Abstract
Purpose: This study aimed to evaluate the safety and efficacy of percutaneous CT-guided radiofrequency ablation (RFA) for unresectable hepatocellular carcinoma pulmonary metastases (HCCPM) and to identify the prognostic factors for survival.
Materials and methods: We reviewed the medical records of 320 patients with HCCPM treated between January 2005 and January 2012. Among them, 29 patients with 68 lesions of unresectable HCCPM underwent 56 RFA sessions. Safety, local efficacy, survival and prognostic factors were evaluated. Survival was analysed using the Kaplan-Meier method. Univariate analyses were evaluated by the log-rank test.
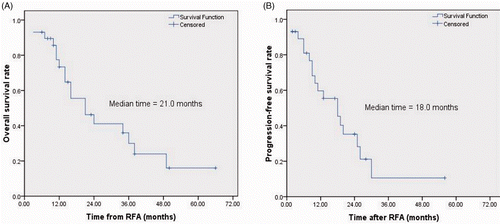
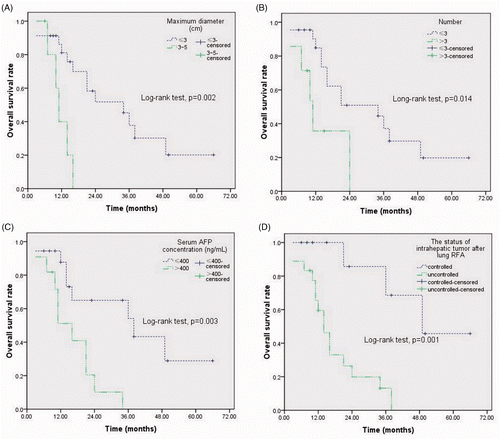
Results: Pneumothorax requiring chest tube placement occurred in five (8.9%, 5/56) RFA sessions. During the median follow-up period of 23 months (range 6–70), 18 patients (62.1%, 18/29) died of tumour progression and 11 (37.9%, 11/29) were alive. The 1-, 2- and 3-year overall survival rates were 73.4%, 41.1% and 30%, respectively. The median progression-free survival was 18 months (95% confidence interval (CI) 9.8–26.2) and the median overall survival time was 21 months (95%CI, 9.7–32.3). The maximum tumour diameter ≤3 cm (p = 0.002), the number of pulmonary metastases ≤3 (p = 0.014), serum AFP level ≤400 ng/mL (p = 0.003), and the controlled status of intrahepatic tumour after lung RFA (p = 0.001) were favourable prognostic factors for overall survival.
Conclusions: Our study indicates that percutaneous CT-guided RFA, as an alternative treatment procedure to pulmonary metastasectomy, can be a safe and effective therapeutic option for unresectable HCCPM.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumour worldwide and the third contributor to cancer-related death Citation[1]. With the advancements of the multidisciplinary applications of surgical resection, transplantation, radiofrequency ablation (RFA), and transcatheter arterial chemoembolisation (TACE) Citation[2–4], the intrahepatic tumour has been well controlled and the survival of patients with HCC has been gradually improved; however, the prognosis of patients with distant metastases remains poor Citation[5].
The lung is the most common site of distant metastasis which occurs in 20–30% of advanced-stage HCC Citation[6]. Lung metastasectomy is currently a standard approach for patients with resectable lung metastases, and the 5-, 10-, and 15-year survival rates are 36%, 26%, and 22%, respectively Citation[7], Citation[8], but 70–75% of hepatocellular carcinoma pulmonary metastases (HCCPM) with multiple or bilateral distributions of pulmonary metastases are excluded from surgical resection, especially for those patients with contraindications to pulmonary metastasectomy or associated with high-risk mortality after open lobectomy, or the patient's refusal to accept the second surgical trauma after liver resection Citation[9]. New targeted agents such as sorafenib have been shown to extend survival in selected advanced HCC patients Citation[10], Citation[11]; however, a recent prospective phase II trial indicated that the presence of lung metastases predicted poor response to sorafenib in advanced HCC patients Citation[12]. Therefore, a novel therapeutic strategy is required to improve the survival of patients with unresectable HCCPM.
Percutaneous CT-guided RFA has been used as a safe and effective therapeutic option in the treatment of kinds of solid tumours Citation[13–15], and pulmonary metastases from colorectal carcinoma Citation[16], nasopharyngeal carcinoma Citation[17], and renal cell carcinoma Citation[18]. The advantages of satisfied local control rate, repeatability, minimal invasion with short hospital stay, and gain in quality of life suggest that RFA might be an alternative to lung metastasectomy for patients with unresectable pulmonary metastases Citation[19]. In this study we evaluated the safety and efficacy of percutaneous CT-guided RFA in treating unresectable HCCPM, which may be useful in accumulating evidence of this practice in clinic.
Materials and methods
This retrospective study was approved by our institutional review board, and all patients provided written informed consent before CT-guided RFA.
Patient selection
Patients with HCCPM were evaluated for RFA by a multidisciplinary team board consisting of thoracic surgeons, hepatobiliary surgeons, medical oncologists, radiation oncologists and interventional oncologists. The selection criteria were (1) age between 18 and 85 years, (2) good Karnofsky performance status of more than 80, (3) radiological or histological diagnosis of pulmonary-only metastases from HCC, (4) intrahepatic tumours were controlled, (5) patients with comorbidities at risk of lung metastasectomy, (6) patient refusing to undergo pulmonary metastasectomy, (7) maximum diameter of metastases ≤5 cm, (8) lesion number ≤5, 9) a distance between the lesion and the pulmonary hilum vessel, main bronchus, or mediastinal organ of 1 cm or more with possibility of safely complete ablation, (10) no coagulopathy.
RFA procedure
Pretreatment work-up included a complete history, physical examination, and imaging modalities including lung, abdomen, and pelvic CT scans with contrast medium, brain MRI, electrocardiogram, and laboratory examinations including complete blood cell counts, blood chemistry, viral titres (such as hepatitis virus and human immunodeficiency virus), and coagulation profile examinations.
Before the procedure all the patients fasted for 12 h. RFA was performed in a hospital CT room by two experienced interventional oncologists, a technician, an anaesthetist and a nurse. All procedures were performed under real-time CT guidance with 5-mm collimation and 10–50 mA. The patient was placed in the appropriate position according to the location of the tumour. Two grounding pads were placed on the proximal thighs. Electrocardiograms, blood pressure, and saturation of blood oxygen were monitored throughout the procedure.
Intraprocedural pain was treated by using a combination of intravenous conscious sedation (midazolam and propofol) and local anaesthesia (subcutaneous 1% lidocaine), or intravenous general anaesthesia without endotracheal intubation. More specifically, after established peripheral venous access, general anaesthesia was induced with intravenous administration of propofol (1.5 mg/kg) and fentanyl (1–1.5 mg/kg) and was maintained with propofol (3–6 mg/kg/h). All anaesthesias were performed by experienced anaesthetists in RFA of pulmonary tumours. Throughout the procedure the patient retained spontaneous breathing via a laryngeal mask airway (nitrous oxide 60% in oxygen, 2–5 L/min). General anaesthesia (26 sessions, 15 patients) was administered when the tumour was close to the pleura or when the patient requested it.
After thoracic CT scanning was performed, the precise location of the target lesion was identified and the puncture angles and depths of electrode insertion were thereby confirmed. Local anaesthesia (subcutaneous 1% lidocaine) was administered at the selected puncture points, and then a 0.5 cm surgical incision (subcostal or intercostal) was made. A monopolar electrode system (Cool-tip; Valleylab, MA, USA) with a single internally cooled electrode (17 gauge, 1.5 mm diameter) with an exposed 3 cm non-insulated tip was used for all procedures. The electrode was carefully inserted into the centre of the tumour at a predetermined angle in a stepwise manner under CT guidance.
Radiofrequency energy was applied with an impedance control algorithm for 12 min at each tumour site. Each patient underwent 1–3 sessions of ablation and the tumours larger than 3 cm received multiple overlapping ablations to obtain the ablative margin. A maximum of three lung tumours were treated on the same side of the lung. The remaining tumours were treated by RFA the following week to minimise possible procedure-related complications, especially for pneumothorax. At the end of each ablation session, the electrode track was ablated to avoid possible bleeding and the risk of puncture-related implantation metastases. The technique successes and possible complications were evaluated by an additional CT scan immediately after the procedure, and ablation was considered successful with the presence of ground-glass opacity of 0.5–1.0 cm in diameter greater than the lesion. Patients were discharged usually 1–3 days after the procedure.
Follow-up and evaluation
The follow-up evaluations were performed at 1, 3, 6, 9 and 12 months; at 6-month intervals thereafter with clinical and radiographic examinations. The radiological examinations included chest and abdomen CT scans, and PET-CT when suspicions of a local relapse or systemic metastasis was detected from contrast CT scans. Local efficacy was assessed according to the modified Response Evaluation Criteria In Solid Tumours (RECIST) Citation[20]. Specifically, complete response (CR) was defined as the target lesion demonstrated cavitations, necrosis and cyst formation with a 25% reduction in size. Partial response (PR) was where the target lesion demonstrated necrosis with liquid density in the central component, or there was >30% reduction in the longest diameter. Stable disease (SD) was where the target lesion demonstrated <30% reduction in the longest diameter or maintained a solid appearance without central necrosis or cavitations; and progressive disease (PD) was where the target lesion increased by > 20% in size and maintained a solid mass with invasion of adjacent structures. Local tumour progression was defined as any detectable tumour activity in the ablation zone, and disease progression was defined as the evidence of new tumour located outside the treated region, and included intrahepatic and extrahepatic sites. Overall survival (OS) was defined as the time from the date of RFA to the date of death or the last follow-up. Progression-free survival (PFS) was defined as the time from the date of RFA to either the first documentation of recurrence or progression or death from any cause.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences program (SPSS version 16.0, Chicago, IL, USA). The OS and PFS were calculated using the Kaplan-Meier method. The survival curves stratified for the possible prognostic factors were compared by the log-rank test. A p value ≤0.05 was considered statistically significant.
Results
Patient sample and tumour characteristics
Between January 2005 and January 2012, 320 patients with HCCPM were reviewed at the institutional database. Among them, 29 patients with 68 unresectable tumours were treated with 56 RFA sessions. There were 16 men and 13 women with a median age of 56 years (range 24–72). The diagnosis of pulmonary-only metastases from HCC was mainly based on typical radiological features of HCCPM on contrast-enhanced CT scan or PET-CT scan with the history of the HCC, except for three patients who underwent CT-guided lung biopsy before RFA as the presentation was atypical.
The intrahepatic tumours were controlled based on radiological images without intrahepatic enhanced lesion lasting for more than 1 month after the multidisciplinary treatments consisted of RFA (n = 3), TACE + RFA (n = 7), liver transplantation + TACE + RFA (n = 9), and hepatectomy + TACE + RFA (n = 10), respectively. The comorbidities at risk of lung metastasectomy were congestive heart failure (n = 4), diabetic nephropathy (n = 2), diabetic cardiopathy (n = 3), and severe chronic obstructive pulmonary disease (n = 8); 12 patients refused to accept lung metastasectomy.
The mean number of lung metastases was 2.3 (range 1–5) and the mean longest diameter was 19.3 mm (range 5–50) at the time of initial lung RFA. The mean disease-free interval between the time of the diagnosis of HCC and development of lung metastases was 17.7 months (range 1–84). The duration of intrahepatic tumour control was 1–164 weeks, with a mean of 25 weeks. The last day of follow-up ended on the date of death or 31 January 2012, and the median follow-up period was 23 months (range 6–70) (further details of the characteristics of patients and tumours are summarised in ).
Table I. Analysis of patient characteristics for overall survival.
Complications
No death was related to the RFA procedure. The major complication was symptomatic pneumothorax requiring chest tube placement occurred in five (8.9%, 5/56) RFA sessions. Minor complications (17.9%, 10/56) included self-limited minor pneumothorax, slight cough, a low-grade fever (<38.5°C), and local pain at the puncture site, were well tolerated, and easily controlled.
Local efficacy
A total of 56 RFA sessions (mean per patient, 1.93; range 1–5) were successfully performed on 68 HCCPM lesions. One month after initial RFA procedure, CR was observed in 24 patients (82.8%), PR in five (17.2%). Additional RFA were performed in all PR patients. The radiological scans in the follow-up period revealed complete necrosis in the re-ablated site. The median time to local tumour progression was 14.3 months (range 2–56). At the end of the study, 25 patients did not have intrapulmonary recurrence and the other four patients suffered intrapulmonary progression with diffuse new metastasis.
Survival
During the median follow-up period after initial lung RFA of 23 months (range 6–70), 18 (62.1%, 18/29) patients died and 11 (37.9%, 11/29) were alive. The cause of death was related to tumour progression in the 18 patients, including intrahepatic recurrence (n = 11, 37.9%), pulmonary metastasis (n = 4, 13.8%), brain metastasis (n = 1, 3.4%), and bone metastasis (n = 2, 6.9%).
The 1-, 2- and 3-year OS rates from initial lung RFA were 73.4%, 41.1%, 30%, respectively for all 29 patients. The 1- and 2-year PFS rates were 59.7% and 28.2%, respectively. The median OS time from initial lung RFA and intrapulmonary PFS time were 21 months (95%CI, 9.7–32.3) and 18 months (95%CI, 9.8–26.2), respectively. The Kaplan-Meier survival curves are depicted in A and B.
Prognostic factors
Univariate analysis based on the characteristics of the population and tumours are summarised in . There were 18 deaths in the present study, which were not sufficient to perform a multivariate analysis for OS.
Analysis of the variables showed that there was no increase in survival related to gender, age, Karnofsky performance status, HBV infection, liver cirrhosis, disease-free interval, or to the presence (metachronous/synchronous), distribution (unilateral/bilateral) and location (central/peripheral) of metastases. Conversely, the maximum diameter ≤3 cm (median survival time 34 months; p = 0.002), the number ≤3 (median survival time 34 months; p = 0.014), the serum AFP level ≤400 ng/mL (median survival time 38 months; p = 0.003), and the controlled status of intrahepatic tumour after lung RFA (median survival time 49 months; p = 0.001) were favourable prognostic factors for OS (A–D).
Discussion
Our study presented a subgroup of patients with unresectable HCCPM who underwent lung RFA. The preliminary results have shown that a long-term PFS of 18 months is similar to that of metastasectomy. The 3-year OS rate for HCCPM after initial lung RFA achieved 30% with a median survival time of 21 months, which was lower than that of pulmonary metastasectomy with a median OS of 29 months (range 16–52) Citation[7], Citation[8], Citation[21–24]. The difference might be caused by the study population in terms of more lung metastatic lesions in bilateral lungs or being multiples in our study in comparison with metastasectomy cases with solitary metastatic nodules.
In previous studies, Kim et al. first reported three patients with HCCPM successfully treated with RFA and all the patients had survived without evidence of recurrence or progression of disease elsewhere during the follow-up of 12, 17, and 35 months, respectively Citation[25]. Hiraki et al. retrospectively evaluated 32 patients with HCCPM from six institutions in Japan treated with RFA. During the median follow-up period of 20.5 months, the 1-, 2-, 3-year OS rates were 87%, 57%, and 57%, respectively, with the median survival times of 37.7 months Citation[26]. The survival outcomes in this study were longer than ours; the reason may be that the patients with unresectable HCCPM in our study were treated with RFA alone without systemic sequential therapies. All of the studies have validated the safety and efficacy of RFA in the treatment of HCCPM.
In univariate analyses, the number and maximum tumour diameter, serum AFP level before lung RFA, and the status of intrahepatic tumour after lung RFA were found to be significant predictors for OS. Several studies have proven that the number and size of tumours are related to the survival of patients treated with lung RFA Citation[27], Citation[28]. The survival benefit in no more than three treated tumours was significantly higher than with four or five metastatic lesions, and the survival benefit in treated tumours smaller than 3 cm was significantly higher than that of metastatic lesions larger than 3 cm. Our study revealed that RFA can ablate multiple lesions to reach the relatively long OS, and repetitive application of RFA is especially beneficial for metastatic tumours of multiple origins.
In our study the serum AFP level before lung RFA was a significant prognostic factor in univariate analysis using the log-rank test (p = 0.003). More than 70% of HCC patients have a high serum concentration of AFP because of the tumour excretion, and serum AFP level is related to HCC cell differentiation Citation[29]. In our study, the rates of CR and PR to RFA were 66.7% (12/18), and 33.3 (6/18) for patients with serum AFP level ≤400 ng/mL, whereas the rates were 27.3 (3/11), and 72.7 (8/11) for patients with serum AFP level >400 ng/mL, respectively. The serum AFP level ≤400 ng/mL had a better CR rate to RFA and lengthened the PFS, then improved the OS time.
As shown in the study, 11 (37.6%) patients with controlled HCC were alive at the end of the follow-up and the others died mainly due to the progression of intrahepatic tumour, which indicates the importance of treatment of HCC when the tumour load in the pulmonary metastases has been eradicated by RFA. As a minimally invasive procedure, RFA can provide a better physical quality of life for patients with HCCPM so that they can cope with the intrahepatic tumour by liver-directed therapies and thus improve survival.
In the present study tumour distribution was not a prognostic factor, which suggests the advantage of lung RFA over surgical intervention as patients with bilateral lung metastases are poor candidates for metastasetomy. The lesser invasiveness appears to support the indication of lung RFA for HCCPM.
The major advantage of RFA therapy is lung sparing and damages a minimal amount of surrounding uninvolved pulmonary parenchyma Citation[30], Citation[31]. This may be useful when patients have limited pulmonary reserve and when multifocal or bilateral metastatic disease requiring significant lung sacrifice outweighs the benefit of surgical resection. Additionally, RFA can be performed in a minimally invasive fashion under percutaneous CT guidance, which avoids a pulmonary metastasectomy in patients with predictable significant comorbidities or in those who refuse surgical resection. However, RFA alone is incapable of complete ablation of the HCCPM with positive mediastinal lymph nodes, which may be better treated with surgical resection Citation[21], Citation[32] or with RFA plus systemic treatments Citation[33], Citation[34].
Complications occurring in this study were well tolerated and easily controlled. The most frequent major complication was pneumothorax in ablating multi-metastatic pulmonary lesions and the incidence was no more than those reported in previous studies Citation[35]. All patients considered themselves free of symptoms within the 1-week follow-up examination. Other reported procedure-related complications such as haemorrhage, pneumonia, tumour seeding along the needle tract and subcutaneous emphysema were not observed in this study. The advantages of minimal invasion and gain in quality of life suggest that RFA can be an alternative option for HCCPM when curative radical resection cannot be achieved.
A problem that is challenging after RFA is the assessment of treatment response. After RFA, the mass comprised of coagulation necrotic tumour in situ and surrounding devitalised lung will usually increase in size compared to the tumour size before procedure. Non-contrast CT imaging based on relative change in mass dimensions using the RECIST criteria will likely be falsely categorised as disease progression, even if the entire tumour has undergone coagulation necrosis. The viable tumour frequently showed nodular perilesional contrast enhancement after administration of contrast material out of proportion to background normal lung parenchyma Citation[36]. In patients with tumours documented to be [18F]fluorodeoxyglucose (FDG) PET-avid before ablation, FDG-PET scans obtained after ablation may provide an additional measure of tumour response and progression Citation[37]. However, uniform FDG-PET scanning of all patients likely will not be feasible because some tumours will not be FDG-avid, and there is considerable variability in reimbursement practices among many third-party payers Citation[36]. In this study we have been using a modification of the RECIST criteria as described by Fernando, et al. Citation[20] to assess tumour progression at follow-up, which has been utilised by many studies Citation[16], Citation[19].
There were some limitations which may affect the clinical value of the study. Firstly, this was a retrospective study with a small sample size. Also, the efficacy of RFA was evaluated by clinical and radiological examinations. However, the encouraging outcomes suggest a useful framework for future larger prospective studies to validate the safety and efficacy of RFA for unresectable HCCPM.
Conclusion
Percutaneous CT-guided RFA is a safe and effective alternative to pulmonary metastasectomy for selected patients with unresectable HCCPM. Prognostic factors identified in this study will help to stratify patients who would benefit from lung RFA.
Declaration of interest: This work was supported by a grant of the Sciences and Technology Committee of Guangdong Province (2011B031800040). This work was supported by a grant of the Sciences and Technology Committee of Guangdong Province (2011B031800040). The authors report no conflicts of interest and the authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300
- Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, et al. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012; 262: 43–58
- Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: Present and future. J Gastroenterol Hepatol 2012; 27: 862–872
- Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer 2011; 117: 4475–4483
- Davidson RS, Nwogu CE, Brentjens MJ, Anderson TM. The surgical management of pulmonary metastasis: Current concepts. Surg Oncol 2001; 10: 35–42
- Nichols FC. Pulmonary metastasectomy. Thorac Surg Clin 2012; 22: 91–99
- Pastorino U, Buyse M, Friedel G, Ginsberg R, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37–49
- Pastorino U. History of the surgical management of pulmonary metastases and development of the International Registry. Semin Thorac Cardiovasc Surg 2002; 14: 18–28
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34
- Yau T, Chan P, Ng KK, Chok SH, Cheung TT, Fan ST, et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: Presence of lung metastasis predicts poor response. Cancer 2009; 115: 428–436
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010; 26: 305–315
- Pan C, Wu P, Yu J, Li W, Huang Z, He N, et al. CT-guided radiofrequency ablation prolonged metastatic survival in patients with liver metastases from nasopharyngeal carcinoma. Int J Hyperthermia 2011; 27: 549–554
- Sung HH, Park BK, Kim CK, Choi HY, Lee HM. Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hyperthermia 2012; 28: 227–234
- Yamakado K, Inoue Y, Takao M, Takaki H, Nakatsuka A, Uraki J, et al. Long-term results of radiofrequency ablation in colorectal lung metastases: Single center experience. Oncol Rep 2009; 22: 885–891
- Pan CC, Wu PH, Yu JR, Li W, Huang ZL, Wang JP, et al. Comparative survival analysis in patients with pulmonary metastases from nasopharyngeal carcinoma treated with radiofrequency ablation. Eur J Radiol 2012; 81: e473–e477
- Soga N, Yamakado K, Gohara H, Takaki H, Hiraki T, Yamada T, et al. Percutaneous radiofrequency ablation for unresectable pulmonary metastases from renal cell carcinoma. BJU Int 2009; 104: 790–794
- Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: An open-labeled prospective trial of 148 patients. Ann Oncol 2010; 21: 2017–2022
- Fernando HC, De Hoyos A, Landreneau RJ, Gilbert S, Gooding WE, Buenaventura PO, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg 2005; 129: 639–644
- Younes RN, Fares AL, Gross JL. Pulmonary metastasectomy: A multivariate analysis of 440 patients undergoing complete resection. Interact Cardiovasc Thorac Surg 2012; 14: 156–161
- Kitano K, Murayama T, Sakamoto M, Nagayama K, Ueno K, Murakawa T, et al. Outcome and survival analysis of pulmonary metastasectomy for hepatocellular carcinoma. Eur J Cardiothorac Surg 2012; 41: 376–382
- Gwak GY, Jung JO, Sung SW, Lee HS. Long-term survival after pulmonary metastatectomy of hepatocellular carcinoma: Treatment outcome or natural history?. Hepatogastroenterology 2004; 51: 1428–1433
- Kawamura M, Nakajima J, Matsuguma H, Horio H, Miyoshi S, Nakagawa K, et al. Surgical outcomes for pulmonary metastases from hepatocellular carcinoma. Eur J Cardiothorac Surg 2008; 34: 196–199
- Kim TS, Lim HK, Lee KS, Yoon YC, Yi CA, Han D. Imaging-guided percutaneous radiofrequency ablation of pulmonary metastatic nodules caused by hepatocellular carcinoma: Preliminary experience. Am J Roentgenol 2003; 181: 491–494
- Hiraki T, Yamakado K, Ikeda O, Matsuoka T, Kaminou T, Yamagami T, et al. Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: Results of a multicenter study in Japan. J Vasc Interv Radiol 2011; 22: 741–748
- Fernando HC. Radiofrequency ablation to treat non-small cell lung cancer and pulmonary metastases. Ann Thorac Surg 2008; 85: S780–S784
- Veltri A, Guarnieri T, Gazzera C, Busso M, Solitro F, Fora G, et al. Long-term outcome of radiofrequency thermal ablation (RFA) of liver metastases from colorectal cancer (CRC): Size as the leading prognostic factor for survival. Radiol Med 2012; 117: 1139–1151
- Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol 2006; 12: 1175–1181
- Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology 2011; 260: 633–655
- Tada A, Hiraki T, Iguchi T, Gobara H, Mimura H, Toyooka S, et al. Influence of radiofrequency ablation of lung cancer on pulmonary function. Cardiovasc Intervent Radiol 2011; 35: 860–867
- Demmy TL, Dunn KB. Surgical and nonsurgical therapy for lung metastasis: Indications and outcomes. Surg Oncol Clin N Am 2007; 16: 579–605
- Jin Y, Cai YC, Cao Y, Cai XY, Tan YT, Shi YX, et al. Radiofrequency ablation combined with systemic chemotherapy in nasopharyngeal carcinoma liver metastases improves response to treatment and survival outcomes. J Surg Oncol 2012; 106: 322–326
- Takao M, Yamakado K, Takeda K. Radiofrequency ablation as an adjunct to systemic chemotherapy for colorectal pulmonary metastases. Cancer 2011; 117: 876–877
- Kashima M, Yamakado K, Takaki H, Kodama H, Yamada T, Uraki J, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: A single center's experiences. Am J Roentgenol 2011; 197: W576–W580
- Rose SC, Dupuy DE, Gervais DA, Millward SF, Brown DB, Cardella JF, et al. Research reporting standards for percutaneous thermal ablation of lung neoplasms. J Vasc Interv Radiol 2009; 20: S474–S485
- Purandare NC, Rangarajan V, Shah SA, Sharma AR, Kulkarni SS, Kulkarni AV, et al. Therapeutic response to radiofrequency ablation of neoplastic lesions: FDG PET/CT findings. Radiographics 2011; 31: 201–213