Abstract
Purpose: The aim of this study was to further understand the effects and mechanism of heat stress on the intestinal mucosal immune system of the rat, including changes in the intestinal mucosal barrier and immune function and their effects on bacterial translocation.
Materials and methods: Sprague Dawley (SD) rats were randomly divided into control and heat-stress groups. Both groups were housed in a 25°C environment of 60% relative humidity. The heat-stress group was subjected to 40°C for 2 h daily over 3 days.
Results: Compared with the control group villi length in the small intestines of the heat-stress group was shortened. Jejunal mucosa were seriously damaged and the number of goblet cells in the epithelia of the duodenum and jejunum was significantly reduced. Electron microscopy revealed intestinal mucosal disorder, a large number of exudates of inflammatory fibrous material, fuzzy tight junction structure between epithelial cells, and cell gap increases in the heat-stress group. Transcription of IFN-γ, IL-2, IL-4, and IL-10, was significantly reduced, as was that of the intestinal mucosal immune-related proteins TLR2, TLR4, and IgA. The number of CD3+ T cells and CD3+CD4+CD8− T cells in the mesenteric lymph nodes (MLNs) was significantly lower, while the number of CD3+CD4−CD8+ T cells was significantly increased. The bacteria isolated from the MLNs were Escherichia coli.
Conclusions: Heat stress damages rat intestinal mechanical and mucosal immune barriers, and reduces immune function of the intestinal mucosa and mesenteric lymphoid tissues, leading to bacterial translocation.
Introduction
Stress can seriously impair an animal's digestive and immune systems Citation[1], Citation[2]. With global climate warming the impact of heat stress on the body, especially with regard to immune function, is increasingly being investigated Citation[3–6]. An increased heterophil to lymphocyte ratio indicates a relationship between heat stress and non-specific immune reactive cells under high ambient temperature Citation[3], Citation[7]. Indeed, the immune status of animals can be improved by relieving heat stress. For example, heat stress causes a reduction in antibody production in broiler chickens Citation[8], while dietary vitamin E supplementation Citation[4] improved immune response of broilers under heat stress.
The intestinal mucosal barrier includes a mucosal mechanical barrier, a biological barrier, and an immune barrier Citation[9]. The intestinal epithelial barrier, also called the mechanical barrier, is composed of the apical plasma membrane of enterocytes (which form the transcellular barrier) and intercellular tight junctions (TJ) (which form the paracellular barrier) Citation[10]. An intact intestinal epithelial TJ barrier is crucial in providing barrier function against paracellular penetration of pathogenic bacteria and toxic luminal antigens, including endotoxins Citation[11], Citation[12]. The intestinal gut flora and their metabolites constitute a biological barrier of the intestine, promoting nutrient digestion, absorption and synthesis, and improving the intestinal immune barrier Citation[13]. The immunological barrier formed by secretory IgA, produced by immune cells in the intestinal mucosa, limits intestinal bacteria and endotoxins to the intestine. Not only various types of stresses, such as haemorrhagic shock Citation[14], Citation[15], endotoxaemia Citation[16–18], psychogenic stress Citation[19–21], exertional stress Citation[22], and heat stroke Citation[23–27] cause an increase in intestinal permeability to luminal endotoxins and lead to bacterial translocation Citation[14], Citation[15], Citation[25], Citation[26], but also hepatic cirrhosis, obstructive jaundice, intestinal ischaemia − reperfusion injury, trauma and infection can increase intestinal mucosal permeability and intestinal mucosal damage, leading to bacterial translocation and subsequent intestinal infection Citation[28–31].
Microarray analysis of gene expression profiles of rat small intestine in response to heat stress has shown that differentially expressed genes are mainly related to stress, immune regulation, and metabolic processes Citation[32]. However, there is little systematic research examining intestinal bacterial migration induced by immune dysfunction and barrier destruction under heat stress. The present study sought to address this.
Materials and methods
Animals and treatment
After adaptation to the environmental conditions of the experimental animal room for 7 days, 12 male Sprague-Dawley (SD) rats were randomly assigned to either a control or heat-stress group (6 rats per group) according to body weight. Rats in the two groups were housed in an artificial climate chamber (HPG-400BX, Harbin Donglian Electronic Technology, Harbin, Heilongjiang, China) (25°C, relative humidity 60%) with free access to food and water. Rats in the heat-stress group were then subjected to 40°C 2 h daily for 3 days.
Rat behavioural changes were observed during heat stress treatment. Rectal and surface temperatures were recorded using a digital thermometer, as well as an infrared and contact thermometer (Fluke 561, Everett, WA, USA). Body weight and food and water intake were recorded during the 7 adaptation days and the 3 days of heat stress. Total serum cortisol concentration was determined by an iodine (I125) cortisol radioimmunoassay kit, performed according to the manufacturer's instructions (Beijing Chemclin Biotech). Rats were sacrificed on day 3 after heat treatment. Normalised to β-actin, the expression of Hsp70 in the jejunum as assessed by real-time PCR was analysed to help establish the heat-stress model. After irrigation by physiological saline, small intestines were sectioned into duodenum, jejunum, and ileum. Each section was prepared for further research: observation of intestinal tract structure and permeability of epithelium, detection of the changes of the number of goblet cells (GCs), and expression of proteins and Th1/Th2 cytokines related to intestinal mucosal immunity. Bacteria were isolated from the mesenteric lymph nodes (MLNs). Lymphocyte sub-populations in the MLNs were detected by flow cytometry.
Microscopic examination and determination of villi dimensions
As previously reported Citation[33], small intestine tissue samples were fixed by a 4% formaldehyde and 2.5% glutaral-paraformaldehyde mixture, and then embedded in paraffin and cut in transverse (5-µm thick) sections. Sections of the duodenum, jejunum, and ileum were stained with haematoxylin and eosin (H&E) after deparaffinisation and dehydration. Mucosal structure was observed using a BH2 Olympus microscope (Tokyo, Japan) and analysed using an image analysis system (Olympus 6.0, Tokyo). Villi height, width, and crypt depth from duodenum, jejunum, and ileum were recorded and measured. Of each section, the mean length of the six longest villi heights was calculated statistically.
Electron microscopy examination
As previously reported Citation[33], tissue sections were fixed for 1 h in cold 1% osmium tetroxide in cacodylate buffer. Preparations were fixed by 2.5% glutaral-paraformaldehyde mixture. After dehydration in graded ethanol solutions, and treatment in isopentyl acetate, preparations were embedded in Araldite (EPON 812, Emicron, Shanghai). Ultra-thin sections were stained with saturated uranyl acetate in 50% ethanol and lead citrate, and examined by transmission electron microscopy (JEM 1230, JEOL, Tokyo).
Counting of goblet cells
Paraffin sections were stained with periodic acid-Schiff (PAS). Six villi heights of each intestinal tract were observed, the numbers of the GCs between 100 consecutive epithelial cells were counted and the mean values computed.
SDS-polyacrylamide gel electrophoresis and western blotting
As assessed by the light and electron microscopic examinations, damage in the jejunum was the most severe compared with the other parts of the intestine. Thus, protein from rat jejunum was extracted by a total protein extraction kit (K3011010, BioChain, San Francisco, CA, USA). The concentration of extracted protein was quantified by a BCA protein assay kit (23225, Pierce, Thermo Scientific, Rockford, IL, USA). Goat anti-rat TLR2 and TLR4 antibodies were from Beijing Biosynthesis Biotechnology. Goat anti-rat β-actin antibody (clone SC-1615) and donkey anti-goat IgG-HRP (clone SC-2033) were from Santa Cruz Biotechnology. Blots were visualised by an AlphaImager HP gelatine imaging system to find differences between the heat-stress and control groups by analysing the grey scale values of blots.
Detection of Th1/Th2 cytokine mRNA related to the intestinal mucosa
Total RNA was extracted as previously reported Citation[33]. RNA was isolated from small intestine tissue using phenol and guanidine isothiocyanate-based TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The concentration and purity of isolated RNA was assessed by spectrophotometry (SmartSpec Plus, Bio-Rad Laboratories, Hercules, CA, USA) based on the OD260/OD280 ratio. Total RNA was reverse transcribed as follows: 2.0 µg RNA isolated from each tissue sample was added to 25 µL reaction solution containing 2.0 µL oligo-dT18, 5.0 µL dNTPs, 1.0 µL RNase inhibitor, 1.0 µL M-MLV transcriptase, 5.0 µL M-MLV RT reaction buffer (Promega, Madison, WI, USA) and RNase-free water. The reverse-transcription procedure was performed based on the manufacturer's instructions; in brief, 70°C for 5 min followed by 42°C for 2 h. IFN-γ, IL-2, IL-4, and IL-10 targets were amplified by PCR with specific primer pairs listed in .
Table 1. Primers used for PCR.
Flow cytometry detection of lymphocytes in the MLNs
MLNs were ground to pieces with a 100-mesh stainless steel screen after washing by aseptic phosphate-buffered saline (PBS) 3 times. Screens were washed with PBS and filtrate collected in a centrifuge tube. Tubes were centrifuged for 5 min at 1400 rpm. The supernatant was discarded and the sediment suspended. Lymphocytes in the MLNs were extracted by hydroxypropylmethylcellulose (TBD Sciences, Tianjin, China). Each sample contained at least 10 000 cells. The percentages of T-cell sub-populations were determined using triple-colour flow cytometry analysis using FITC mouse anti-rat CD3 (G4.18, BD Pharmingen, San Diego, NJ, USA), APC mouse anti-rat CD4 (OX-35, BD Pharmingen), and PE mouse anti-rat CD8a (OX-8, BD Pharmingen) in a one-step procedure with an electronic gate set on CD3+ cells. This permitted the identification of the T-cell sub-populations that included CD3+CD4+CD8− (naïve Th cells), CD3+CD4−CD8+ (Tc cells), and CD3+ T cells. Data are expressed as percent-positive T cells, Th cells, or Tc cells for the different groups.
Separation and identification of bacteria in the MLNs
Bacteria were separated from MLNs under aseptic conditions. They were identified according to the features of bacterial colonies, Gram staining, API 20 E kit (API, Bois d'Arcy, France), and API web software.
Statistical analysis
All results are presented as mean ± SD. Statistical analysis was performed by one-way ANOVA using SPSS 16.0 software. A p value < 0.05 was considered significant.
Results
Assessment of heat treatment
Compared with the control group, rat body and rectal temperatures were significantly increased after the 2-h exposure to at 40°C (data not shown). The cortisol levels of rats in the heat-stress group (17.69 ± 2.94 ng/mL, p < 0.05) increased significantly on day 3 in contrast to rats in the control group (9.47 ± 5.09 ng/mL), consistent with Yu et al.'s report Citation[33]. Heat stress increased the water intake of rats, and decreased the food consumption. The average body weight of rats decreased significantly after heat stress (p < 0.05). The expression of Hsp70 in the jejunum increased 15.6-fold changes after heat stress (p < 0.01). All the results indicated that the heat-stress model was established, and that samples collected could be used for further research.
Changes of mucosal structure in the small intestine under heat stress
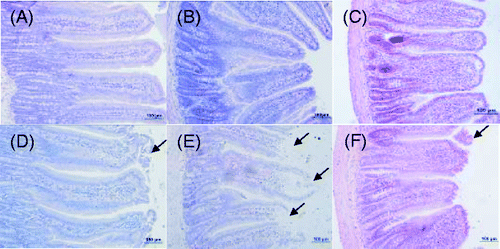
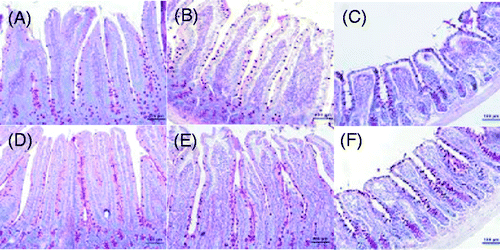
Photomicrographs of H&E-stained sections of the rat small intestine are shown in . The structure of the small intestine was intact, and the mucosal epithelial cells regularly arranged (A–C) in the control rats. However, villi of the duodenum, jejunum, and ileum in the heat-stressed rats were all damaged, indicated by shortening of villi height and crypts, especially in the jejunum where it was most severe. Desquamation of mucosal epithelia was exhibited at the tips of the intestinal villi, exposing the lamina propria, where inflamed cells infiltrated into the mucosa (D–F; arrows represent damage to the mucosal epithelial cells).
Figure 1. Photomicrographs of H&E-stained sections of rat jejunum, duodenum, and ileum on day 3 (200 × magnification); top row, control group; lower row, heat-stress group (A, D, duodenum; B, E, jejunum; C, F, ileum). Villi of the duodenum, jejunum, and ileum in the heat-stress rats were all damaged, especially in the jejunum. Scale bar represents 100 µm. Arrows represent damage to the mucosal epithelial cells.
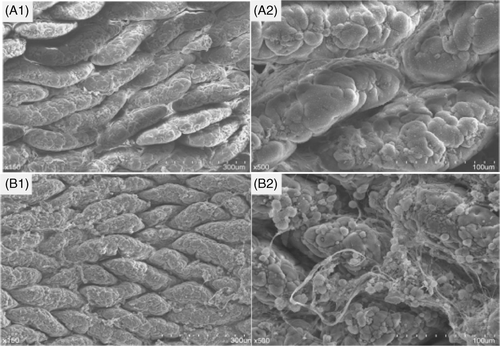
Transmission electron microscopic examination of changes in jejunal mucosa
As changes in the jejunal mucosa in the heat-stress group were most obvious under light microscopy, we performed ultrastructure examination of rat jejunal epithelia. In the control group, fingered or lingulate apophysis could be seen on the surface of intestinal mucosal villi, arranged regularly and gathered tightly. However, in the heat-stress group, the intestinal mucosa was abnormal and exudates containing a large amount of inflamed substances flowed out (A1B1, 1500 × magnification). Under the high-power lens, villi height and micro-villi heights from the control group were arranged regularly, with clear delimitation. In the heat-stress group, TJs between epithelial cells were obscure and intercellular spaces were loosened, indicating enhanced permeability of cells and occurrence of inflammation (A2B2, 5000 × magnification).
Figure 2. Ultrastructure examination of rat jejunal epithelium on day 3; top row, control group; lower row, heat-stress group (A1, B1, 1500 × magnification; A2, B2, 5000 × magnification). Abnormal microstructure was found after heat stress. A large amount of inflamed fibrous substances flowed out, tight junctions between epithelial cells became obscure, and intercellular spaces were loosened.
Observation of goblet cells in intestinal mucosa
Paraffin sections of the small intestine were stained with PAS (), and the numbers of the GCs in the duodenum, jejunum, and ileum were counted. The numbers of GCs in the duodenum and jejunum in the heat-stress group were less than that in the control group (p < 0.05 for the duodenum), though the number of GCs in the ileum was significantly increased (p < 0.05).
Figure 3. Photomicrographs of PAS-stained sections of rat jejunum, duodenum and ileum on day 3 (200 × magnification). Heat stress significantly decreased the numbers of the goblet cells in the duodenum and jejunum, but significantly increased the number of goblet cells in the ileum. Scale bar represents 100 µm.
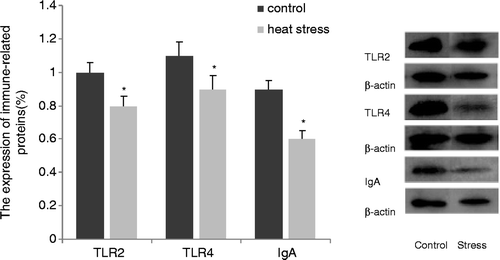
Heat stress reduced the expressions of TLR2, TLR4, and sIgA in the jejunum
Changes in the structure of the intestinal mucosa after heat stress indicated that mechanical barriers were damaged. Furthermore, jejunal damage was the most severe and was therefore selected for the detection of the expression of proteins related to the mucosal immune response. After heat-stress treatment, the expression of TLR2 and TLR4, which play a role in stabilising intestinal ecology, as well as the expression of sIgA, which produces a marked effect on local immunity, were significantly decreased (p < 0.05, ).
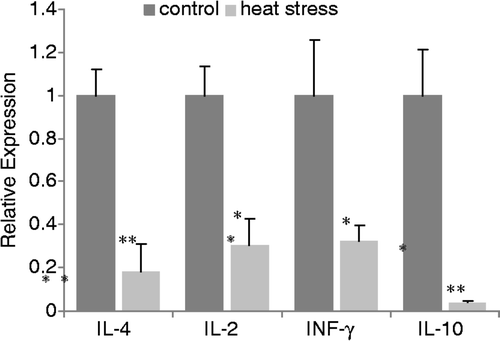
Heat stress reduced expression of cytokine mRNA related to Th1/Th2 cells in the mucosa
Heat stress significantly decreased the expression of TLR2/TLR4 and sIgA, which are related to mucosal immunity, indicating that heat stress had impaired the non-specific immune responses of the intestine. Cytokine mRNAs in the mucosa were quantified by real-time PCR. Results revealed that the expression of Th1 cytokines (IL-2 and IFN-γ) were significantly decreased (p < 0.05, ) after heat stress, as were Th2 cytokines (IL-4 and IL-10) (p < 0.05, ).
Flow cytometry analysis of quantitative changes of lymphocytes in the MLNs
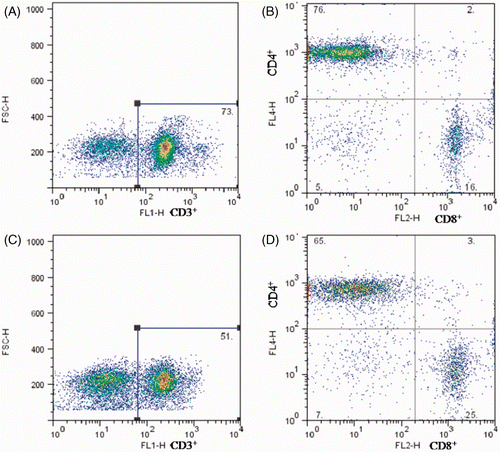
shows the flow cytometry diagrams of the total numbers of T cells and their sub-populations in the MLNs. Compared with the control group (CD3+ T cells 72.43 ± 3.03, Th cells 75.24 ± 1.61), the number of CD3+T cells (54.06 ± 3.15) and Th cells (68.95 ± 3.13) in the heat-stress group was significantly lower (p < 0.05). The number of Tc cells (23.46 ± 1.69) was significantly higher than that in the control group (18.43 ± 2.03) (p < 0.05), however. These data indicate that after heat-stress treatment, humoral immune function in the MLNs is impaired, concomitant with a decline of total T cells and an increase in Tc cells.
Figure 6. Flow cytometry diagrams of quantitative changes of lymphocytes in the MLNs on day 3 after heat stress. Top row, control group; lower row, heat-stress group. A and C represent the numbers of CD3+ T cells in the MLNs. B and D represent the percentages of T-cell sub-populations, top left section represent CD3+CD4+CD8− Th cells and low right section represent CD3+CD4-CD8+ Tc cells.
Heat stress induced bacterial migration
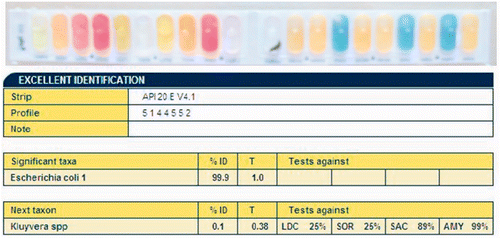
We observed single bacterial colonies from MLNs cultivated for bacteria on agar medium. Gram staining and light microscopic examination revealed moderate-sized Gram-negative bacilli. We used an API 20 E kit to perform bacterial biochemical tests; positive or negative results as judged by colour were input into the API web software system and analysed (). Results revealed that all the characteristics were 99% consistent with Escherichia coli, thus showing that the isolated bacteria from the MLNs were Escherichia coli.
Discussion
Previously we have shown that, compared with rats not exposed to heat stress, serum cortisol increased significantly on days 1, 3 and 6 after heat stress, with damage in the small intestine most pronounced on day 3 Citation[34]. In the present study, we thus chose day 3 to study changes in the rat immune response, subjecting rats to high temperature 2 h per day for 3 days. After heat treatment, the rats’ surface and rectal temperatures in the heat-stress group were significantly higher than those in the control group, and serum cortisol levels were significantly increased on the third day. Mean body weights of rats decreased significantly on the first and second day after heat stress, and the expression of Hsp70 in the jejunum increased significantly, consistent with previous reports Citation[32], Citation[34–38]. This indicated that the heat-stress model was successfully established. We then showed that heat stress could damage the rat intestinal barrier and mucosal immune barrier, altering the functions of intestinal mucosa and gut-associated lymphoid tissue, consequently leading to bacterial translocation.
The pathogenesis of stress-induced intestinal dysfunction has yet to be fully elucidated. Under heat stress, internal body heat is dissipated by increased peripheral blood flow, which dramatically reduces blood flow to the GI tract Citation[39], Citation[40]. Heat stress (41.5°C) increases the intestinal permeability of rats to portal LPS Citation[26] or FITC-dextran Citation[41], indicating loss of TJ integrity and likely enterocyte membrane damage, possibly setting the precondition for bacterial migration Citation[42]. To maintain the integrity of the barrier function of the intestine under normal circumstances, intestinal bacteria and endotoxins must be confined to the enteric cavity, and not result in transfer of bacteria to the peripheral organs Citation[12], Citation[20]. In the present study, light and electron microscopic observations revealed that heat stress severely destroyed intestinal epithelial cells and the mucosal barrier, which could account for the subsequent migration of bacteria.
Intestinal GCs play an important role in the protection of the intestinal mucosa and the reconstruction of the intestinal mucosa early after injury Citation[43]. Mucus can protect the intestinal mucosa and maintain the integrity of intestinal epithelial cells by directly inhibiting toxins from contacting GCs Citation[44], Citation[45]. In the present study, the desquamations were observed at the tips of villi in the duodenum and jejunum of heat-stressed rats, and the numbers of GCs in the duodenum and jejunum were also lower compared with the control group. The expression of typical Th1 (IFN-γ/IL-2) and Th2 (IL-4/IL-10) cytokines from the intestinal mucosa were also significantly lower in the heat-stress group compared with the control group. This indicated injury of the intestinal mechanical barrier and immune barrier and weakening of the ability to prevent bacterial invasion. Some members of the TGF-β superfamily are up-regulated after heat stress Citation[33], and TGF-β is able to down-regulate both Th1 and Th2 responses in mucosal tissues Citation[46]. Dysregulated Th2 cells cause goblet cell transformation in the small intestinal epithelium mediated by excess IL-4 Citation[47]. We found that after heat-stress treatment, the expression of IL-4 in the ileum was significantly lower compared with the control group, while the expression of IL-4 in the duodenum and jejunum showed no significant differences (data not shown). The decreased expression of IL-4 thus might increase the number of GCs in the ileum.
Toll-like receptors (TLRs) widely expressed in the innate immune system recognise pathogen-associated molecular patterns in symbiotic bacteria or pathogens and play an important role in mucosal tolerance or protection and maintenance of intestinal homeostasis Citation[48–51]. The expression of TLR2 and TLR4 reflects the consequences of the interaction of intestinal epithelial cells with intestinal microbiota Citation[52]. Secretory IgA is secreted by gastrointestinal mucosa and determines the immune level of the gastrointestinal tract Citation[53–55]. In our study, heat stress reduced the expressions of TLR2, TLR4 and intestinal mucosal IgA, which implies decrease in immune surveillance and sIgA secretion, immune barrier damage of the intestinal mucosa and weakening of the ability to resist bacterial invasion, which subsequently led to bacterial translocation.
Organisms maintain immune functions by mediating between CD4+ T cells and CD8+ T cells. CD4+ T cells, which can recognise antigens presented by major histocompatibility complex (MHC) II, participate in an assistant role and in delayed-type hypersensitivity. CD8+ T cells recognise antigen peptides presented by MHC I and can directly kill specific target cells, thus playing roles in cell toxicity and immune suppression Citation[56]. MLNs are not only the first line of defence to resist intestinal bacterial invasion, but also play roles in humoral and cellular immunity Citation[57], Citation[58]. Detection of bacteria from MLNs is one of the most direct and sensitive methods to estimate barrier destruction and bacterial migration Citation[59]. In the present study, the expression of typical Th1 cytokines (IFN-γ/IL-2) and Th2 cytokines (IL-4/IL-10) associated with cell-mediated and humoral immunity were down-regulated. The numbers of T cells and Th cells in MLNs were significantly decreased under heat stress. Bacteria isolated from MLNs of heat-stressed rats were identified as Escherichia coli. These results collectively demonstrate reduction of the intestinal mucosal immune function in rats due to heat stress, thus leading to the translocation of bacteria from the intestine.
In summary, we have shown that heat stress can damage the rat intestinal mucosa, increase intestinal permeability, injure the immunological and biological intestinal barriers, and significantly decrease the numbers of T cells and CD3+CD4+CD8- Th cells in MLNs, which could explain the reasons for Escherichia coli translocation.
Acknowledgements
We are thankful for the help from the members of CAU-BUA TCVM teaching and research team. Liu Fenghua and Xu Jianqin contributed equally to this work.
Declaration of interest: This work was supported by grants from the Twelve-Five National Technological Support Plan of China (2011BAD34B01), PHR (IHLB), the Research Fund for the Doctoral Program of Higher Education of China and the Ministry of Agriculture, Public Service Sectors Agriculture Research Projects (201003060-9/10).
References
- Choo D, Khwaja K, Nori K, Rewinski M, Zeff R, Perdrizet G. In vivo characterization of the molecular-genetic changes in gastric mucosa during the development of acute gastritis and stress ulceration. J Trauma 2002; 52: 720–725, discussion 725–726
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol 2006; 66: 552–563
- Mashaly MM, Hendricks GR, Kalama MA, Gehad AE, Abbas AO, Patterson PH. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 2004; 83: 889–894
- Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci 2009; 88: 2101–2107
- Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa LR, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci 2010; 89: 1905–1914
- Starkie RL, Hargreaves M, Rolland J, Febbraio MA. Heat stress, cytokines, and the immune response to exercise. Brain Behav Immun 2005; 19: 404–412
- McFarlane JM, Curtis SE. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and the heterophil:lymphocyte ratio. Poult Sci 1989; 68: 522–527
- Zulkifli I, Che NM, Israf DA, Omar AR. The effect of early age feed restriction on subsequent response to high environmental temperatures in female broiler chickens. Poult Sci 2000; 79: 1401–1407
- Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care 2002; 5: 685–694
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17: 375–412
- Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol 2000; 2: 305–315
- Fasano A, Nataro JP. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv Drug Deliv Rev 2004; 56: 795–807
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10: 159–169
- Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma 1988; 28: 896–906
- Deitch EA, Bridges W, Baker J, Ma JW, Ma L, Grisham MB, et al. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery 1988; 104: 191–198
- O’Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg 1988; 123: 1459–1464
- Salzman AL, Wang H, Wollert PS, Vandermeer TJ, Compton CC, Denenberg AG, et al. Endotoxin-induced ileal mucosal hyperpermeability in pigs: Role of tissue acidosis. Am J Physiol 1994; 266: G633–G646
- Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg 1988; 123: 1313–1319
- Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: Role of mast cells. Am J Physiol Gastrointest Liver Physiol 2000; 278: G847–G854
- Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 2000; 119: 1019–1028
- Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci 2002; 47: 208–215
- Bosenberg AT, Brock-Utne JG, Gaffin SL, Wells MT, Blake GT. Strenuous exercise causes systemic endotoxemia. J Appl Physiol 1988; 65: 106–108
- Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Antilipopolysaccharide improves survival in primates subjected to heat stroke. Circ Shock 1987; 23: 157–164
- Shapiro Y, Alkan M, Epstein Y, Newman F, Magazanik A. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Appl Physiol Occup Physiol 1986; 55: 410–412
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 2001; 280: H509–H521
- Gathiram P, Wells MT, Brock-Utne JG, Wessels BC, Gaffin SL. Prevention of endotoxaemia by non-absorbable antibiotics in heat stress. J Clin Pathol 1987; 40: 1364–1368
- Gathiram P, Gaffin SL, Brock-Utne JG, Wells MT. Time course of endotoxemia and cardiovascular changes in heat-stressed primates. Aviat Space Environ Med 1987; 58: 1071–1074
- Nakagawa H, Tsunooka N, Yamamoto Y, Yoshida M, Nakata T, Kawachi K. Intestinal ischemia/reperfusion-induced bacterial translocation and lung injury in atherosclerotic rats with hypoadiponectinemia. Surgery 2009; 145: 48–56
- Chiva M, Guarner C, Peralta C, Llovet T, Gomez G, Soriano G, et al. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol 2003; 15: 145–150
- Nakagawa H, Tsunooka N, Yamamoto Y, Yoshida M, Nakata T, Kawachi K. Pitavastatin prevents intestinal ischemia/reperfusion-induced bacterial translocation and lung injury in atherosclerotic rats with hypoadiponectinemia. Surgery 2009; 145: 542–549
- Ara C, Esrefoglu M, Polat A, Isik B, Aladag M, Gul M, et al. The effect of caffeic acid phenethyl ester on bacterial translocation and intestinal damage in cholestatic rats. Dig Dis Sci 2006; 51: 1754–1760
- Lu A, Wang H, Hou X, Li H, Cheng G, Wang N, et al. Microarray analysis of gene expression profiles of rat small intestine in response to heat stress. J Biomol Screen 2011; 16: 655–667
- Yu J, Yin P, Liu F, Cheng G, Guo K, Lu A, et al. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp Biochem Physiol A Mol Integr Physiol 2010; 156: 119–128
- Yu J, Yin P, Yin J, Liu F, Zhu X, Cheng G, et al. Involvement of ERK1/2 signalling and growth-related molecules’ expression in response to heat stress-induced damage in rat jejunum and IEC-6 cells. Int J Hyperthermia 2010; 26: 538–555
- Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp Biochem Physiol B Biochem Mol Biol 2004; 137: 35–42
- Srikandakumar A, Johnson EH. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop Anim Health Prod 2004; 36: 685–692
- Sinha RK. Study of changes in some pathophysiological stress markers in different age groups of an animal model of acute and chronic heat stress. Iran Biomed J 2007; 11: 101–111
- Elez D, Vidovic S, Matic G. The influence of hyperthermic stress on the redox state of glucocorticoid receptor. Stress 2000; 3: 247–255
- Deja M, Ahlers O, Macguill M, Wust P, Hildebrandt B, Riess H, et al. Changes in hepatic blood flow during whole body hyperthermia. Int J Hyperthermia 2010; 26: 95–100
- Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol 1988; 64: 2582–2588
- Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 2002; 92: 1750–1761
- Tekbas OF, Ceylan S, Hamzaoglu O, Hasde M. An investigation of the prevalence of depressive symptoms in newly recruited young adult men in Turkey. Psychiatry Res 2003; 119: 155–162
- Masuda K, Ikeda H, Kasai K, Fukuzawa Y, Nishimaki H, Takeo T, et al. Diversity of restitution after deoxycholic acid-induced small intestinal mucosal injury in the rat. Dig Dis Sci 2003; 48: 2108–2115
- Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 2003; 221: 42–49
- Matovelo JA, Sund RB, Landsverk T. Morphological and functional recovery following exposure to deoxycholic acid. A study in the rat small intestine in vivo. Apmis 1989; 97: 798–810
- Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003; 33: 1205–1214
- Dohi T, Fujihashi K, Koga T, Shirai Y, Kawamura YI, Ejima C, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology 2003; 124: 672–682
- Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr 2005; 40: 589–595
- Michaelsson G, Kristjansson G, Pihl LI, Hagforsen E. Palmoplantar pustulosis and gluten sensitivity: A study of serum antibodies against gliadin and tissue transglutaminase, the duodenal mucosa and effects of gluten-free diet. Br J Dermatol 2007; 156: 659–666
- Pasare C, Medzhitov R. Toll-like receptors: Balancing host resistance with immune tolerance. Curr Opin Immunol 2003; 15: 677–682
- Pasare C, Medzhitov R. Toll-like receptors: Linking innate and adaptive immunity. Adv Exp Med Biol 2005; 560: 11–18
- Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem 2008; 56: 267–274
- Tagliabue A, Nencioni L, Villa L, Keren DF, Lowell GH, Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature 1983; 306: 184–186
- Magnusson KE, Stjernstrom I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology 1982; 45: 239–248
- Beagley KW, Fujihashi K, Aicher W, Xu J, Kiyono H, Eldridge JH, et al. Mucosal homeostasis: Role of interleukins, isotype-specific factors and contrasuppression in the IgA response. Immunol Invest 1989; 18: 77–89
- Yamashita U. [Antigen-recognition mechanism of T cells]. J UOEH 1998; 20: 153–162
- Robinson K, Chamberlain LM, Lopez MC, Rush CM, Marcotte H, Le Page RW, et al. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect Immun 2004; 72: 2753–2761
- Zimecki M, Artym J, Chodaczek G, Kocieba M, Kruzel M. Effects of lactoferrin on the immune response modified by the immobilization stress. Pharmacol Rep 2005; 57: 811–817
- de Souza LJ, Sampietre SN, Assis RS, Knowles CH, Leite KR, Jancar S, et al. Effect of platelet-activating factor antagonists (BN-52021, WEB-2170, and BB-882) on bacterial translocation in acute pancreatitis. J Gastrointest Surg 2001; 5: 364–370