Abstract
Purpose: To evaluate the influence of regional hyperthermia on rates of complete pathological response (pCR) and sphincter-sparing surgery in the context of an up-to-date radiochemotherapy protocol for locally advanced rectal cancer.
Methods: Between 2007 and 2010, 106 patients with locally advanced cancer of the middle and lower rectum were admitted to neoadjuvant radiochemotherapy either with (n = 61) or without (n = 45) regional hyperthermia. A retrospective comparison was performed between two groups: 45 patients received standard treatment consisting of 5040 cGy in 28 fractions to the pelvis and 5-fluorouracil (RCT group) and 61 patients received the same treatment in combination with regional hyperthermia (HRCT group). Target temperature was 40.5°C for at least 60 min. Total mesorectal excision was performed routinely.
Results: pCR was seen in 6.7% of patients in the RCT group and 16.4% in the HRCT group. Patients who received at least four hyperthermia treatments (n = 40) achieved a significantly higher pCR rate (22.5%) than the remaining 66 patients (p = 0.043). Rates of sphincter-sparing surgery were similar in both groups with 64% in the RCT group and 66% in HRCT. When considering only low-lying tumours located within 8 cm of the anal verge prior to treatment, the rate of sphincter-sparing surgery was 57% in the HRCT group compared with 35% in the RCT group (p = 0.077).
Conclusion: The combination of regional hyperthermia and neoadjuvant radiochemotherapy may lead to an increased pCR rate in locally advanced rectal cancer. Patients with low-lying tumours especially may benefit when additional downsizing allows sphincter-preserving surgery.
Introduction
Preoperative 5-fluorouracil-based radiochemotherapy followed by total mesorectal excision (TME) has become a mainstay in the treatment of locally advanced rectal cancer Citation[1]. The combination of TME with modern neoadjuvant radiochemotherapy concepts provides local control rates of more than 90% in International Union Against Cancer (UICC) stage II and III rectal cancer. In comparison to post-operative radiochemotherapy alone, it has been shown, that sphincter preservation rates are improved with preoperative chemoradiotherapy Citation[1]. However, high risk subgroups including T4 or node positive tumours and tumours close to the anal verge remain a challenge with regard to local control and sphincter preservation. In this regard radiochemo sensitisation by means of additional regional hyperthermia is a promising concept. Regional hyperthermia can improve tumour oxygenation by increasing blood flow and thus increasing susceptibility of the tumour to radiation. In addition, hyperthermia has been proven in in vitro experiments to enhance the effects of chemotherapy Citation[2], Citation[3]. Beneficial effects of hyperthermia in addition to radiotherapy on local tumour control and response rates have been observed in prospective randomised studies for advanced stage cervical cancer, recurrent breast cancer and recurrences or metastasis of malignant melanoma Citation[4–6].
For rectal cancer, a recent review performed by the Cochrane Collaboration has shown improved complete response rates after preoperative radiotherapy with hyperthermia Citation[4], Citation[7–12]. However, none of the trials in the review has applied a fluorouracil-based radiochemotherapy protocol as described above Citation[1].
Data for feasibility and toxicity of a trimodal neoadjuavant treatment for advanced rectal cancer including radiotherapy, chemotherapy and hyperthermia are sparse. Moreover, a direct comparison of radiochemotherapy with a trimodal approach including hyperthermia is still lacking.
The present retrospective study is the first study to compare neoadjuvant radiochemotherapy performed according to an up-to-date treatment protocol including TME and 5-FU-based chemotherapy with and without hyperthermia. The main goals of this two-armed, non-randomised, retrospective trial were to assess the influence of hyperthermia on the rates of pathological complete response, acute treatment-related toxicity and sphincter-sparing surgery.
Patients and methods
Patients
A total of 106 patients with locally advanced (T3 or T4 or node positive) adenocarcinoma of the middle and lower rectum were treated with preoperative radiochemotherapy or radiochemotherapy plus regional hyperthermia at the University Hospital Tübingen (Germany) between 2007 and 2010. Three patients had solitary liver metastases which were resected prior to neoadjuvant treatment. Inclusion criteria for this retrospective study were biopsy-proven adenocarcinoma of the rectum and tumour location within 12 cm distance from the anal verge. Pre-therapeutic staging included proctoscopy with transrectal ultrasound and pelvic CT and/or MRI. During initial patient briefing, regional hyperthermia was offered to eligible patients as an additional treatment modality. Contraindications for hyperthermia treatment were bleeding tumours, pelvic metal implants and relevant cardiac comorbidities. A total of 61 patients gave informed consent for hyperthermia treatment (HRCT group), and 45 patients received radiochemotherapy without hyperthermia (RCT group). In the latter group hyperthermia was not performed either because of patient refusal or contraindications. Radiotherapy or chemotherapy-related toxicity was assessed according to the Common Toxicity Criteria, National Cancer Institute, version 3.0.
Radiochemotherapy
Over a period of 5 weeks, 50.4 Gy in 28 fractions using 15 MV photons were delivered to the pelvis in prone position five times weekly. 5-Fluorouracil was given as a continuous infusion over 120 h at a dose of 1000 mg/m2/day in the first and fifth week of treatment. All patients received anti-emetic premedication.
Hyperthermia
Hyperthermia was delivered immediately after radiation once to twice weekly with a Sigma Eye or Sigma-60 applicator (BSD 2000/3D, BSD Medical Systems, Salt Lake City, Utah, USA). Quality assurance guidelines according to the recommendations of the European Society for Hyperthermic Oncology were considered Citation[13]. Temperatures in the bladder, rectum and vagina were measured continuously with endoluminal catheters (Bowman Probes, BSD Medical Systems, Salt Lake City, Utah, USA). Target temperature was 40.5°C for at least 60 min and the maximum treatment duration per hyperthermia session was 90 min. Two index temperatures were determined: the T90 which indicates the temperature achieved in 90% of tumour-related measurement points, and the CEM T43 representing the cumulative equivalent minutes at a reference temperature of 43°C Citation[14].
Surgery and post-operative treatment
Low anterior or abdomino-perineal resection with total mesorectal excision was performed four to six weeks after completion of radiochemotherapy. Adjuvant chemotherapy was applied according to a protocol described elsewhere Citation[1].
Assessment of pathological response
A central pathological review of all resection specimens was carried out by the department of Pathology, University Hospital of Tübingen, Germany. Clinical (cTNM) and pathological stage (ypTNM) was recorded according to the UICC TNM system, version 6.0. Tumour regression was reported according to Becker's regression score, see Citation[15]. Complete pathological response corresponds with grade 1 a and is defined as the absence of any vital malignant cells in the primary tumour and lymph nodes.
Table I. Grades of tumour regression according to Becker's regression scale.
End points and statistical analysis
The primary end points of this study were pathological complete response and sphincter-sparing surgery. Secondary end-point was hyperthermia-related acute toxicity. Statistical analysis was performed using commercial software (SPSS 19, IBM, Armonk, NY, USA). Chi-square test was used to compare rates of complete pathological response and sphincter-sparing surgery between the treatment arms. To compare patient and tumour characteristics between groups, a two-tailed t-test was performed.
Results
Pretreatment patient and tumour characteristics
Patient and tumour characteristics are shown in . The groups were well balanced with regard to patient gender, age, tumour stage and grade. Tumours in the HRCT group were located closer to the anal verge. As the number of hyperthermia treatments varied widely, patients in the HRCT group were subdivided into two groups: patients who received at least four hyperthermia treatments, and patients who received one to three hyperthermia treatments.
Table II. Patients and tumour characteristics.
Feasibility and treatment compliance
In 21 of 61 patients (34%) hyperthermia was discontinued within the first three sessions. Of these 21 patients, 15 (71%) discontinued treatment because of local pain or hot-spot phenomena, three patients because of urinary tract infections after hyperthermia and two patients because of hypertension and tachycardia during treatment. One patient experienced more severe skin toxicity (maceration of the anal cleft) which did not permit any further hyperthermia treatment. The addition of hyperthermia did not compromise the feasibility of radiochemotherapy. In both groups, with or without hyperthermia, all patients regularly completed radiotherapy. In the HRCT group 58 of 61 patients (95%) received 100% of the target chemotherapy dose, three of 61 patients (5%) required dose reduction of the second chemotherapy cycle to 75% because of grade III oral mucositis. In the RCT group 41 of 45 patients (91%) received both courses of chemotherapy at full dose. Two of 45 patients refused the second cycle because of nausea. In another two of 40 patients the second cycle was omitted because of grade III oral mucositis.
Hyperthermia
The median number of treatments in the HRCT group was four (range 1–9). Median T90 was 39.3°C and median CEM T43 was 1.1 min. In the subgroup of patients receiving at least four treatments (40 of 61 patients), median T90 was 39.44°C (range 38.52–40.48°C) and median CEM T43 was 1.5 min (range 0.09–9.22 min). For the 21 of 61 patients who discontinued hyperthermia within the first three sessions, median T90 was 39.1°C (range 37.1–40.6°C) and median CEM T43 was 0.3 min (range 0–3.69 min). In the latter subgroup three patients discontinued hyperthermia within the first 35 min of treatment without reaching a temperature above 38°C.
Urinary tract infections occurred in eight of 61 (13.1%) patients and required oral antibiotic treatment. Grade III skin toxicity occurred in four of 61 (6.5%) patients. However, due to the multimodal approach, treatment toxicities cannot reliably be assigned to any individual treatment modality.
Surgical outcome
Surgery was scheduled 4–6 weeks after the completion of RCT or HRCT. Sphincter preservation was achieved in 40 of 61 patients (66%) in the HRCT group and 29 of 45 patients (64%) in the RCT group. For tumours located within 8 cm of the anal verge sphincter preservation was achieved in 28 of 49 patients (57%) in the HRCT group and eight of 23 patients (35%) in the RCT group (p = 0.077). The mean distance from the anal verge for these patients was 4.85 cm in the HRCT group and 4.76 cm in the RCT group. All patients in the HRCT group had clear margins, while pathological evaluation showed positive margins in two of 45 (4.4%) specimens in the RCT group.
Pathological outcome
In the HRCT group 10 of 61 patients (16.4%) compared with three of 45 patients (6.7%) in the RCT group had a pCR. In the subgroup of patients who received at least four hyperthermia treatments, the pCR rate was 22.5% (9/40) and significantly higher than in the subgroup of patients who received no more than three hyperthermia treatments or no hyperthermia at all (p = 0.043). See for the distribution of regression grades in all subgroups. For clinically node positive patients, sterilisation of the primary tumour predicted sterilisation of lymph nodes for all patients with at least four hyperthermia treatments, but for only 50% of the remaining patients (p = 0.024). provides a detailed summary of pre- and post-operative tumour stages.
Figure 1. Distribution of tumour regression grades after preoperative radiochemotherapy without pelvic hyperthermia (RCT), with 1–3 hyperthermia treatments (HRCT 1–3) and with four or more hyperthermia treatments (HRCT > 4). With the application of at least four hyperthermia treatments significantly more patients achieved pathological complete response (see ).
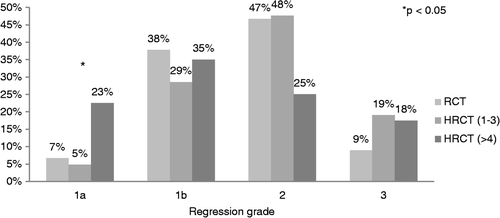
Table III. Clinical (pretreatment) and pathological tumour stages.
Mean T90, mean CEM T43 and mean pretreatment haemoglobin levels had no significant influence on the distribution of regression grades. See for detailed information about hyperthermia related parameters.
Table IV. Tumour regression grades and hyperthermia related parameters.
Discussion
In the present retrospective two-armed study, we assessed feasibility, treatment response and rates of sphincter-sparing surgery for patients who underwent preoperative radiochemotherapy with and without local hyperthermia for locally advanced rectal cancer. We combined modern standard treatment with local hyperthermia in order to further improve tumour downstaging and the rate of sphincter preservation. Though retrospective, our study provides two well-balanced groups. A central pathological review of all surgical specimens warrants a high accuracy of tumour regression grading.
However, as with all retrospective studies, the risk of selection bias must be considered when interpreting the results. In particular, it can be assumed that patients who gave informed consent for hyperthermia are more motivated and predominantly in a better health condition. A total of 21 of 61 patients discontinued hyperthermia within the first three treatments. As patients were informed prior to treatment that hyperthermia is an optional addendum to radiochemotherapy, a low threshold to discontinue hyperthermia can be supposed. A high number of treatment discontinuation in pelvic hyperthermia has already been described by others Citation[4], Citation[16]. The addition of hyperthermia did not compromise patient compliance with radio- or chemotherapy. The observed number of urinary tract infections (13.1%) cannot be allocated to a single treatment modality. For pelvic radiotherapy, increased rates of urinary tract infections have already been described by others Citation[17]. Additionally bladder catheterisation is a potential risk factor.
In this non-randomised comparison, the application of at least four hyperthermia treatments does significantly improve downstaging and increases the rate of pCR. The rate of pCR was significantly higher in the group receiving preoperative radiochemotherapy with at least four hyperthermia treatments (22.5%) compared with a pathological complete response rate of 6.7% for patients receiving radiochemotherapy alone and 4.7% for patients treated with radiochemotherapy and one to three hyperthermia treatments (p = 0.043). This finding is in accordance with a variety of randomised trials showing an increased pCR rate when hyperthermia is added to radiotherapy alone without chemotherapy Citation[7], Citation[8], Citation[10], Citation[12].
The assumption that the addition of hyperthermia increases the efficacy of local treatment is additionally supported by the fact that the pCR rate of 22.5% is higher than in most published neoadjuvant radiochemotherapy trials, including modern phase II trials with experimental polychemotherapy approaches Citation[18–22].
Controversy exists about the predictive value of pCR for disease-free and overall survival. While some studies demonstrated pCR to be a positive prognosticator for disease-free or overall survival Citation[23–25], others could not reproduce this observation Citation[26], Citation[27]. However, a recent pooled analysis including 3,105 patients with rectal cancer treated with preoperative radiochemotherapy (without hyperthermia) showed a significantly improved long-term outcome for patients who achieved a pCR with neoadjuvant treatment Citation[28].
Whether an increased rate of pCR achieved by the addition of hyperthermia translates into improved local control and/or even overall survival has yet to be determined. In a retrospective trial of neoadjuvant radiochemotherapy with regional hyperthermia, Maluta et al. observed a local control rate of 100% after 5 years for all 18 of 76 patients who achieved pCR Citation[29].
Regardless of whether the intensification of local neoadjuvant treatment by means of hyperthermia translates into improved local control or disease-free survival, the potential increased rate of sphincter preservation is an important issue.
In our study the rate of sphincter-sparing surgery was similar in both groups: 64% in the RCT group and 66% in the HRCT group, when all patients are considered. This is in accordance with previously reported trials of neoadjuvant radiochemotherapy for locally advanced rectal cancer, which have reported similar rates of conservative surgery in the range of 50% and 72% Citation[1], Citation[30–32]. The trial reported by Sauer et al. demonstrated that tumour downstaging can improve the rate of sphincter preservation, when surgeons include the results of restaging procedures in the decision for or against sphincter-sparing surgery Citation[1]. When only the subgroup of patients with tumours close to the anal verge is considered, our trial points to a trend that sphincter preservation may be improved by the addition of hyperthermia. However, potential biases have to be considered. For instance, patients with low-lying tumours are more likely to complete hyperthermia as planned in order to achieve relevant downsizing.
A very recent Dutch trial suggests that downstaging to pCR will become an important issue for treatment decisions in the future. This study showed promising results for a wait-and-see policy in patients without residual tumour on imaging and endoscopy after radiochemotherapy for rectal cancer. After a mean follow-up of 25 months only one of 21 patients developed local recurrence Citation[33]. In this regard intensification of local treatment by hyperthermia will possibly gain additional importance, especially since efforts to improve rates of pCR by intensifying systemic therapy were largely disappointing Citation[18–22].
In our study a higher number of hyperthermia treatments was associated with a significantly higher pCR rate. While other authors reported a significant correlation between the T90 or CEM T43 and rates of pCR Citation[29], Citation[34], this was not the case in the present study. Higher levels of T90 or CEM T43 did not translate into higher rates of pCR. A possible explanation for this observation might be the lower median temperature applied in our study. A correlation between temperature and response rate might be observed only after a certain threshold.
In summary, we observed that regional pelvic hyperthermia with at least four treatments in addition to 5-fluorouracil-based radiochemotherapy significantly increases the rates of pathological complete response and thus might play an important role in future treatment strategies. A randomised trial comparing RCT and HRCT with well defined inclusion criteria for high-risk patients is warranted.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740
- Klostergaard J, Leroux ME, Tomasovic SP. Clonogenic survival studies of human colon tumor cell lines in vitro: Combined hyperthermia, 5-fluorouracil/leucovorin, carboplatin and tumor necrosis factor. Radiat Res 1995; 141: 44–48
- Klostergaard J, Leroux E, Siddik ZH, Khodadadian M, Tomasovic SP. Enhanced sensitivity of human colon tumor cell lines in vitro in response to thermochemoimmunotherapy. Cancer Res 1992; 52: 5271–5277
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia 1990; 6: 881–890
- Berdov B, Kurpeshev OK, Mardinsky YSM. Influence of hyperthermia and hyperglycemia on efficiency radiation therapy for oncological patients. Russ J Oncol 1996; 20: 20
- Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, et al. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia 1990; 6: 719–740
- You QS, Wang RZ, Suen GQ, Yan FC, Gao YJ, Cui SR, et al. Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: Long-term results of 44 patients. Int J Hyperthermia 1993; 9: 19–24
- Trotter JM, Edis AJ, Blackwell JB, Lamb MH, Bayliss EJ, Shepherd JM, et al. Adjuvant VHF therapy in locally recurrent and primary unresectable rectal cancer. Australas Radiol 1996; 40: 298–305
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, van Mastrigt GA, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev 2009; 3: CD006269
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, Wust P, De Leeuw AC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998; 14: 125–133
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003; 98: 1521–1530
- Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R, et al. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia 2006; 22: 301–318
- Bialas I, Bessell EM, Sokal M, Slack R. A prospective study of urinary tract infection during pelvic radiotherapy. Radiother Oncol 1989; 16: 305–309
- Velenik V, Ocvirk J, Oblak I, Anderluh F. A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. Eur J Surg Oncol 2010; 36: 244–250
- Velenik V, Ocvirk J, Music M, Bracko M, Anderluh F, Oblak I, et al. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: Results of an open-label phase II study. Radiat Oncol 2011; 6: 105
- Resch G, De Vries A, Ofner D, Eisterer W, Rabl H, Jagoditsch M, et al. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer – A two stage phase II clinical trial. Radiother Oncol 2012; 102: 10–13
- Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011; 29: 2773–2780
- Gerard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010; 28: 1638–1644
- Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005; 62: 752–760
- Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 2002; 53: 664–674
- Das P, Lin EH, Bhatia S, Skibber JM, Rodriguez-Bigas MA, Feig BW, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: A matched-pair analysis. Int J Radiat Oncol Biol Phys 2006; 66: 1378–1383
- Pucciarelli S, Toppan P, Friso ML, Russo V, Pasetto L, Urso E, et al. Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum 2004; 47: 1798–1807
- Diaz-Gonzalez JA, Calvo FA, Cortes J, Garcia-Sabrido JL, Gomez-Espi M, Del Valle E, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys 2006; 64: 1122–1128
- Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–844
- Maluta S, Romano M, Dall’oglio S, Genna M, Oliani C, Pioli F, et al. Regional hyperthermia added to intensified preoperative chemo-radiation in locally advanced adenocarcinoma of middle and lower rectum. Int J Hyperthermia 2010; 26: 108–117
- Gerard JP, Chapet O, Nemoz C, Romestaing P, Mornex F, Coquard R, et al. Preoperative concurrent chemoradiotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: The Lyon R0-04 phase II trial. J Clin Oncol 2003; 21: 1119–1124
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–1123
- Bujko K, Kolodziejczyk M. The 5 × 5 Gy with delayed surgery in non-resectable rectal cancer: A new treatment option. Radiother Oncol 2008; 87: 311–313
- Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011; 29: 4633–4640
- Rau B, Wust P, Hohenberger P, Loffel J, Hunerbein M, Below C, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: A phase II clinical trial. Ann Surg 1998; 227: 380–389
