Abstract
Purpose: Chronic heat exposure in mice has cellular and physiological effects that improve thermal tolerance [1], but also modifies innate immune responses with potential adverse consequences [2]. While male and female mice are known to respond differently to acute exposure to severe hyperthermia, sex-based differences in responses to chronic moderate heat exposure have not been reported. The major objective of this study was to compare the tolerance of male and female mice for chronic heat exposure.
Materials and methods: We used a mouse model of 5-day moderate heat exposure (ambient temperature ∼37°C) to compare the physiological and cellular heat shock response in male and female mice. Core temperature, heart rate, and activity were monitored telemetrically and heat shock protein levels were measured in brain and lung by western blotting.
Results: Adult CD-1 female mice maintained a 1.2°C lower core temperature (38.31 ± 0.64 versus 39.51 ± 0.72°C; p = 0.002), experienced less weight loss (1.54 ± 0.45 versus 4.54 ± 1.97 g; p = 0.0007), and had improved survival (16/16 survived versus 13/21, p < 0.006) than male mice of the same age. After 5 days of moderate heat exposure Hsp72 levels in brain and lung increased 2.1-fold (p = 0.007) and 5-fold (p = 0.048) in male mice compared with 1.3- (p = 0.054) and 1.5-fold (p = 0.134) in female mice.
Conclusions: This study reveals previously unknown and potentially important differences between male and female mice in physiological and cellular responses to chronic heat exposure, which had consequences for survival. Future studies may identify biomarkers of differential heat tolerance and treatments to improve heat tolerance in humans.
Introduction
As efficient homeotherms, humans regulate body temperature within a narrow range Citation[3]. When excess heat is generated by increased metabolic activity or heat elimination is impaired by increased ambient temperature and/or humidity, humans compensate to maintain a thermal steady state. Repeated exposure to such conditions improves subsequent tolerance of such conditions through a process called heat acclimation Citation[4]. We have previously shown that the physiological responses to heat acclimation in humans are accompanied by modifications in the heat shock gene expression profile Citation[5] that may contribute to the beneficial effects of heat acclimation. Uncompensable heat stress occurs when heat generation exceeds the capacity for heat elimination and results in progressively increasing core temperature and heat-related illness Citation[6]. We have shown that exposure to the same temperatures that cause heat acclimation can also modify critical barrier function of endothelial cells Citation[7], Citation[8], which contribute to increased lung injury during exposure to febrile-range hyperthermia Citation[2], Citation[9], Citation[10].
Chronic compensable heat exposure and acute uncompensable heat stress are often unavoidable, especially in individuals living in hot environments without access to air conditioning and in those engaged in particular professions such as professional athletes and military personnel. Progression of global warming and increased competition for resources is likely to expand the population at risk for heat-related illness Citation[11]. Since prevention is the most effective approach to minimising the impact of heat-related illness Citation[11], identifying high-risk individuals would be of great value. Several demographic and physiological characteristics are clearly associated with reduced thermal tolerance including very young and very old age, obesity, and reduced physical fitness Citation[12], Citation[13]. The influence of gender on thermal tolerance is less clear.
Men generally demonstrate smaller increases in core temperature and greater tolerance to acute heat exposure whether at rest Citation[14] or during exercise Citation[15], Citation[16], but much of the difference in tolerance may be caused by confounding factors, including greater adipose content and reduced fitness in women. Completing a heat acclimation protocol has a greater impact on subsequent thermal tolerance in women than men Citation[17], thereby reducing the gender difference in heat acclimated populations. In a study of heat acclimated individuals, Shapiro et al. Citation[18] found that women exhibited greater thermal tolerance than men during exposure to moderately high ambient temperature (37°C) at high relative humidity (80%), but men had greater tolerance for hot, dry conditions (49°C, 20% humidity). In a small study of four physically fit men and women, Avellini et al. Citation[4] found a similar advantage in women to moderate heat and high humidity. In addition to anthropometrics, physical fitness, and previous heat acclimation, the menstrual cycle also modifies thermal tolerance in women. For example, women not using oral contraceptives exhibited reduced thermal tolerance during the luteal phase of the menstrual cycle Citation[19], Citation[20].
These studies suggest that men generally exhibit greater thermal tolerance than women in response to acute uncompensable heat exposure but identify a multitude of confounding factors that may account for most or all of the differences. Much less is known about the relative tolerance of men and women for chronic exposure to moderate heat.
In this study we used adult (10 to 14 weeks old) male and female mice to study sex-based differences in tolerance for chronic exposure to moderate heat and acute uncompensable heat exposure.
Materials and methods
Animals
Ten- to 12-week-old male and female CD-1 mice were purchased from Charles River (Wilmington, MA, USA) in three separate lots and housed in the Animal Care Facility in the Veterans Administration Medical Center, Baltimore, under AALAC-approved conditions and under the supervision of a full-time veterinarian. Male and female mice were housed separately to maintain females in an anestrus state and studied in parallel. Mice were adapted to standard plastic cages with standard bedding and a plastic igloo for at least 7 days before being subjected to heating protocols and were used within 4 weeks of arrival. To avoid the influence of diurnal cycling, all experiments were started at approximately the same time each day (between 08:00 and 10:00). Lights were cycled on and off at 07:00 and 19:00, respectively. Mice were implanted with intraperitoneal telemetric thermistors (ETA-F10, Data Sciences International (DSI), St Paul, MN, USA) 7 days prior to heat exposure. A sterilised ETA-F10 transmitter was placed into the peritoneal cavity and subcutaneous electrodes secured under isoflurane anaesthesia as described in the manufacturer's protocol. Postoperatively, the mice were housed one mouse per cage, received 0.1 mg/kg buprenorphine analgesia subcutaneously every 12 h for two post-operative days with food and water provided ad libitum immediately after surgery, and allowed to recover for 7 days in standard animal facility conditions, ∼22°C ambient temperature and 43–65% relative humidity.
Heating protocols
All procedures were approved by the Baltimore Veterans Administration and the University of Maryland, Baltimore, Institutional Animal Care and Use Committee.
Five-day moderate heat exposure was achieved by transferring the cage containing one mouse with standard bedding and a plastic igloo into modified Air Shields™ (Bloomfield, CT, USA) infant incubators set to 37°C with food and water provided ad libitum. No additional humidification was provided so the relative humidity was calculated to be 18.1–27.4% using the August-Roche-Magnus approximation Citation[21]. We have previously shown that exposing mice to ambient temperature of 37°C with access to drinking water of the same temperature causes core temperature to increase above 37°C and have attributed the temperature increase to a reduction in the heat elimination gradient Citation[1], Citation[9], Citation[10]. Core temperature, heart rate, and a semi-quantitative assessment of activity were continuously measured at 20 s intervals using the DSI Automated Data Acquisition System. Activity was measured using the DSI proprietary algorithm that predominantly assesses locomotor activity by measuring change in transmitter signal strength. Normothermic controls were housed under identical conditions except at standard room temperature (∼22°C; 43–65% relative humidity).
Acute uncompensable thermal stress was achieved by exposing mice to 43°C ambient temperature for 45 min. An Air Shields™ infant incubator was modified to reach ambient temperatures of 43°C (calculated relative humidity 13.2–19.9%) using three radiant heating panels (Boaphile model 1611) and controlled with an electronic temperature controller (Ranco, London, UK) as we have previously described Citation[1]. Mice implanted with transmitters were transferred to a standard mouse cage in the 43°C incubator for 45 min with continuous monitoring of core temperature, heart rate, and activity. The igloo and most of the bedding were removed to allow even heat distribution within the cage. Water but not food was available ad libitum during the thermal stress exposure. Mice were then transferred back to their original cages with bedding, igloo, water, and food at room temperature (∼22°C) and physiological parameters monitored for an additional 3 h.
Body weight was measured manually using an electronic balance before surgery and prior to and following heat exposures. The food and water bottles were weighed prior to and immediately following the 5-day heat exposure and at comparable times in the normothermic controls.
Analysis of heat shock protein levels
Following euthanasia by exsanguination via cardiac puncture and cervical dislocation under deep isoflurane anaesthesia, lungs and brain were harvested from normothermic and 5-day-heat exposed mice and snap frozen in liquid nitrogen for western blot analysis. These organs were shown to be affected in our previous study of male mice subjected to the same 5-day heat exposure Citation[1] and clinical and mouse studies suggest the brain and lung are two organs that are most vulnerable to the adverse effects of hyperthermia Citation[22], Citation[23]. Frozen organs were homogenised (Precellys® 24, Bertin Technologies, Montigny-le-Bretonneux, France) using four 20-s cycles at 6,000 rpm in RIPA buffer solution containing 10 µg/mL 4-(2-aminoethyl benzenesulfenylo-fluoride hydrochloride), 20 µg/mL aprotinin (20 µg/µL), phosphatase inhibitors 1 and 2 (Sigma, St Louis, MO, USA), and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). The samples were clarified by centrifugation. Protein levels were measured using a commercial Bradford reagent (Thermo Fisher Pierce, Rockford, IL, USA), 10–20 µg of protein per lane was separated by 10% SDS–PAGE, and electrostatically transferred to PVDF membrane. The membranes were blocked for 1 h at room temperature in blocking buffer (TBS-T) (10 mM Tris HCl, pH 7.5, 136 mM NaCl, 2.0 mM KCl, 0.1% Tween 20) containing 5% non-fat dry milk. Following blocking, the membranes were washed with TBS-T and incubated with antibodies against Hsp72 (SPA812; 1:10,000 dilution, Stressgen, Ann Arbor, MI, USA), Hsp25 (SPA801; 1:10,000 dilution, Stressgen), Hsp90 (SC 7947; 1:10,000 dilution, Santa Cruz Biotechnology, CA, USA), or the control housekeeping gene β-tubulin (MAB3408; 1:20,000 dilution, Millipore, Billerica, MA, USA) in blocking buffer overnight. After incubation with primary antibody, the membranes were washed with TBS-T, incubated for 1 h with horseradish peroxidase-conjugated secondary antibody, developed with a chemiluminescence detection system (Western Lightning Plus – ECL; Perkin-Elmer, Waltham, MA, USA), and quantified by direct imaging (Fuji LS4000 gel documentation system and ImageGauge software (Bedford, MA, USA)). Density of each HSP band was normalised to β-tubulin density after stripping and probing the same gel and standardised to the levels in normothermic males from the same experiment.
Data analysis
Data are presented as mean ± standard error of the mean (SEM). During the 5-day moderate heat exposure, physiological measurements were recorded every 20 s, and 1-hour means calculated. During the 45 min thermal stress, 1-min means of core temperature and heart rate and 15-min means of locomotor activity were calculated. During the recovery period, 2-min means of core temperature were calculated. Differences in physiological measures between male and female mice were analysed by repeated measures analysis of variance (ANOVA) using the JMP statistical package (SAS, Cary, NC, USA). Differences in standardised western blot band densities between the two groups were analysed by unpaired student t-test. Differences among more than two groups were analysed by applying the post hoc Tukey honestly significant difference (HSD) test to a one-way ANOVA.
Results
Female mice exhibit smaller increases in core temperature than male mice during 5-day moderate heat exposure
At 22°C ambient temperature male and female mice exhibited circadian fluctuations in core temperature, heart rate, and activity with nadirs between 08:00 and 10:00 and peaks between 22:00 and 24:00 (). Under these conditions, female mice maintained a mean core temperature 0.55°C higher than the male mice () (). Increasing ambient temperature from 22°C to 37°C caused an increase in core temperature in male and female mice without modifying circadian fluctuations. However, female mice increased mean core temperature by only 1.0°C compared with a 2.8°C increase in the male mice ().
Figure 1. Physiological response to 5-day exposure to moderate hyperthermia. Mice were implanted with intraperitoneal thermal sensors, recovered for 7 days, and core temperature (A, B), heart rate (C, D), and activity level (E, F) in male (A, C, E) and female (B, D, F), respectively, were continuously monitored during 5-day exposure to either 22°C (NT) or 37°C (cHT) ambient temperature. Core temperature and heart rate were measured every 20 s and 1-h means calculated. Activity was measured every 20 s and 4-h means calculated. Four experiments, each with four mice of each sex per group, were pooled. Data are presented as means ± SEM. The differences between NT and cHT mice for each sex are indicated on the figures. NT female mice maintained a 0.55°C higher core temperature than NT males (p = 0.005), but cHT female mice maintained 1.2°C lower core temperature than cHT males (p = 0.002). There were no significant differences in heart rate or activity level between the two sexes.
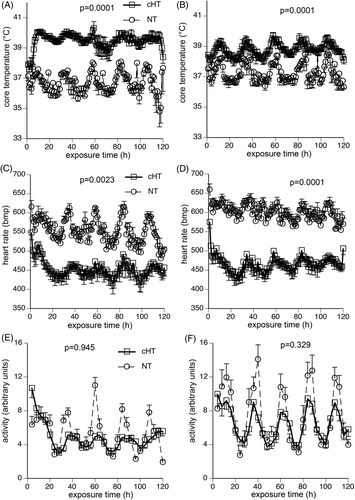
Table I. Change in weight, water and food intake, and survival during 5-day exposure to Normothermic or Hyperthermic conditions1.
Although global activity level tended to be lower during chronic heat exposure compared with normothermic controls for both sexes, there were no significant differences between normothermic and heat-exposed male mice (p = 0.945) and female mice (p = 0.329), and no difference in activity between male and female mice under normothermic (p = 0.0982) or hyperthermia conditions (p = 0.1711) (). Similar reductions in heart rate occurred during the 5-day heat exposure in male mice (453 ± 48 versus 552 ± 49 bpm; p = 0.0023) and female mice (466 ± 42 versus 599 ± 48; p = 0.0001) ().
Mortality during the 5-day heat exposure was substantially greater in the male mice than female mice (). All 16 female mice survived the 5-day exposure compared with only 13 of 21 males with five of the eight male mice dying within the first 48 h. As expected Citation[24], the weight of male mice prior to heat exposure was 23% higher than comparably aged female mice (36.8 ± 0.75 g versus 29.8 ± 0.43 g; p < 0.0001). The male mice that survived the 5-day heat exposure lost 12.3% of their starting weight compared with only a 5.1% loss in female mice during the 5-day exposure (). Normothermic male and female mice gained 0.46 ± 0.39 g and 0.14 ± 0.4 g during the same 5-day period. The male mice that survived the 5-day heat exposure and those that died had similar starting body weight (36.48 ± 1.1 versus 35.48 ± 0.37 g). A linear regression analysis of weight loss during the 5-day heat exposure and pre-exposure body weight in all surviving male and female mice showed an r2 value of only 0.32. Both female and male mice subjected to the 5-day heat exposure tended to have lower 5-day food intake than normothermic controls over the same time period, but the difference did not reach statistical significance (). Female mice had greater water intake during the 5-day heat exposure compared to normothermic female controls but the 5-day heat exposed male mice did not exhibit a similar increase in water intake compared with normothermic male controls ().
Effect of 5-day heat exposure on HSP expression
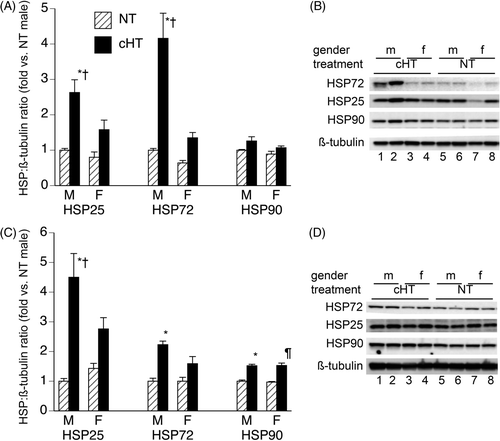
HSP protein levels in lung and brain were measured in mice exposed to chronic 5-day hyperthermia (cHT) and in normothermic (NT) controls (). Male mice that completed a 5-day moderate heat exposure had higher levels of Hsp25 and Hsp72 protein in brain and lung than normothermic males, whereas female mice completing a 5-day moderate heat exposure and normothermic females had similar levels of Hsp25 and Hsp72. Hsp90 levels were ∼1.5-fold higher in brains of 5-day moderate heat-exposed males and females compared with normothermic controls, but Hsp90 levels were similar in the lungs of 5-day heat-exposed and normothermic mice of both sexes.
Figure 2. Effect of 5-day chronic moderate heat exposure on expression of heat shock proteins in lung and brain. Male and female mice exposed to moderate hyperthermia for 5-days (cHT) and normothermic (NT) control mice were euthanised, the lungs (A, B) and brain (C, D) snap-frozen, and tissue homogenates analysed for Hsp25, Hsp72, and Hsp90 levels by immunoblotting. HSP band densities were expressed as ratio to ß-tubulin band densities and standardised to NT male levels for each experiment. (A, C) Mean ± SEM of 16 female and 13 male mice are displayed. (B, D) Representative blots. *, †, and ¶ indicate p < 0.05 versus NT male, cHT female, and NT female, respectively.
Acute exposure to uncompensable heat stress stimulates similar physiological responses in male and female mice
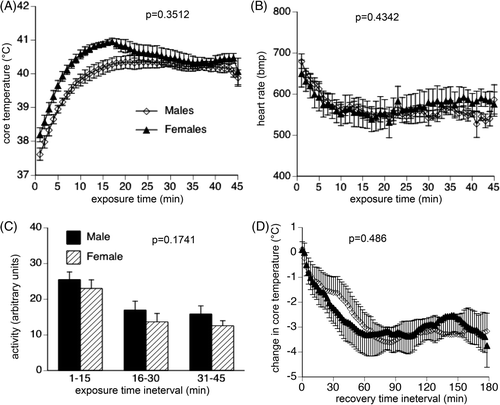
Exposure to 43°C temperature stimulated a rapid ∼3°C increase in core temperature in male and female mice (). Core temperature in female mice tended to be higher for the first 15 min of acute heat exposure, but this difference did not reach statistical significance (p = 0.3512) and the male and female mice maintained a similar core temperature for the remainder of the 45-min heat exposure and similarly returned to normal baseline within 60 min of transfer back to 22°C ambient temperature (; p = 0.486). Male and female mice exhibited similar reductions in heart rate (; p = 0.4342) and persistence of activity (; p = 0.1741) during the acute heat stress. All mice survived the 45-min acute heat stress and the 4-h recovery.
Figure 3. Physiological response to acute exposure to uncompensable heat stress. Following implantation of intraperitoneal thermal sensors, male and female mice with were exposed to 43°C for 45 min, then returned to 22°C while continuously monitoring core temperature, heart rate and activity level from the intraperitoneal sensors. Core temperature (A) and heart rate (B) were measured every 20 s, and 1-min means calculated. Activity (C) was measured every 20 s, and 15-min means calculated. (D) During recovery, core temperature was measured every 20 s, 2-min means calculated and expressed as change from the core temperature measured at the end of the acute heat exposure. Data are expressed as mean ± SEM of 16 male and 16 female mice. P values are indicated in the figure panels.
Discussion
We previously showed that male mice continuously exposed to 37°C ambient temperature for 5 days exhibited a physiological response that closely resembled the human response to heat acclimation protocols Citation[1]. However, 32% (7 of 22) of the mice in that study died during the 5-day heating protocol. In the present study, 38% (8 of 21) of the male mice died during the same 5-day heating protocol (p = 0.75 for comparison of two studies by chi square). In striking contrast, all 16 of the female mice survived the same 5-day heat exposure. The improved survival in the female mice was associated with maintenance of ∼1.2°C lower core temp and less weight loss. The female mice increased their water intake during the heat exposure more than the male mice. Both the male and female mice maintained their underlying circadian patterns and had similar levels of activity during the 5-day heat exposure. In contrast with the substantial difference between male and female mice in tolerance for 5-day moderate heat exposure, we found no sex-based difference in core temperature, heart rate, or activity level during the 45-min exposure to 43°C ambient temperature and 2-h recovery. Since increased physical activity level causes greater heat generation, which would increase core temperature when heat elimination is limited, it was important to exclude sex-based differences in activity during hyperthermia exposure. Although the method used, which detects change in location of the intraperitoneal transmitter within the cage, is a crude measure of physical activity, it suggests that there were no large differences in activity that would explain the difference in core temperature between the male and female mice.
The male mice in the current study lost more weight (12.3% versus 5.8% of starting weight), had higher core temperatures (39.51 ± 0.72°C versus 38.88 ± 0.14°C), and exhibited a smaller increase in water intake (38% versus 65%) than the mice in our previous study Citation[1]. The mice in the current study were exposed to higher relative humidity (18.1–27.4% versus 16.5–19.4% at 37°C ambient temperature) compared with our previous study. Since higher relative humidity impairs heat elimination Citation[25], the higher relative humidity in the current study may have contributed to the poorer outcome in the 5-day heat-exposed male mice compared with our previous study. In the current study male and female mice were exposed to the same temperature and humidity levels. The moderately high temperature and relative humidity used in these studies may have favoured the female mice as in humans women have superior tolerance for moderate heat at high humidity compared with men Citation[18] while men tolerate exposures to hot, dry conditions better than women Citation[18], Citation[26].
Smaller body size and associated increased surface area:mass ratio has been shown to improve tolerance to moderately high heat and high humidity but not high dry heat Citation[27], and may have contributed to the improved tolerance of female mice to 5-day moderate heat exposure. However, the male mice in the current study were smaller than the male mice used in our previous study (36.8 ± 0.75 g versus 40.8 ± 0.5 g), yet the mice in the current study did not exhibit improved thermal tolerance. Adiposity is an independent determinant of thermal tolerance in humans Citation[28]. We used mice between ages 10 and 14 weeks. Male C57B6 mice increase both weight and adiposity faster between 8 and 16 weeks of life than female mice Citation[24], but similar data about body size and composition for the CD-1 mice we used in this study are not available. We did not measure body composition and cannot rule out a contribution of higher adipose content in the male than female mice. However, as discussed above, the male mice used in our previous study were heavier than the male mice in our current study yet exhibited superior thermal tolerance.
As we previously showed Citation[1], male and female mice exhibited similar reductions in heart rate upon transfer to 37°C ambient temperature. We had also found that humans participating in a 10-day heat acclimation protocol exhibited a 12% decrease in mid-exercise heart rate after day 5 of the protocol compared with their pre-acclimated state. Since the human subjects in that study were exercising while the mice in the present study were exposed to passive hyperthermia, it is not clear whether the negative chronotropic effect of thermal stress was mediated by the same mechanisms in the two species. However, Spielman and Lyman Citation[29] reported a similar rapid onset of bradycardia in rats in response to thermal stress, which has been shown to be mediated by both direct effects on intrinsic pacemaker activity Citation[29] and by altered autonomic tone Citation[30]. Whatever the mechanism, there does not appear to be a difference in this response in male and female mice.
The lower water intake and greater weight loss in male than female mice in this study suggest relative impairment of water intake may be an important contributor to the reduced thermal tolerance. Dehydration can contribute to core temperature increase by reducing evaporative heat loss Citation[31], but core temperature curves for male and female mice diverged within the first few hours of heat exposure before significant dehydration would have occurred. Kaufman reported that female rats drank more water than male rats after 48 h of water deprivation or angiotensin II administration Citation[32]. Other groups reported that oestrogen administration reduces water intake in oophorectomised female rats Citation[33–35]. Igbokwe and Obika Citation[36] found similar thirst perception in men and women but these individuals were not studied in the context of heat exposure. Whether males and females have intrinsically different dipsogenic responses during heat exposure is not yet known.
Alternatively, the decrease in water intake by male mice may have been the result rather than the cause of the greater increase in core temperature. The increased expression of Hsp25 and Hsp72 in the brains of the male mice subjected to the 5-day-heat exposure suggests the male mice, but not the female mice exceeded the brain tissue temperature threshold for the cellular stress response. Hyperthermia can cause release of neurotransmitters Citation[37], some of which, like gamma-aminobutyric acid (GABA), can suppress thirst Citation[38]. However, the association of higher brain temperature in the 5-day heat-exposed male mice with reduced water intake does not prove a mechanistic link.
Conclusion
In summary, our data suggest that gender may be an independent determinant of tolerance for chronic heat exposure. Considering the consequences of heat-related illness and the large population exposed to high temperatures for prolonged periods, evaluating sex-based differences in chronic heat tolerance in humans and elucidating the mechanisms of such differences would have important public health implications.
Declaration of interest: This study was supported by US National Institutes of Health grants HL69057 and HL085256 and a Veterans Administration Merit Review grant to J.D.H. The authors alone are responsible for the content and writing of the paper.
References
- Sareh H, Tulapurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperone 2011; 16: 297–307
- Tulapurkar ME, Hasday JD, Singh IS. Prolonged exposure to hyperthermic stress augments neutrophil recruitment to lung during the extended post-exposure period. Int J Hyperthermia 2011; 25: 717–725
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 2008; 93: 773–797
- Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol 1980; 49: 254–261
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, et al. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of Hsp72 and Hsp90 in peripheral blood mononuclear cells. Am J Physiol Reg 1 2008; 294: R185–191
- Sawka MN, Latzka WA, Montain SJ, Cadarette BS, Kolka MA, Kraining KKN, et al. Physiologic tolerance to uncompensable heat: Intermittent exercise, field vs laboratory. Med Sci Sports Exerc 2001; 33: 422–430
- Shah NG, Tulapurkar ME, Damarla M, Singh IS, Goldblum SE, Shapiro P, et al. Febrile-range hyperthermia augments reversible TNF-alpha-induced hyperpermeability in human microvascular lung endothelial cells. Int J Hyperthermia 2012; 28: 627–635
- Tulapurkar ME, Almutairy EA, Shah NG, He JR, Puche AC, Shapiro P, et al. Febrile-range hyperthermia modifies endothelial and neutrophil functions to promote extravasation. Am J Respir Cell Mol Biol 2012; 46: 807–814
- Hasday J, Garrison A, Singh I, Standiford T, Ellis G, Rao S, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol 2003; 162: 2005–2017
- Rice P, Martin E, He J-R, Frank M, DeTolla L, Hester L, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental Gram-negative bacterial pneumonia. J Immunol 2005; 174: 3676–3685
- O’Neill MS, Carter R, Kish JK, Gronlund CJ, White-Newsome JL, Manarolla X, et al. Preventing heat-related morbidity and mortality: New approaches in a changing climate. Maturitas 2009; 64: 98–103
- Kilbourne EM, Choi K, Jones TS, Thacker SB. Risk factors for heatstroke. A case-control study. JAMA 1982; 247: 3332–3336
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, et al. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 1996; 335: 84–90
- Wyndham CH, Morrison JF, Williams CG. Heat reactions of male and female Caucasians. J Appl Physiol 1965; 20: 357–364
- Cunningham DJ, Stolwijk JA, Wenger CB. Comparative thermoregulatory responses of resting men and women. J Appl Physiol 1978; 45: 908–915
- McLellan TM. Sex-related differences in thermoregulatory responses while wearing protective clothing. Eur J Appl Physiol Occup Physiol 1998; 78: 28–37
- Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol 1981; 50: 65–70
- Shapiro Y, Pandolf KB, Avellini BA, Pimental NA, Goldman RF. Physiological responses of men and women to humid and dry heat. J Appl Physiol 1980; 49: 1–8
- Kolka MA, Stephenson LA. Interaction of menstrual cycle phase, clothing resistance and exercise on thermoregulation in women. J Thermal Biol 1997; 22: 137–141
- Tenaglia SA, McLellan TM, Klentrou PP. Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol 1999; 80: 76–83
- Lawrence MG. The relationship between relative humidity and the dew point temperature in moist air: A simple conversion and applications. Bull Am Meteorol Soc 2005; 86: 225–233
- Hasday JD, Shah N, Mackowiak PA, Tulapurkar M, Nagarsekar A, Singh I. Fever, hyperthermia, and the lung: It's all about context and timing. Trans Am Clin Climatol Assoc 2011; 122: 34–47
- Shah NG, Hasday JD. Does temperature make a difference? It depends on how hot (or cold), for how long, and in what clinical context. Crit Care Med 2012; 40: 326–327
- Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 2009; 150: 1353–1360
- Coris EE, Ramirez AM, Van Durme DJ. Heat illness in athletes: The dangerous combination of heat, humidity and exercise. Sports Med 2004; 34: 9–16
- Shoenfeld Y, Udassin R, Shapiro Y, Ohri A, Sohar E. Age and sex difference in response to short exposure to extreme dry heat. J Appl Physiol 1978; 44: 1–4
- Shvartz E, Saar E, Benor D. Physique and heat tolerance in hot-dry and hot-humid environments. J Appl Physiol 1973; 34: 799–803
- Bar-Or O, Lundegren HM, Buskirk ER. Heat tolerance of exercising obese and lean women. J Appl Physiol 1969; 26: 403–409
- Spielman RS, Lyman CP. Thermal bradycardia in the mildly stressed rat. Am J Physiol 1971; 221: 948–951
- Horowitz M, Meiri U. Central and peripheral contributions to control of heart rate during heat acclimation. Pflug Arch 1993; 422: 386–392
- Garby L, Lammert O, Nielsen E. 24-h evaporative and sensible heat losses in relation to ambient humidity at 24°C in clothed human subjects. Acta Physiol Scand 1986; 127: 167–170
- Kaufman S. A comparison of the dipsogenic responses of male and female rats to a variety of stimuli. Can J Physiol Pharmacol 1980; 58: 1180–1183
- Fujisawa S, Tanaka J, Nomura M. Estrogen attenuates the drinking response induced by activation of angiotensinergic pathways from the lateral hypothalamic area to the subfornical organ in female rats. Behav Brain Res 2001; 122: 33–41
- Jonklaas J, Buggy J. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol 1984; 247: R167–172
- Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol 1999; 276: R90–96
- Igbokwe VU, Obika LFO. Thirst perception and dryness of mouth in healthy young adults Nigerians. African J Biomed Res 2008; 11: 39–46
- Frosini M, Sesti C, Palmi M, Valoti M, Fusi F, Mantovani P, et al. The possible role of taurine and GABA as endogenous cryogens in the rabbit: Changes in CSF levels in heat-stress. Adv Exp Med Biol 2000; 483: 335–344
- Jones DL, Mogenson GJ. Central injections of spiperone and GABA: Attenuation of angiotensin II stimulated thirst. Can J Physiol Pharmacol 1982; 60: 720–726
