT-43 (R = 2 when T ≥ 43°C; R = 4 when T ≤ 43°C; 43°C is the critical temperature above which heated cells are more susceptible to apoptosis) Citation[11]. We previously showed that infrared local hyperthermia (44°C for 30 min, given once daily for 3 consecutive days and then for 2 more days, 2 weeks later) resolved plantar warts in >50% of patients within 3 months Citation[4].
Darier disease is an autosomal dominant disorder that may present with multiple keratotic papules or plaques in seborrhoeic regions. Characteristic histopathological findings include dyskeratosis of keratinocytes with formation of corps ronds (characteristic of apoptosis) and acantholysis (focal areas of separation between suprabasal epidermal cells). A mutation in the ATP2A2 gene, which encodes a calcium pump (type 2) sarco-endoplasmic reticulum Ca2+-ATPase (SERCA2) highly expressed in epidermal keratinocytes, is responsible for Darier disease Citation[12]. As a result of perturbed calcium ion signalling in Darier disease, the function of junctional complexes in keratinocytes are adversely affected, leading to acantholysis, a distinct histological feature of the disease. Topical steroids, or topical or oral retinoids, may improve the symptoms of Darier disease Citation[13]. However, Darier disease may be associated with bacterial or fungal infections because of small wounds in the skin. Darier disease lesions are rarely infected by HPV Citation[14–16], possibly because the HPV life cycle is intimately linked to epithelial differentiation Citation[17]. In Darier disease, there is perturbed differentiation and a loss of cell to cell adhesion, which might be an unfavorable condition for HPV genome replication Citation[18]. Initial cutaneous infection by HPV is through a microscopic wound.
We report a case of a patient recently treated for extensive warts from HPV infection associated with longstanding Darier disease. Treatment included hyperthermia at 40°C for 30 min, for 3 consecutive days, and the warty lesions were resolved by 15 days after treatment, much sooner than expected. Furthermore, both treated and untreated lesions at remote sites were resolved.
Case history
A 35-year-old Chinese man was referred to the outpatient clinic with a 2-month history of warty lesions in the genital and posterior neck regions that had not resolved with occasional wet dressings with nitrofurazone (1:5000) solution ( and ). He had a 27-year history of pruritic, malodorous red patches on his trunk, worse during the summer. He was single, heterosexual, and sexually active with a regular partner, and he denied sexual promiscuity. Family history was notable for similar skin disorders in his maternal grandmother, mother, sister, and sister's daughter. A diagnosis of Darier disease had been made based on clinical appearance and histopathological findings (). He had been treated with oral retinoic ethylester (0.03 mg, once daily) for the past 6 years, with control of the lesions except for frequent exacerbation during the summer.
Figure 1. Clinical and histologic appearance of neck lesions in a 35-year-old man with warts and Darier disease. (a) Warts (cycled) superimposed on lesions of Darier disease. (b) Diffuse vacuolated keratinocytes in the upper epidermis were characteristic of HPV-infected skin; epidermal invagination and suprabasal lacunae with dyskeratotic acantholytic cells were characteristic of Darier disease (hematoxylin-eosin, frozen section of neck lesion, original magnification ×200). (c) DNA segment specific for HPV type 6. (d) At 15 days after hyperthermia treatment, all papules and plaques in the neck disappeared completely, but Darier disease persisted.
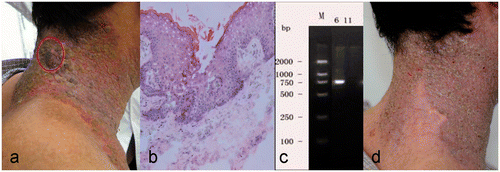
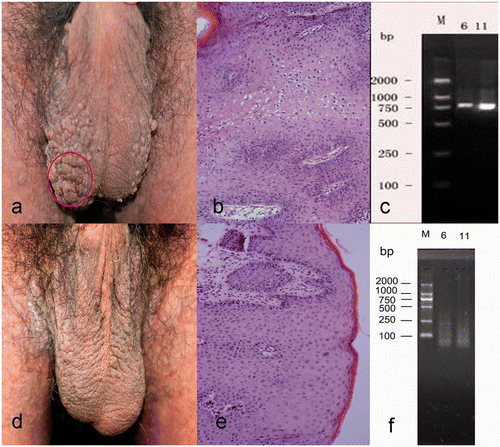
Figure 2. Clinical and histologic appearance of scrotal lesions in a 35-year-old man with warts and Darier disease. (a) Papillary and cauliflower-shaped papules and plaques in the scrotum. (b) Patches of vacuolated keratinocytes in thickened epidermis were characteristic of HPV-infected skin (hematoxylin-eosin, original magnification ×200). (c) DNA segments specific for HPV types 6 and 11. (d, e) Clinical and histologic findings after 2 months hyperthermia treatment, showing resolution of the warty lesions. (f) No HPV-specific DNA was detected after 2 months treatment.
Physical examination showed irregularly shaped, slightly reddish, hyperkeratotic or crusted, malodorous patches or plaques on his face, neck, axilla, trunk, suprapubic region, and groin. There were brownish, warty papules and plaques on his scrotum. There also were warty plaques superimposed on the reddish patches on right side of the neck ( and ). Laboratory tests including complete blood count, urinalysis, and hepatic and renal function tests were normal. Serologic tests for syphilis, HIV, and hepatitis B were negative. Chest radiography and ultrasonography of the abdomen and lymph nodes were normal.
Biopsy of the warty lesions of the scrotum and neck showed features of viral warts, and the neck lesion also had features of Darier disease ( and ). Polymerase chain reaction showed DNA segments specific for HPV types 6 and 11 in the scrotal lesions and HPV type 6 in the neck lesions ( and ), confirming the diagnosis of HPV viral warts and Darier disease Citation[19].
Method and result
The patient was treated with a patented local hyperthermia generator (patent no. ZL 200720185403.3, China Medical University) after informed consent was obtained from the patient and approval was obtained from the Ethics Committee of China Medical University (2009, no. 22). The device had been installed with an energy source of infrared light from a halogen lamp (most (>90%) wavelengths from 760 to 2300 nm; peak wavelength, 1200 nm) (dichroic reflector halogen lamp, MR16, 50 W, Hua Xu Lighting, Lianyungang, China). The heat was concentrated and delivered through a metal tube to the skin surface without direct skin contact, the end of the tube being 2 to 3 cm from the skin surface. A confluent plaque on the scrotum was selected as the target lesion (), and the lesion (1.77 cm2) was heated to 40°C for 30 min, once daily for 3 consecutive days. By 15 days after the 3-day treatment, all of the papules and plaques in the scrotum and neck had disappeared completely, with residual areas of hyperpigmentation ( and ). No adverse effects were observed except for a slight burning sensation during the treatment that was well tolerated.
A biopsy from the scrotum at 2 months after complete clearance of the warty lesions showed no residual features of the wart (), and polymerase chain reaction showed no further HPV-specific DNA detected (). Follow-up evaluation 2 years later showed no recurrence of the warty lesions.
Discussion
The patient had resolution of warty lesions with hyperthermia treatment. Previous reports of hyperthermia treatment of HPV-infected skin lesions have shown that hyperthermia is effective, but there is no consensus about methodology because of variation between studies in the clinical profile of patients, type of heat generating devices, hyperthermia parameters, and protocols Citation[3–10]. In the present patient, there were extensive warty lesions associated with erosive Darier disease plaques of the neck and with scrotal skin that was not affected by Darier disease.
A placebo-controlled trial of hyperthermia treatment of 29 cases of hand warts with a radiofrequency device (surface temperature, 50°C; ≤4 treatments, each 30 to 60s, every 4 to 6 weeks) showed that treatment was effective (clearance frequency: treatment, 86%; control, 41%); the method was slightly ablative, because anaesthesia was required to relieve pain and some patients had scarring Citation[5]. Recalcitrant common warts have been successfully treated with Nd:YAG laser hyperthermia (surface temperature, 40°C; treatment duration, 30 s; treatment repeated after 6 weeks) Citation[6], and this method may result in 100% clearance of HPV DNA in the treated lesion, even when clinical improvement is not clearly documented Citation[3]. A randomised placebo-controlled trial of plantar wart treatment also showed that infrared hyperthermia (surface temperature, 44°C; treatment duration, 30 min, once daily for 3 consecutive days plus 2 more treatments after 2 weeks) was effective (frequency of cure at 3 months: treatment, 54%; control, 12%), with tolerable burning sensation but no severe side effects Citation[4].
In the present patient the warty lesion was quite extensive, especially in the genital region ( and ), and a destructive option for viral warts was not appropriate. Hyperthermia treatment resulted in clearance of lesions at both the treated and remote sites, which may be explained by the development of an immune response against HPV infection Citation[4]. Cell mediated immunity may be important for elimination of HPV infection, but the molecular and cellular mechanisms are not fully characterised Citation[20]. Hyperthermia may promote the release of heat shock proteins from damaged cells, resulting in stimulation of antigen-presenting cells, cytokine release and expression of cell surface molecules, presentation of heat shock protein-bound peptide antigens to major histocompatibility complex class I molecules in dendritic cells, and induction of antigen-specific cytotoxic T lymphocytes Citation[21]. Furthermore, hyperthermia at 40°C may increase antigen uptake and phagocytosis by dendritic cells and macrophages, increase migration of dendritic cells to regional lymph nodes, and promote lymphocyte movement to lymphoid and tumour tissue Citation[22]. Whole body hyperthermia (39.5°C to 40°C for 6 h) may affect the severity of murine contact hypersensitivity, an established model of T cell mediated immune response in the skin Citation[1]. Local hyperthermia at 42°C and 45°C for 30 min promotes migratory maturation of Langerhans cells in HPV-infected skin Citation[23]. The migration of Langerhans cells may be coupled with decreased expression of CCL-20, which is a potent chemokine for recruitment of Langerhans cells to the skin Citation[24]. In condyloma acuminata, local hyperthermia could modulate antiviral activity by an endogenous interferon-dependent pathway Citation[25], Citation[26]. In addition, hyperthermia at 42°C and 45°C for 30 min may promote apoptosis in both HPV-infected and normal keratinocytes, even though there may be different apoptosis signalling pathways in HPV-infected and normal skin Citation[27]. Warts that respond to photodynamic therapy have dense CD4+ and CD8+ T lymphocytes in the lesion Citation[28], confirming participation of immune cells in the clearance of the lesions. We recently observed that in condyloma acuminata, which may regress after local hyperthermia treatment, dense CD4+ and CD8+ T lymphocytic infiltration was noted in lesions (unpublished observation).
Other factors may account for the rapid clearance of distant lesions in the present case. Spontaneous resolution may have occurred, because HPV-infected lesions may have a self-limiting course, with 30% warts regressing spontaneously within 3 months Citation[29]. The rare occurrence of HPV infection with Darier disease may imply a skin condition in Darier disease unfavourable for HPV infection Citation[18]. The patient had been on long-term use of oral retinoic ethyl ester for Darier disease, and retinoid may be effective in eliminating warty lesions Citation[15]. Hyperthermia may stimulate cytotoxic and apoptotic effects, which may help eliminate HPV-infected tissue Citation[30–32]. Furthermore, the virus or viral replication may be inhibited directly by heat; copies of HPV E6 and E7 transcripts in HPV-infected tissue may be decreased after hyperthermia treatment (unpublished observation). Despite incomplete understanding of the mechanism, the present case shows that heat therapy may be another option to treat warts when other therapies fail or are not applicable.
Acknowledgement
Written informed consent was obtained from the patient for submission and publication of this case report and accompanying images. X.H. Gao and H.D. Chen contributed equally to this work.
Declaration of interest: This work was supported in part by the Program for Changjiang Scholars, Innovative Research Team in University (IRT0760), National Natural Science Foundation of China (No. 30740082) and Shenyang Science and Technology Bureau (No.1081267-9-00). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data, or in the preparation, review, or approval of the manuscript. The authors alone are responsible for the content and writing of the paper.
References
- Ostberg JR, Gellin C, Patel R, Repasky EA. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J Immunol 2001; 167: 2666–2670
- Klein A, Bäumler W, Landthaler M, Babilas P. Laser thermal therapy of benign skin tumours: Review and update. Int J Hyperthermia 2011; 27: 762–770
- El-Tonsy MH, Anbar TE, El-Domyati M, Barakat M. Density of viral particles in pre and post Nd:YAG laser hyperthermia therapy and cryotherapy in plantar warts. Int J Dermatol 1999; 38: 393–398
- Huo W, Gao XH, Sun XP, Qi RQ, Hong Y, Mchepange UO, et al. Local hyperthermia at 44 degrees C for the treatment of plantar warts: A randomized, patient-blinded, placebo-controlled trial. J Infect Dis 2010; 201: 1169–1172
- Stern P, Levine N. Controlled localized heat therapy in cutaneous warts. Arch Dermatol 1992; 128: 945–948
- Pfau A, Abd-el-Raheem TA, Bäumler W, Hohenleutner U, Landthaler M. Nd:YAG laser hyperthermia in the treatment of recalcitrant verrucae vulgares (Regensburg's technique). Acta Derm Venereol 1994; 74: 212–214
- Dvoretzky I. Hyperthermia therapy for warts utilizing a self-administered exothermic patch. Review of two cases. Dermatol Surg 1996; 22: 1035–1039
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed 2003; 19: 228–234
- Gao XH, Gao D, Sun XP, Huo W, Hong YX, Li XD, et al. Non-ablative controlled local hyperthermia for common warts. Chin Med J (Engl) 2009; 122: 2061–2063
- Kang S, Fitzpatrick TB. Debilitating verruca vulgaris in a patient infected with the human immunodeficiency virus. Dramatic improvement with hyperthermia therapy. Arch Dermatol 1994; 130: 294–296
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular bases of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 1999; 21: 271–277
- Pani B, Singh BB. Darier's disease: A calcium-signaling perspective. Cell Mol Life Sci 2008; 65: 205–211
- Borgogna C, Zavattaro E, Dell’Oste V, Mondini M, Valente G, Colombo E, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol 2009; 36: 1005–1009
- Li YH, Gao XH, He CD, Zhang G, Dong X, Chen HD. Detection of human papillomavirus and response to oral arotinoid ethylester in two cases of Darier disease. Arch Dermatol 2002; 138: 695–696
- Orihuela E, Tyring SK, Pow-Sang M, Dozier S, Cirelli R, Arany I, et al. Development of human papillomavirus type 16 associated squamous cell carcinoma of the scrotum in a patient with Darier's disease treated with systemic isotretinoin. J Urol 1995; 153: 1940–1943
- Hebner CM, Laimins LA. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol 2006; 16: 83–97
- Savignac M, Edir A, Simon M, Hovnanian A. Darier disease: A disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta 2011; 1813: 1111–1117
- Saegusa M, Hashimura M, Takano Y, Ohbu M, Okayasu I. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in oesophageal and gastric carcinomas in Japan. Mol Pathol 1997; 50: 101–104
- Frazer IH. Interaction of human papillomaviruses with the host immune system: A well evolved relationship. Virology 2009; 384: 410–414
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int J Hyperthermia 2009; 25: 610–616
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: A temperature's story. Cancer Lett 2008; 271: 191–204
- Li X, Gao XH, Jin L, Wang Y, Hong Y, McHepange UO, et al. Local hyperthermia could induce migrational maturation of Langerhans cells in condyloma acuminatum. J Dermatol Sci 2009; 54: 121–123
- Wang X, Gao XH, Hong Y, Li X, Chen HD. Local hyperthermia decreases the expression of CCL-20 in condyloma acuminatum. Virol J 2010; 7: 301
- Zhu LL, Gao XH, Qi R, Hong Y, Li X, Wang X, et al. Local hyperthermia could induce antiviral activity by endogenous interferon-dependent pathway in condyloma acuminata. Antiviral Res 2010; 88: 187–192
- Payne J, Nair MP, Ambrus JL, Chadha KC. Mild hyperthermia modulates biological activities of interferons. Int J Hyperthermia 2000; 16: 492–507
- Wang X, Gao XH, Li X, Hong Y, Qi R, Chen HD, et al. Local hyperthermia induces apoptosis of keratinocytes in both normal skin and condyloma acuminata via different pathways. Apoptosis 2009; 14: 721–728
- Giomi B, Pagnini F, Cappuccini A, Bianchi B, Tiradritti L, Zuccati G. Immunological activity of photodynamic therapy for genital warts. Br J Dermatol 2011; 164: 448–451
- Lipke MM. An armamentarium of wart treatments. Clin Med Res 2006; 4: 273–293
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Gerner EW, Cress AE, Stickney DG, Holmes DK, Culver PS. Factors regulating membrane permeability alter thermal resistance. Ann N Y Acad Sci 1980; 335: 215–233
- Roti Roti JL, Wright WD, VanderWaal R. The nuclear matrix: A target for heat shock effects and a determinant for stress response. Crit Rev Eukaryot Gene Expr 1997; 7: 343–360
