Abstract
Because of its minimally invasive nature, thermal ablation is increasingly performed in patients with renal cell carcinoma (RCC) who are poor surgical candidates. Thermal ablation has been associated with excellent outcomes, and thus has been regarded as a viable alternative to nephron-sparing surgery. Many papers report minimal to no reduction in renal function after ablation therapies. However, in order to achieve good local control, normal renal tissue must be sacrificed, subsequently leading to reduced renal function. The amount of normal renal tissue to be ablated depends on the size, location, and number of RCCs, as well as the type of thermal ablation applied. However, there are few reports about what reduces renal function following thermal ablation therapies. The purpose of this review was to discuss factors that affect reduction in renal function and to assess the relationship between local tumour control and renal function.
Introduction
The incidence of renal cell carcinoma (RCC) has increased due to the increasing use of cross-sectional imaging studies. Incidental RCC is often characteristic of asymptomatic patients with organ-confined tumours and is typically staged as T1a cancer with a good prognosis Citation[1], Citation[2]. Nephron-sparing surgery is considered the treatment of choice for small RCC but can cause severe morbidity and mortality to patients who are poor surgical candidates.
Currently, thermal ablation is used to treat RCC in selected patients with co-existing medical diseases Citation[3–6]. The long-term outcomes of thermal ablation are not well characterised, Citation[7] but the short-term or intermediate outcomes are excellent Citation[3–6]. To reduce local recurrence, an ablation zone should cover adequate normal renal tissue beyond the edge of the tumour. The loss of renal tissue is necessary to prevent local recurrence, but it may be associated with reduced renal function. Intuitively, the amount of renal tissue to be ablated depends on whether a RCC is small or large, parenchymal or non-parenchymal, and single or multiple. It is still unclear what tumour factors contribute to reduced renal function following ablation therapies Citation[8]. However, many studies report that thermal ablation has minimal effects on renal function Citation[9–13].
The purpose of this review was to discuss changes in renal function related to size, location, tumour number and treatment modality using a theoretical mathematical model. The relationship between local recurrence and renal function was also assessed.
Lesion size
Lesion size is closely related to changes in renal function. As the size of RCCs increases, the amount of normal tissue to be ablated also increases (). Generally, radiofrequency ablation (RFA) requires a 0.5 cm tumour margin to prevent local recurrence. If spherical parenchymal RCCs of 1−4 cm in diameter are treated with RFA and if the tumour volume is calculated by 4πr3/3, the tumour volume ranges from 0.5–32 cm3 (mean, 12.5 cm3) but the volume of tumour margin ranges from 3.5–30.5 cm3 (mean, 15.5 cm3) on the assumption that 4π/3 out of 4πr3/3 is regarded as 4. In other words, the loss of normal tissue is slightly greater than the corresponding tumour volume.
Table 1. Comparison of RFA and cryoablation in terms of total ablated volume.
Ablation of a larger RCC may lead to decreased renal function compared to a smaller RCC because a greater volume of tumour margin is ablated. The loss of renal tissue is not a problem in healthy patients with good renal function, but this is not the case in patients with chronic kidney disease, a solitary kidney, or repeated nephron-sparing surgery. Patients with marginal renal function are more susceptible to renal failure after thermal ablation of a large RCC. Some studies have reported large RCCs being re-treated after a first session of RFA because of higher local recurrence rates Citation[3], Citation[4]. In order to lower rates of local recurrence, re-ablation requires ablation of extended tumour margins, leading to reduction in renal function.
Lesion location
The volume of tumour margin can differ according to lesion location (). RCCs can be classified as exophytic, parenchymal, central, or mixed lesions Citation[14]. Although exophytic, parenchymal, central, and mixed RCCs are similar in size, the extent of normal renal tissue adjacent to the tumour varies in volume; thus decreased renal function may vary from one lesion to the other. A round parenchymal RCC of 2 cm in diameter requires 9.5 cm3 of tumour margins. In contrast, a 2 cm exophytic RCC that is projecting 70% out of the kidney requires only 2.9 cm3 of tumour margin ().
Figure 1. Schematic diagram of a renal tumour and tumour margin according to the location of the tumour. A parenchymal tumour (A) has a greater volume of tumour margin than an exophytic (B) or central (C) tumour in cases where all the tumours are the same size. The parenchymal tumour requires double the ablation of the tumour margin than an exophytic or central tumour that is projecting 50% from the renal parenchyma to the peri-renal space or renal sinus. RP indicates renal parenchyma.
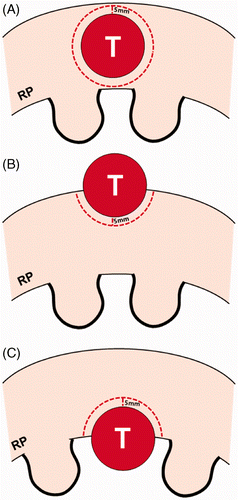
Previous studies showed that there were different proportions of RCCs in terms of tumour location Citation[9–13]. If there is a higher proportion of exophytic RCC, the reduction in renal function will be minimal compared to other types of RCC. Inversely, if one has a higher proportion of parenchymal RCCs, renal function will be more impaired. It is meaningless to evaluate or compare renal function before and after thermal ablation without matching tumour location.
A central or mixed RCC abuts small vessels in the renal sinus. When an artery measures less than 3 mm in diameter it is likely to be occluded during the ablation, subsequently resulting in segmental renal infarction Citation[15], Citation[16]. To ablate a central or mixed RCC, an applicator should traverse the renal sinus. This procedure may be complicated by an arteriovenous fistula requiring embolisation of the arterial feeder that also supplies normal renal parenchyma Citation[17]. When ablating central lesions, collecting system injury is another potential cause of renal damage. In these clinical situations, inevitable loss of normal renal tissue leads to worsening renal function compared to uneventful RCC ablations.
Lesion number
Lesion number may have a greater influence on renal function than other tumour factors (). If there are five parenchymal RCCs 2 cm in diameter, a total of 48 cm3 of normal renal tissue must be ablated to prevent local recurrence. If these RCCs develop in a solitary kidney measuring 150 cm3 and the patient's glomerular filtration rate (GFR) is 80 mL/min/1.73 m2, the GFR may drop below 60 mL/min/1.73 m2.
Figure 2. Schematic diagram of a renal tumour and tumour margin according to the number of tumours. (A) An exophytic tumour is projecting 50% out from the renal parenchyma. RP indicates renal parenchyma. (B) There are three tumours consisting of one parenchymal tumour (left) and two exophytic tumours, all the same size as figure A. These exophytic tumours are projecting 70% (middle) and 30% (right) from the parenchyma into the peri-renal space, respectively. Renal tumours in figure B require four times the normal renal tissue for thermal ablation than in figure A.
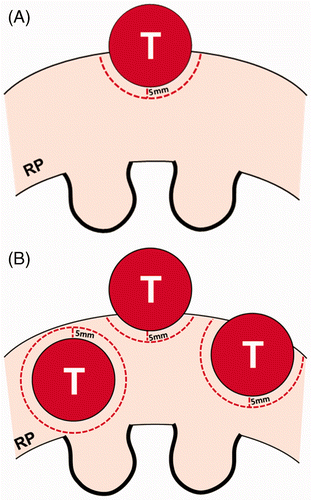
Some studies reported no significant difference in renal function before and after thermal ablation Citation[10], Citation[12]. Paired t-test is frequently used to determine whether renal function decreases after treatment. The values of the paired GFR may not follow a normal distribution in retrospective studies with small populations. Thus, non-parametric tests such as Wilcoxon signed-rank test are preferred for analysis. Because loss of normal renal tissue is accompanied by ablation of tumour margins, GFR will decrease after thermal ablation. If there were no reduction in GFR after thermal ablation, patients with multiple hereditary RCCs would not be reluctant to undergo thermal ablations for fear of impaired renal function. Indeed, patients with von Hippel Lindau disease have gradually worsening renal function as the number of ablation sessions increases Citation[18–20].
Treatment modality
Change in renal function may vary according to type of thermal ablation therapy. Currently, RFA and cryoablation are the main thermal ablation therapies for treating RCC. Cryoablation requires a wider tumour margin than RFA. RFA and cryoablation therapies ablate 1 cm and 0.5 cm tumour margins, respectively Citation[21], Citation[22]. When a spherical parenchymal RCC of 2 cm in diameter is treated, tumour margins are 28 cm3 and 9.5 cm3 for cryoablation and RFA, respectively (). Therefore, cryoablation requires more renal tissue to be ablated than RFA. Reduction in renal function following cryoablation seems to be more common than in RFA. In contrast, local recurrence rate is lower in cryoablation than RFA Citation[12], Citation[23]. However, comparisons between RFA and cryoablation were not matched for size, location and number of renal tumours.
Indeed, the tumour margin ablation volume in RFA or cryoablation is larger than the theoretical volume of tumour margin described above. The shape of a RFA zone is not spherical but oval (). In cases of spherical RCC, the volume of tumour margin to be ablated is different at different points along the lesion. For this reason, some renal tissue around the tumour must be over-ablated beyond the tumour margin in order to achieve a complete ablation. Subsequently, this over-ablation of renal tissue may be involved in reduction of renal function. The shape of a cryoablation zone is also not spherical. Similarly, cryoablation cannot avoid over-ablation of normal renal tissue (). Practically, complete ablation of tumour margins requires more injury to normal renal tissue than theoretical tissue loss.
Figure 3. Over-ablation beyond the tumour margin during the RFA procedure. Arrowheads indicate RFA electrodes. (A) For radiofrequency ablation (RFA) of a spherical RCC (red), the shape of ablation zone (yellow) is not a circle but oval. Thus, the lateral areas of the RCC and tumour margin (dotted line) require additional ablations to prevent a residual or recurrent lesion. (B and C) Additional ablations (blue and violet) are performed to completely cover the tumour margin (dotted line) as well as the RCC (red). However, some over-ablation of normal tissue is created beyond the lateral tumour margin.
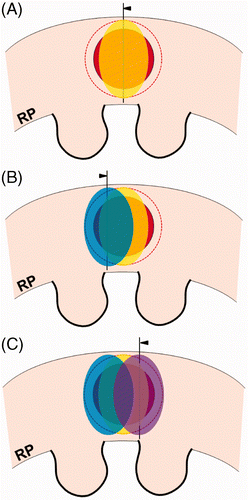
Figure 4. Over-ablation beyond the tumour margin during the cryoablation procedure. (A) For cryoablation of a spherical RCC (red), the ends (black dots) of three cryoprobes are seen within the tumour. The ice balls (yellow, blue, and violet) do not cover the entire tumour margin (arrowheads). (B) The ice balls grow to completely cover the tumour margin. However, some over-ablation of normal renal tissue (arrows) is noted beyond the tumour margin.
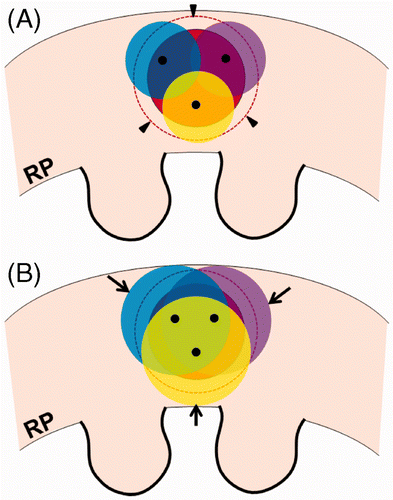
Thermal ablation and compensatory hypertrophy
Compensatory hypertrophy of remaining renal tissue may occur after thermal ablation as well as partial or radical nephrectomy. However, it does not imply the complete recovery of renal function. Hypertrophy improves GFR per renal tissue weight. Therefore, post-ablation or post-surgery GFR may increase but cannot reach pre-treatment status. Several recent studies from the urological literature found that the percentage of remaining renal parenchyma is the most important factor in post-operative renal function Citation[24–26]. These results seem to relate to our results, given that size, location, and number of RCC determine the amount of remaining renal parenchyma following thermal ablation. Many studies dealing with renal function following thermal ablation and/or nephrectomy reported decreased renal function after treatment Citation[27–30]. However, the degree of renal function reduction was much lower after thermal ablation than partial nephrectomy Citation[27–29].
Local recurrence and renal function
The relationship between local recurrence and renal function involves a balancing act between two conflicting goals. Minimising local recurrence requires adequate ablation of tumour margins and subsequently results in the reduction of renal function. Inversely, achieving minimum ablation of tumour margin may be accompanied by unwanted local recurrence. Some studies that achieved an excellent local control rate ranging between 88–92% with thermal ablation showed a significant difference in renal function before and after treatment Citation[31] or did not mention it Citation[7], Citation[32]. In contrast, other studies that achieved low recurrence-free survival rates of 33−70% with thermal ablations reported minimal or no changes in renal function Citation[12], Citation[29].
Local recurrence and renal function should be weighed according to circumstances. In cases of a sporadic RCC in a healthy patient, sufficient ablation of tumour margin around the tumour is acceptable for prevention of locally recurrent tumour. Since sporadic RCC is barely recurrent, even the over-ablation of renal tissue will not lower GFR less than 60 mL/min/1.73 m2 Citation[28]. Although thermal ablation therapies sufficiently ablate normal renal tissue, these minimally invasive treatments are more effective than partial nephrectomy in preserving renal function Citation[27–29].
In the case of hereditary RCC in a patient with von Hippel Lindau disease, over-ablation of normal renal tissue is not recommended in order to prolong the dialysis-free survival period. The best approach to manage hereditary RCCs is to simultaneously preserve renal function as much as possible and to remove as many RCCs as possible. For treatment of hereditary RCCs, thermal ablation as well as surgery should be conducted with the understanding that microscopic cancer cells will likely remain Citation[18], Citation[19], Citation[33]. Therefore, the goal of treating hereditary RCCs is not to cure, but to control multifocal or recurrent renal tumours to prevent cancer progression and to delay dialysis as long as possible.
Conclusion
It is difficult to avoid impaired renal function in the setting of thermal ablation since tumours should be ablated with wide tumour margins to prevent local recurrence. The degree of reduced renal function varies according to the size, location and number of RCC and the type of ablation modality. Therefore, thermal ablation therapies should be appropriately designed to achieve complete local control and to minimise loss of normal renal tissue, as well.
Declaration of interest: None of the authors have conflicts of interest or financial support for conducting this study. The authors alone are responsible for the content and writing of the paper.
References
- Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: Impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol 1999; 162: 1930–1933
- Kim EY, Park BK, Kim CK, Lee HM. Clinico-radio-pathologic features of a solitary solid renal mass at MDCT examination. Acta Radiol 2011; 51: 1143–1148
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: Part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol 2005; 185: 64–71
- Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol 2007; 189: 429–436
- Silverman SG, Tuncali K, vanSonnenberg E, Morrison PR, Shankar S, Ramaiya N, et al. Renal tumors: MR imaging-guided percutaneous cryotherapy – Initial experience in 23 patients. Radiology 2005; 236: 716–724
- Atwell TD, Farrell MA, Leibovich BC, Callstrom MR, Chow GK, Blute ML, et al. Percutaneous renal cryoablation: Experience treating 115 tumors. J Urol 2008; 179: 2136–2140, discussion: 2140–2131
- Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology 2011; 77: 1393–1397
- Sung HH, Park BK, Kim CK, Choi HY, Lee HM. Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hyperthermia 2012; 28: 227–234
- Bourne AE, Kramer BA, Steiner HL, Schwartz BF. Renal insufficiency is not a contraindication for cryoablation of small renal masses. J Endourol 2009; 23: 1195–1198
- Pettus JA, Werle DM, Saunders W, Hemal A, Kader AK, Childs D, et al. Percutaneous radiofrequency ablation does not affect glomerular filtration rate. J Endourol 2010; 24: 1687–1691
- Mues AC, Landman J. Results of kidney tumor cryoablation: Renal function preservation and oncologic efficacy. World J Urol 2010; 28: 565–570
- Altunrende F, Autorino R, Hillyer S, Yang B, Laydner H, White MA, et al. Image guided percutaneous probe ablation for renal tumors in 65 solitary kidneys: Functional and oncological outcomes. J Urol 2011; 186: 35–41
- Wehrenberg-Klee E, Clark TW, Malkowicz SB, Soulen MC, Wein AJ, Mondschein JI, et al. Impact on renal function of percutaneous thermal ablation of renal masses in patients with preexisting chronic kidney disease. J Vasc Interv Radiol 2012; 23: 41–45
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: Clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 2003; 226: 417–424
- Park BK, Kim CK, Lim HK. Renal infarction resulting from segmental arterial injury during radiofrequency ablation of renal tumor in patient with a single kidney. Urology 2009; 73: 442 e9–11
- Park BK, Kim CK. Complications of image-guided radiofrequency ablation of renal cell carcinoma: Causes, imaging features and prevention methods. Eur Radiol 2009; 19: 2180–2190
- Park BK, Kim CK, Moo HL. Arteriovenous fistula after radiofrequency ablation of a renal tumor located within the renal sinus. J Vasc Interv Radiol 2007; 18: 1183–1185
- Park BK, Kim CK. Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: Preliminary results. J Urol 2010; 183: 1703–1707
- Park SY, Park BK, Kim CK, Lee HM, Jeon SS, Seo SI, et al. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease previously undergoing a radical nephrectomy or repeated nephron-sparing surgery. Acta Radiol 2011; 52: 680–685
- Iwamoto Y, Kanda H, Yamakado K, Soga N, Arima K, Takeda K, et al. Management of renal tumors in Von Hippel-Lindau disease by percutaneous CT fluoroscopic guided radiofrequency ablation: Preliminary results. Fam Cancer 2011; 10: 529–534
- Aron M, Gill IS. Minimally invasive nephron-sparing surgery (MINSS) for renal tumours. Part II: Probe ablative therapy. Eur Urol 2007; 51: 348–357
- Park BK, Morrison PR, Tatli S, Govindarajulu U, Tuncali K, Judy P, et al. Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur J Radiol 2012; 81: 1702–1706
- Goel RK, Kaouk JH. Probe ablative treatment for small renal masses: Cryoablation vs radio frequency ablation. Curr Opin Urol 2008; 18: 467–473
- Simmons MN, Fergany AF, Campbell SC. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J Urol 2011; 186: 405–410
- Porpiglia F, Fiori C, Bertolo R, Morra I, Russo R, Piccoli G, et al. Long-term functional evaluation of the treated kidney in a prospective series of patients who underwent laparoscopic partial nephrectomy for small renal tumors. Eur Urol 2012; 62: 130–135
- Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012; 79: 356–360
- Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008; 179: 75–79, discussion: 79–80
- Raman JD, Raj GV, Lucas SM, Williams SK, Lauer EM, Ahrar K, et al. Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int 2010; 105: 496–500
- Turna B, Kaouk JH, Frota R, Stein RJ, Kamoi K, Gill IS, et al. Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol 2009; 182: 2150–2157
- Song C, Bang JK, Park HK, Ahn H. Factors influencing renal function reduction after partial nephrectomy. J Urol 2009; 181: 48–53, discussion: 53–44
- Weisbrod AJ, Atwell TD, Frank I, Callstrom MR, Farrell MA, Mandrekar JN, et al. Percutaneous cryoablation of masses in a solitary kidney. Am J Roentgenol 2010; 194: 1620–1625
- Klatte T, Grubmuller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: Systematic review and cumulative analysis of observational studies. Eur Urol 2011; 60: 435–443
- Reed AB, Parekh DJ. Surgical management of von Hippel-Lindau disease: Urologic considerations. Surg Oncol Clin N Am 2009; 18: 157–174,x
