Abstract
Background: Currently known as a metastatic disease, stage IV gastric cancer cannot be cured by surgery, but treatments are recommended to relieve symptoms such as pain and to prolong survival.
Methods: With better access to metastases and certain large or inoperable tumours, we applied two treatment sessions of combined therapy of whole-body hyperthermia and hyperthermic intraperitoneal chemo-perfusion in the treatment group, while patients in the control group were treated with oxaliplatin combined with 5-fluorouracil chemotherapy or Xeloda. We used the RECIST criteria for outcome evaluation.
Results: With the combined treatment, we found the complete and partial remission rate of patients to be 61.5%, and the rate of stable disease was 19.2%. Symptoms such as pain and a large volume of ascites were alleviated, and the quality of life was correspondingly improved. In addition, the combined treatment had a significant therapeutic benefit against the primary tumour and the metastases to the lymph nodes and liver. Survival time was also significantly prolonged (the 1-year survival rate was 38.5% compared to the control group rate of 19%).
Conclusions: These results suggest that whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy is an effective treatment for patients with advanced gastric malignancies.
Introduction
Digestive tract malignancies (including gastric cancer, colorectal cancer and pancreatic cancer) in advanced stages are mainly treated with systemic chemotherapy. Capecitabine, oxaliplatin and paclitaxel (or docetaxel), proven chemotherapy drugs, have had some success in the extension of survival time and improvement in patient quality of life. However, some obvious toxic side effects have limited their widespread clinical application, and the survival rate still needs improvement Citation[1], Citation[2]. Patients with advanced gastric malignant tumours mainly present with manifestations such as incomplete obstruction, ascites, pain, weight loss, and cachexia. A lack of effective treatment approaches has been the biggest challenge for these patients over recent decades. These patients have experienced a great burden of body and heart; therefore, the goal of our study was to improve patient quality of life and to extend survival by finding a truly effective treatment method. Whole-body hyperthermia (WBH) in animal models with colon cancer has been shown to significantly inhibit tumour growth and therefore may prolong survival. The mechanism of action of hyperthermia might be through enhancement of cytotoxic lymphocyte (CTL) activity Citation[3]. In the case of peritoneal metastases of colorectal cancer, the application of hyperthermia for the whole body was feasible after surgical tumour removal or radiotherapy; however, the synergistic effect on radiation therapy was not without its limitations Citation[4].
The hyperthermic intraperitoneal chemotherapy (HIPEC) regimen has been suggested to be useful in treating advanced peritoneal metastatic cancer, and following tumour resection, HIPEC significantly prolonged survival time Citation[5], Citation[6].
Therefore, in our study, we focused on the clinical measures of WBH combined with the hyperthermic intraperitoneal chemo-perfusion and especially its therapeutic roles for advanced, malignant stage IV gastric tumours, hoping to provide an alternative approach for better treatments for patients with advanced cancer.
Methods
Patient and clinical data
The group comprised 26 patients (16 male and 10 female, aged 38 to 65 years) from the Oncology Department in Clifford Hospital of Guangzhou University of Traditional Chinese Medicine. The patients had advanced malignant gastrointestinal cancer, and the study period was scheduled from February 2005 to March 2011 (). All diagnoses were confirmed through pathological examination. Among the gastric cancer cases, 22 were adenocarcinomas, three were signet ring cell carcinoma, and there was one case of squamous cell carcinoma. The disease staging was determined in accordance with the Union for International Cancer Control criteria, and the diagnosis of distant metastases was confirmed by CT, MRI and PET-CT examination.
Table 1. Clinical data.
All cases were at stage IV with metastases to the abdominal lymph nodes and other organs; there were 15 cases of metastases to the liver, three cases to the lung, eight cases to bone and one case with brain metastases. Regarding disease manifestation, there were 17 cases of ascites, 12 cases of cancerous pain (abdominal pain with no significant relief with the application of analgesic drugs), and incidences of incomplete intestinal obstruction in eight cases. The KPS scores were ≥60, and there were no cases of skin inflammation or ulceration. Before the thermotherapy course all patients were free of cardiovascular disease and lung, liver or renal functional abnormalities; no brain metastases or vascular tumour thrombus were found. Three days before treatment, all subjects were given conventional nutritional support and water supplements; the day of therapy initiation, all were prescribed abrosia.
The control group comprised 21 patients with stage IV gastric cancer. There were no significant differences between the two groups with regard to the clinical data criteria before the study. The control group received basic care (oxaliplatin combined with 5-fluorouracil or Xeloda chemotherapy) as a baseline for comparing pain control and improvements in psychological state and nutritional support.
Procedures
Preparation for the therapy
Abdominocentesis was performed before thermal therapy, and an indwelling tube was retained for the intraperitoneal injection of saline and drugs during the treatment course. If the volume of ascites was too great, we removed it through the tube. The saline and chemotherapy drugs were placed in a water bath box warmed to 42°C before treatment.
Chemotherapy drugs
At the beginning of the whole-body heat therapy, the body temperatures of the subjects measured below 38.5°C. The patients were given intraperitoneal hyperthermic perfusion of 1000 to 2000 mL normal pre-heated saline while the chemotherapy drugs were injected into the abdominal cavity. The chemotherapy regimen comprised cisplatin (40 mg/m2) plus 5-fluorouracil (0.75 mg/m2). If the ascites volume was too great, we discontinued the saline perfusion.
Protocols
Protective measures were prepared well in advance. First, the patients were instructed to wear cotton clothes and lay supine in the heat shield of the machine, for comfort. Some parts of the body and any surgical scars that might feel sensitive were covered by a small towel, and the patients wore specialised cotton hand gloves that were approximately 2 cm thick and socks to protect their skin.
The subjects were treated with the whole body inside the machine except for the head, which was outside in a heat-free compartment. Upon the initiation of the treatment course the patients were prescribed fluid administration, oxygen inhalation, and electrocardiographic (ECG) monitoring of body temperature, heart rate, blood pressure, respiration, and blood oxygen saturation. Temperature monitoring included a probe for surface temperature, which was placed at the spot where the heat converged on the abdomen, and a rectal probe, which was placed in the rectum. During the entire treatment process vital signs were closely observed, as well as consciousness, urine output and skin conditions. When signs of irritation occurred we administered propofol at 0.3–0.4 mg/kg/h as intravenous anaesthesia; if the symptoms were very obvious we added 10 mg midazolam to the intravenous infusion. When the temperature reached the preset level of 41°C we stopped the machine and maintained the treating temperature at 41.8°C for 120 min. Then we gradually opened the heat-free shield, so that the body temperature could drop naturally. When the body temperature reached approximately 39°C we ended the use of sedatives for a gradual recovery. Ultimately, the patients regained consciousness to finish the course.
During the heating process we closely observed the body temperature with the workstation of the multi-probe temperature measurement. One was placed at the rectum, the other at the anterior abdominal wall. We measured the rectum temperature with two probe detectors to form the average value, which was the main parameter to consider the temperature of patients. For the anterior abdominal wall we put a probe in the area of the direct heat-radiation region, and another at the perimeter for comparison. When the temperature probe reached 42°C, cooling measures were taken by giving the topically hottest spots a small spray of cold water, thus reducing the surface temperature to avoid scalding the skin. Cerebral vascular leakage increased during the heating and could induce various degrees of cerebral oedema. Therefore, when the rectal temperature reached 40.0°C, an ice pillow could be given to avoid an overly high head temperature. Of the 26 patients in the treatment group, the range of temperature examined was maintained from 41.8°C to 42°C. Temperature of 41.8°C was the highest value for the 14 cases; 41.9°C appeared as the highest degree in four cases and 42°C in eight cases. The temperature probe was regularly checked in case the wrong error would be over 0.3 degree.
During the treatment course we inserted a catheter to evaluate the urine volume of patients. In addition, we set two venous access lines, one in an upper extremity vein and one in a subclavian deep vein, to facilitate the measurement of central venous pressure and to ensure the circulation was adequate. First aid equipment and medicine must be prepared and ready at the bedside.
Every 10 min the body temperature, heart rate, blood pressure, respiration and blood oxygen saturation were monitored. When the heart rate reached 130 beats per min drugs were administered to control it. Other measures included the replenishment of electrolytes and a colloidal solution to avoid hypovolaemia leading to hypotension; if necessary, we prescribed vasopressors.
Complications
Of the 26 patients in the treatment group, hypotensive symptoms occurred in one case. We immediately implemented rehydration steps through the infusion of vasopressors, and the patient's blood pressure recovered. The next day, the skin of another patient presented with second degree burns; the area was <0.5% (whole body surface area), and he was administered topical burn ointments. Three days later the wound scabbed and healed. Herpes presented in three cases; however, one week later, all symptoms had disappeared. A total of 12 cases manifested with anorexia, but no nausea, vomiting or other gastrointestinal reactions were found. There were no side effects such as bone marrow suppression after chemotherapy.
Results
The evaluations for the clinical efficacy of the two cycles of treatment followed the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The body status was assessed by the Karnofsky score standard. The Visual Analogue Scale score (VAS) was adopted to measure pain levels.
Infrared ray thermotherapy for the whole body combined with intraperitoneal hyperthermic chemo-perfusion (the complete and partial remission rate was 61.5%, the stable disease rate was 19.2%) significantly surpassed the optimal basic chemotherapy group (; ).
Figure 1. Treatment effect assessment. In the treatment group, the number of CR + PR was 16, the complete and partial remission rate of patients was 61.5%, and the stable rate was 19.2%. However, in the control group, the number of CR + PR was 5, the complete and partial remission rate of patients was 23.8%, and the stable disease rate was 28.5%. All data were tested using χ2, χ2 = 8.04, P = 0.045. According to the level α = 0.05, there was a significant difference between the two groups. Notes: Complete Response (CR): disappearance of all target lesions. Partial Response (PR): at least a 30% decrease in the sum of LD of target lesions. Progressive Disease (PD): at least a 20% increase in the sum of LD of target lesions or the appearance of one or more new lesions. Stable Disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase.
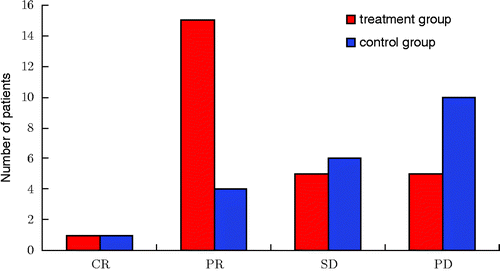
Table 2. Treatment effect assessment.
In addition, the treatment used in this clinical study significantly improved patient quality of life, which was reflected by the Karnofsky score (; ). After two cycles of treatment the patient Karnofsky scores increased significantly and were superior to the basic chemotherapy control group.
Figure 2. Karnofsky score. As the histogram above shows, whole-body hyperthermia combined with hyperthermic intraperitoneal chemo-perfusion in the treatment group increased Karnofsky scores.
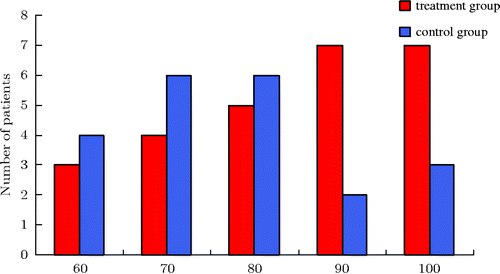
Table 3. Karnofsky score.
In terms of metastases assessment we found that the size of the liver metastases and abdominal lymph nodes were significantly reduced or eliminated and the size of the local masses decreased (; ).
Figure 3. Treatment effect assessment. Primary focus: in the treatment group, the complete and partial remission rate of patients was 50%, while the rate in the control group was 33%. All data were tested using χ2, χ2 = 1.69, P = 0.43. According to the level α = 0.05, there is no significant difference between the two groups. Hepatic metastases: in the treatment group the complete and partial remission rate of patients was 66.7%, while the rate in the control group was 28.6%. All data were tested using χ2, χ2 = 5.25, P = 0.023. According to the level α = 0.05, there is significant difference between the two groups. Lymph node: in the treatment group, the complete and partial remission rate of patients was 57.7%, while the rate in the control group was 42.1%. All data were tested using χ2, χ2 = 1.28, P = 0.53. According to the level α = 0.05, there is no significant difference between the two groups. Ascites: the complete and partial remission rate of patients was 70.6%, while the rate in the control group was 33.3%. All data were tested using χ2, χ2 = 6.89, P = 0.017. According to the level α = 0.05, there is a significant difference between the two groups. Notes: Complete Response (CR): disappearance of all target lesions. Partial Response (PR): at least a 30% decrease in the sum of LD of target lesions. Progressive Disease (PD): at least a 20% increase in the sum of LD of target lesions or the appearance of one or more new lesions. Stable Disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase.
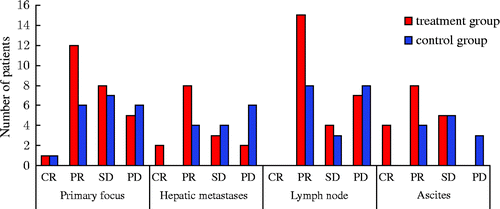
Table 4. Assessment of therapeutic efficacy.
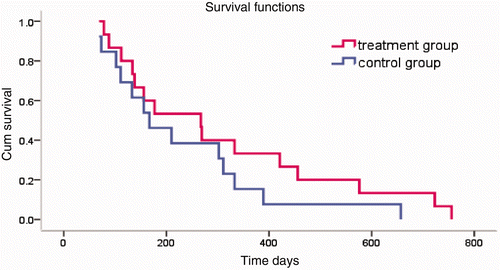
The therapy also prolonged patient survival (). The patients whose survival time was prolonged over 12 months in the treatment group significantly outnumbered the patients with similar survival rates in the control group.
Figure 4. Survival time. In the treatment group, the 6-month survival rate was 69.2%, and the 1-year survival rate was 38.5%. In the control group, the 6-month survival rate was 66.7%, and the 1-year survival rate was 19%. Tested using the log-rank test, χ2 = 1.32, P = 0.25. According to the level α = 0.05, there is no significant difference between the two groups.
Discussion
Currently, palliative chemotherapy is the main approach for treating stage IV gastric cancer. With the development of recent chemotherapy drugs, clinical efficacy has improved, but the systemic side effects cause many patients to be unable to complete the entire treatment course. The toxic effects of second-line therapy also lead to treatment termination. The causes of death in patients with stage IV malignant gastric tumours were primarily obstruction, ascites and liver failure. The control of abdominal tumours is the principal goal of treatment, which can prolong survival period and improve quality of life. Peritoneal perfusion chemotherapy may have a positive therapeutic effect on local recurrence and metastasis of gastrointestinal malignancies and thus has been widely used in clinical practice. This treatment is currently listed in the clinical guidelines of the National Comprehensive Cancer Network Citation[7]. In the clinic, the local infusion of chemotherapeutic agents, combined with topical hyperthermia, can improve clinical efficacy. The primary reason might lie in the principle that hyperthermia accelerates blood circulation, thereby improving local drug concentration in the tumour so that the heat and drugs can be more evenly distributed Citation[8]. Furthermore, drug concentration in the liver increased by nearly ten-fold, thus minimising the side effects of chemotherapy drugs.
Whole-body thermotherapy is intended to kill tumour cells by raising the body temperature. Studies have shown that when the body temperature reaches 41.8°C, tumour cells undergo apoptosis, and the moderate temperature enhances the immune function of the body against the tumour. In addition, studies have shown that whole-body thermotherapy improves the immune system by stimulating the production of neutrophils to control tumour growth Citation[9]. The combination of WBH with systemic chemotherapy can boost the effect of chemotherapy drugs and the reversal of the anti-inflammatory response during chemotherapy (the number of NK cells and T lymphocytes was significantly increased, whereas the levels of interleukin-10 and interleukin-12 were reduced Citation[10]). For certain patients who were not responding well to the initial chemotherapy we used WBH in addition to chemotherapy. WBH strengthened the chemotherapy effects and has been used for the treatment of digestive tract tumours Citation[11].
The principle behind the therapy was to administer local chemotherapy drugs to kill the tumour and to perform WBH to increase the abdominal temperature at a specific target location and enhance the effect of local chemotherapy drugs. In addition, moderate systemic thermotherapy would strengthen the immune function, reducing the inhibition of chemotherapy drugs on the immune function. WBH therapy has a direct role in inducing tumour cell apoptosis and otherwise enhancing the concentration of chemotherapy drugs in the liver, which has synergistic effects on the killing of tumour cells. In advanced gastric tumours, WBH can also induce tumour cells to migrate into the blood circulation, but without clinical significance Citation[12]. Oxaliplatin has been commonly used as an intra-abdominal perfusion chemotherapy drug for advanced colorectal cancer, and HIPEC can increase its concentration in the peritoneum and the tumour spots but lessen the systemic absorption Citation[13], Citation[14]. The most common drugs in the study we adopted were cisplatin, fluorouracil and mitomycin due to fewer side effects. Meanwhile, WBH combined with hyperthermic intraperitoneal perfusion treatment reduced the side effects of the chemotherapy drugs in the treatment group, in which no bone marrow suppression, no liver or kidney damage and no hair loss was observed for any of the 26 cases in the study.
Whole-body hyperthermia combined with hyperthermic intraperitoneal chemo-perfusion treatment significantly improved patient quality of life, as suggested by the Karnofsky scores. Patient pain was also alleviated in the treatment group as assessed from the VAS scores. We consider that the improved quality of life was mainly due to the effective control of primary lesions or the metastatic tumours.
During the treatment course, various complications needed to be carefully monitored and prevented, especially the prevention of burns. The heart and lung functions also needed to be closely monitored in the rapid rehydration process due to excessive sweating during the hyperthermia therapy; therefore, attention should be focused on patients’ cardiopulmonary reserve in case of heart failure.
Our finding serves to stress some strong points in favour of this therapeutic method for pain mitigation, extended survival, and amelioration of quality of life for patients with advanced gastric tumours. The concerns regarding the treatment of certain incurable diseases such as advanced tumours have increasingly focused upon patient quality of life, moods, and spiritual and mental well-being. However, there is still more that needs to be accomplished to help patients live a better life, and our searches and efforts have to be considered in these terms.
In this study, whole-body hyperthermia combined with hyperthermic intraperitoneal chemo-perfusion treatment showed obvious advantages in terms of clinical efficacy, reduced side effects of chemotherapy drugs, improvement of patient quality of life, and the extension of the survival period, all of which reflect that this treatment is a proven way to effectively cope with advanced gastric cancer.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ajani JA. Optimizing docetaxel chemotherapy in patients with cancer of the gastric and gastroesophageal junction: Evolution of the docetaxel, cisplatin, and 5-fluorouracil regimen. Cancer 2008; 113: 945–955
- Cen P, Ajani JA. Medical treatment for advanced gastroesophageal adenocarcinoma. Curr Opin Gastroenterol 2007; 23: 631–635
- Hattori T, Kokura S, Okuda T, Okayama T, Takagi T, Handa O, et al. Antitumor effect of whole body hyperthermia with alpha-galactosylceramide in a subcutaneous tumor model of colon cancer. Int J Hyperthermia 2007; 23: 591–598
- Aarts F, Hendriks T, Boerman OC, Oyen WJ, Bleichrodt RP. Hyperthermia and fibrinolytic therapy do not improve the beneficial effect of radioimmunotherapy following cytoreductive surgery in rats with peritoneal carcinomatosis of colorectal origin. Cancer Biother Radiopharm 2008; 23: 301–309
- Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008; 98: 263–267
- Portilla Gómez A. Peritoneal carcinomatosis. Ten years of applying the new combined triple therapy. Personal experience. Cir Esp 2007; 82: 346–351
- Hadi R, Saunders V, Utkina O, Clingan P, Kam P, Links M, et al. Review of patients with peritoneal malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J Surg 2006; 76: 156–161
- El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 2004; 6: 117–127
- Ostberg JR, Ertel BR, Lanphere JA. An important role for granulocytes in the thermal regulation of colon tumor growth. Immunol Invest 2005; 34: 259–272
- Ahlers O, Hildebrandt B, Dieing A, Deja M, Böhnke T, Wust P, et al. Stress induced changes in lymphocyte subpopulations and associated cytokines during whole body hyperthermia of 41.8–42.2°C. Eur J Appl Physiol 2005; 95: 298–306
- Hildebrandt B, Dräger J, Kerner T, Deja M, Löffel J, Stroszczynski C, et al. Whole-body hyperthermia in the scope of von Ardenne's systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: A phase I/II study. Int J Hyperthermia 2004; 20: 317–333
- Hegewisch-Becker S, Braun K, Otte M, Corovic A, Atanackovic D, Nierhaus A, et al. Effects of whole body hyperthermia (41.8°C) on the frequency of tumor cells in the peripheral blood of patients with advanced malignancies. Clin Cancer Res 2003; 9: 2079–2084
- Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann Oncol 2002; 13: 267–272
- Ferron G, Dattez S, Gladieff L, Delord JP, Pierre S, Lafont T, et al. Pharmacokinetics of heated intraperitoneal oxaliplatin. Cancer Chemother Pharmacol 2008; 62: 679–683
