Abstract
Purpose: The efficacy of anticancer drugs in solid tumours is impaired by their inability to reach all cancer cells in sufficient concentration to cause cytotoxicity. Hyperthermia-triggered release of drugs from thermosensitive liposomes can increase tumour drug concentration, but tumour-specific drug delivery requires precise temperature control, and effects on microregional distribution of anticancer drugs in tumours are unknown. Here we evaluate thermally triggered release of doxorubicin in a rabbit tumour model by comparing free versus thermosensitive liposomal doxorubicin administered systemically during magnetic resonance imaging (MRI)-controlled focused ultrasound hyperthermia.
Materials and methods: Twelve rabbits with a transplanted VX2 tumour in each thigh had a 10 mm diameter region in one tumour heated to 43°C using focused ultrasound with temperature control by MRI thermometry. Delivery of doxorubicin to tumours and normal tissues was quantified by fluorescence in tissue homogenates, and by fluorescence microscopy.
Results: Using thermosensitive liposomal doxorubicin (2.5 mg/kg), doxorubicin concentrations in heated tumours were 26.7 times higher than in unheated tumours (n = 7, p = 0.017, two-sided Wilcoxon signed-rank test). There was no significant enhancement with free doxorubicin in heated versus unheated tumours (n = 3, p = 0.5). With thermosensitive liposomes (8.3 mg/kg), fluorescence microscopy demonstrated increased doxorubicin fluorescence in heated versus unheated tumours, co-localised with nuclear staining throughout the tumour.
Conclusions: Localised image-guided delivery of high concentrations of doxorubicin to cancer cells was achieved non-invasively in implanted tumours with temperature-sensitive drug carriers and a preclinical MRI-controlled focused ultrasound hyperthermia system.
Introduction
The clinical efficacy of chemotherapy in solid tumours is impaired by systemic toxicity and the inability of anticancer drugs to reach all cancer cells in sufficient concentration to cause cytotoxicity Citation[1]. To treat tumour cells that are inadequately perfused by their disorganised vasculature, drugs must cross the vessel wall and penetrate the dense extracellular matrix, overcoming sequestration in perivascular cells Citation[2]. Anticancer drugs must reach tumour cells in a bioavailable form at a cytotoxic dose; however, the administered dose must not exceed levels that cause systemic toxicity arising from efficient delivery through highly organised vasculature in well-perfused normal tissues such as the liver and kidney Citation[3].
Encapsulating chemotherapeutic agents in long-circulating liposomal drug carriers prevents extravasation from normal microvasculature while allowing preferential extravasation from leaky tumour vessels Citation[4]. However, passive liposome accumulation occurs over a period of 24–48 h; to prevent toxicity from systemic drug release, liposomes are designed to degrade slowly after accumulating in tumours, thereby delivering sustained, but low, concentrations of the bioavailable drug Citation[5]. Peak concentrations of the bioavailable drug at the target site can be increased with thermosensitive liposomes, which remain in the bloodstream for about 4 h, but release encapsulated cytotoxic agents when heated to a critical temperature of approximately 41°C Citation[6–9]. In preclinical studies using regional heating in small animal tumour models, heat-triggered release results in increased overall drug concentrations, reduced blood flow Citation[10], and enhanced anti-tumour effect Citation[11]; however, the underlying mechanisms remain unclear. One theory is that rapid drug release from thermosensitive liposomes in heated tumour vessels creates a localised concentration gradient from the vessel into the tumour interstitium, thereby increasing the penetration of anticancer drugs through the tumour microenvironment Citation[12]. With prolonged heating, thermally triggered release from intact liposomes continually circulating into the heated region could maintain exposure to high levels of the bioavailable drug Citation[13]. However, little is known about how triggered release affects the microregional distribution of anticancer drugs in solid tumours in vivo.
In ongoing clinical trials, thermosensitive liposomes are being combined with two heating techniques: percutaneous radiofrequency thermal ablation to increase the ablation volume when treating liver tumours Citation[14], and superficial microwave hyperthermia to increase the specificity and efficacy of doxorubicin for local breast cancer recurrence Citation[15]. Both heating techniques typically use interstitial probes to monitor tissue temperature during treatment Citation[16], Citation[17], and offer limited spatial control of temperature elevations and hence drug release. In order to increase the number of treatable sites, it would be desirable to use a non-invasive heating and monitoring system with increased spatial and temporal temperature control.
One promising heating technique is focused ultrasound guided by magnetic resonance imaging (MRI). Ultrasound has been used clinically to apply thermal therapy non-invasively to targets that are inaccessible with other heating techniques Citation[18], and can be combined with real-time MRI thermometry to achieve precise spatial and temporal temperature control Citation[19–22]. MRI can also be used to non-invasively monitor drug release from liposomes that co-encapsulate MRI contrast agents Citation[8], Citation[23], Citation[24]. Using MRI-controlled focused ultrasound to localise heating and drug release from thermosensitive liposomes could thus add a non-invasive treatment option for many patients with locally advanced solid tumours.
Previously, hyperthermia-mediated drug delivery using focused ultrasound has been demonstrated in normal tissue Citation[25], small animal tumour models Citation[8], Citation[26], and one recent study demonstrating feasibility in rabbit tumours Citation[27]. Here we report the effects of thermally triggered doxorubicin release in rabbit tumours using a commercially developed thermosensitive drug carrier and MRI-controlled focused ultrasound hyperthermia. We demonstrate temperature control across a large area of each tumour, compare drug concentrations in heated and unheated tumours, and investigate the microregional distribution of released drug within the tumour microenvironment.
Materials and methods
Animals and VX2 tumours
All experiments were approved by the Animal Care Committee at Sunnybrook Research Institute in accordance with the Canadian Council of Animal Care. Thirteen days before treatment, male New Zealand white rabbits (2.5–3.5 kg) were injected in both thigh muscles with a 0.4 mL suspension of 3 to 4 million VX2 carcinoma cells in Hank's balanced salt solution. One tumour was heated in each rabbit, the other served as the control.
Prior to ultrasound heating, rabbits were anaesthetised by intramuscular injection of ketamine (50 mg/kg/h) and xylazine (10 mg/kg/h), and had one ear vein and one ear artery cannulated. Both legs were depilated to enable transmission of ultrasound into the thigh, and then rabbits were placed on a stage above the degassed water tank of an MRI-compatible focused ultrasound system (). During treatment, rectal and skin temperatures were monitored by fibre-optic temperature probes (3100, Luxtron, Santa Clara, CA, USA), and maintained at the same temperature by manually regulating the temperatures of a hot water blanket covering the animal and the degassed water reservoir below (T/Pump Model TP-500, Gaymar, Orchard Park, NY, USA).
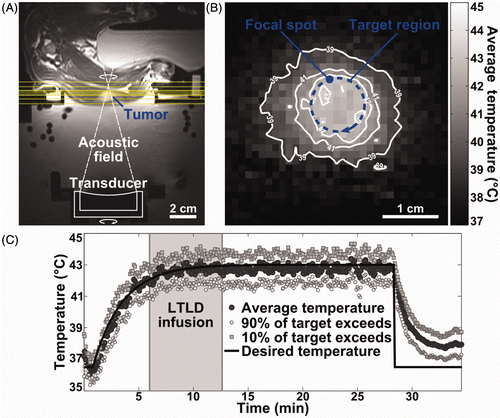
Figure 1. MRI-controlled focused ultrasound hyperthermia in rabbit VX2 tumour. (A) Axial T2-weighted MR image depicting experimental set-up with tumour-bearing rabbit lying on its side above a degassed water bath containing a mechanically positioned focused ultrasound transducer. Coronal image planes in which MR thermometry was used to measure temperature during heating are indicated; the middle plane, set at the depth of the ultrasound focus, was used to control treatment. (B) Time-averaged MRI temperature measurements in a coronal image plane through the tumour, demonstrating spatial temperature distribution achieved using MRI-controlled focused ultrasound hyperthermia. Desired temperature was 43°C in the 10 mm diameter targeted region (blue overlay). The focal spot width and scan trajectory are shown. (C) Experimental timeline for thermosensitive liposome administration and temperature control. Mean temperatures measured using MR thermometry within the 10 mm diameter target region are shown across three image planes (dark circles), as well as the temperatures that 90% (white circles) and 10% (grey squares) of the region exceeds. Thermosensitive liposome infusion started when target temperatures reached 42°C (shaded).
MRI-controlled focused ultrasound hyperthermia
Heating was administered by continuous sonication at 2.787 MHz with a spherically focused air-backed ultrasound transducer (curvature radius 10 cm; aperture diameter 5 cm) incorporated into a computer-controlled MRI-compatible positioning system Citation[28] in a clinical 1.5 T MRI (Signa, GE Healthcare, Waukesha, WI, USA) as described previously Citation[25] and illustrated in . The focal spot was 1.4 mm wide by 20.7 mm long, centred 94 mm from the transducer surface (full-width half-maximum of the relative pressure amplitude squared, measured in water with a fibre-optic hydrophone). MRI-controlled focused ultrasound was used to heat 10 mm diameter tumour regions to 43°C for 20 min. This temperature elevation and exposure duration were chosen to be sufficient to trigger drug release from thermosensitive liposomes Citation[6] and increase local perfusion within the heated region of tissue Citation[29] without causing significant tissue damage Citation[30].
Before treatment, anatomical MR images were acquired with T1-weighting and T2-weighting to identify the tumour location, verify acoustic coupling, and to define a 10 mm diameter target encompassing all or most of the tumour to be heated. The ultrasound transducer was positioned to set the focus at the depth of the targeted tumour, and three imaging planes for MR thermometry were prescribed perpendicular to the ultrasound beam, centred at the depth of the focus.
To heat the 10 mm diameter target region with the approximately 1 mm diameter acoustic focus, the ultrasound transducer was rapidly repositioned along a 10 mm diameter circular scan path perpendicular to the beam direction at one revolution per second during continuous sonication. The focus was thus scanned along the perimeter of the target region in the focal plane (), heating the interior of the region by conduction. During heating, spoiled gradient-echo MR images were acquired continuously, transferred to a control computer, reconstructed, corrected for magnetic field drift, and temporally averaged, producing temperature maps in all three imaging planes every 5 s, as described previously Citation[31]. In the MR thermometry plane through the centre of the tumour, temperatures measured at eight points along the periphery of the target region were used in a proportional-integral feedback control loop to automatically update the ultrasound power applied as the focus crossed through those points Citation[25], thus maintaining temporally and spatially uniform heating in the entire targeted region.
Following sonication, T2-weighted and contrast-enhanced T1-weighted images (0.2 mmol/kg gadodiamide, GE Healthcare) were acquired to evaluate tissue and perfusion changes related to thermal damage and drug release.
The temporal and spatial uniformity of heating were evaluated based on temperatures measured within MR thermometry image regions matching the intended target diameter for all images after the target temperature reached 42°C. The temporal average of the spatial mean, 10th percentile and 90th percentile temperatures at each time point are reported, as well as the diameter receiving a time-averaged temperature of greater than 41°C, and the duration over which a spatially averaged temperature of greater than 41°C was achieved. Thermal dose in the target region was calculated in cumulative equivalent minutes at 43°C (CEM43) using the Sapareto and Dewey time–temperature equation Citation[32].
Drug concentration in unheated tissue and VX2 tumours
Tumour-bearing rabbits were administered either lyso-thermosensitive liposomal doxorubicin (LTLD) (Celsion, Lawrenceville, NJ, USA) or free doxorubicin (Doxorubicin HCl, Teva Novopharm, Toronto, ON, Canada) at a dose of 2.5 mg/kg body weight diluted with equal parts 5% dextrose sterile solution. Infusion at 1.2 mL/min into the ear vein during hyperthermia was initiated once the mean temperature in the target region reached 42°C.
Prior to drug infusion, and at 30-min intervals afterwards, blood (1.5 mL) was collected from a catheterised ear artery and transferred to a 2 mL tube containing 167 µL of 0.109 M sodium citrate to prevent clotting. Plasma was isolated by centrifugation at 4°C for 10 min at 2000 g, and stored at −20°C.
Approximately 2 h after drug infusion, liposomal and free doxorubicin in the systemic circulation were eliminated by cardiac perfusion with saline under deep anaesthesia, effectively isolating extravasated drug deposited in tissue. For evaluation of tissue drug concentrations, samples of tumour and adjacent muscle were harvested from the heated and unheated legs, frozen in liquid nitrogen and stored at −80°C. Samples were also acquired from the skin, heart, lung, liver, kidney and spleen.
Tissue doxorubicin concentrations were measured by the fluorescence intensity of doxorubicin extracted from homogenised tissue samples as described previously Citation[25], Citation[33]. Tissue samples were diced, weighed to 75 mg, and added to 20 volumes of acidified ethanol extraction solvent (0.3 N HCl in 50% ethanol) before homogenisation with either a tissue grinder (PYREX® Ten Broeck, Corning Inc., Corning, NY, USA) or with 500 µL each of 1 mm and 2 mm zirconia beads in a bead mill homogeniser (Mini-BeadBeater 16, Biospec, Bartlesville, OK, USA). Tissue homogenates, as well as plasma samples added to 20 volumes of acidified ethanol, were refrigerated overnight prior to centrifugation (16,000 g, 30 min) and subsequent storage of supernatants in the dark at −20°C. Fluorescence intensity of doxorubicin was measured in 0.5 mL aliquots of supernatant added to 1.5 mL of acidified ethanol in 3 mL fluorometry cuvettes using a bench top fluorometer (VersaFluor, Bio-Rad Laboratories, Hercules, CA, USA) with 480 nm excitation and 590 nm emission filters. Relative fluorescence intensities were scaled to doxorubicin concentrations using a fluorescence calibration curve of a serial dilution of free doxorubicin added to 0.5 mL blank tissue or plasma supernatants in 1.5 mL of acidified ethanol.
Doxorubicin concentrations in tissue are summarised by the mean and SD across all animals. The two-sided Wilcoxon signed-rank test was used to compare drug concentrations in heated and unheated tissues for samples of tumour and adjacent muscle. A two-sided Wilcoxon rank-sum test was used to compare enhancement ratios for thermosensitive liposomes versus free doxorubicin. Differences were considered statistically significant for values of p < 0.05.
Drug distribution in the tumour microenvironment
The microregional distribution of doxorubicin with respect to the tumour microvasculature was measured using quantitative fluorescence microscopy Citation[34]. To improve detection of doxorubicin fluorescence two animals were administered increased doses of 8.3 mg/kg of thermosensitive liposomal doxorubicin during MRI-controlled focused ultrasound hyperthermia. Following post-treatment imaging, these rabbits were sacrificed without saline perfusion. Heated and unheated tumours were excised, embedded in optimum cutting temperature compound (OCT, Sakura Finetek, Torrance, CA, USA), frozen in liquid nitrogen, and stored at −80°C prior to cryostat sectioning (6 µm).
Blood vessels in tissue sections were recognised by the expression of CD31 membrane protein on endothelial cells, and doxorubicin distribution was identified by its fluorescence. Thawed sections were imaged and tiled at 10× and 40× using an Olympus BX50 upright microscope (Olympus Canada Inc., Richmond Hill, ON, Canada) equipped with a 100 W mercury light source, a Photometrics CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ, USA), and a motorised stage. Doxorubicin fluorescence was detected using 3000 ms exposures with 475 to 495 nm excitation and 589 to 625 nm emission filters prior to immunohistochemical staining. Following doxorubicin imaging, sections were fixed in acetone for 20 min, washed in PBS, and blocked with a 1:10 dilution of donkey serum for 30 min to prevent non-specific antibody binding. Sections were then incubated with mouse anti-human CD31 monoclonal antibodies that show cross-reactivity with rabbit CD31 antigen (ab9498, Abcam, Cambridge, MA, USA) at a dilution of 1:100 for 1 h in a humidified chamber, washed in Tris-buffered saline, and subsequently stained with donkey anti-mouse IgG secondary antibody conjugated to an Alexa Fluor 488 fluorophore (Jackson ImmunoResearch, West Grove, PA, USA) at a dilution of 1:200 for 1 h. Finally, slides were cover-slipped with a mounting medium containing DAPI nuclear dye (Vector, Burlingame, CA). Using the same microscope stage positions used for doxorubicin imaging, anti-CD31 staining of endothelial cells was detected using 1000 ms exposures with 475 to 495 nm excitation and 510 to 540 nm emission filters, and DAPI-stained nuclear DNA localisation was detected using 100 ms exposures (381–392 nm excitation, 420–460 nm emission).
Drug delivery in the tumour microenvironment was evaluated based on the distribution of doxorubicin in relation to the distribution of tumour blood vessels. Tiled 10× images of CD31-stained tumour vasculature were thresholded, aligned with the DAPI images based on overlays of the CD31 and DAPI images, and overlaid on the corresponding images of doxorubicin fluorescence, which were corrected by subtracting the background reading measured in blank slide regions. Each tissue section was divided into a grid of 0.5 × 0.5 mm2 regions (0.4 µm2/pixel), excluding areas with staining artefacts and muscle surrounding the tumour. In each gridded region, the mean doxorubicin fluorescence intensity was recorded, as well as the microvessel density (measured by the number of vessels per mm2). Doxorubicin fluorescence in gridded sections of similar vascular density was compared between heated and unheated tumours using the two-sided Wilcoxon rank-sum test.
Results
Animals and VX2 tumours
Of 24 rabbits inoculated with bilateral VX2 tumours, six were excluded due to tumours that were poorly positioned or too small to identify on MR imaging. Tumours of at least 8 mm in their largest dimension were detectable as well-defined regions of heterogeneous signal increase on T2-weighted MR images. Treated tumours were situated either in the fascia between the biceps femoris and semimembranosus muscles or infiltrating into one of the two; the largest dimension of treated tumours ranged between 11 and 28 mm ().
Table I. Temperatures measured non-invasively with MR thermometry during MRI-controlled focused ultrasound hyperthermia in rabbit VX2 tumours. Temperatures reported as the temporal mean from when the desired target temperature reached 41°C until the end of heating. Summary data are reported as mean ± standard deviation across all rabbits.
MRI-controlled focused ultrasound hyperthermia in VX2 tumours
The precision of MR temperature measurements used to control heating was 0.25 ± 0.04°C, measured as the standard deviation of the temperature noise in MR thermometry images. A linear background magnetic field drift of −0.26 ± 0.06°C/min was corrected online by subtracting MR temperature measurements made in a mineral oil phantom from temperatures in the rest of the image. Initial rabbit body temperatures ranged from 34.0°C to 36.7°C and were maintained within 1°C during treatment.
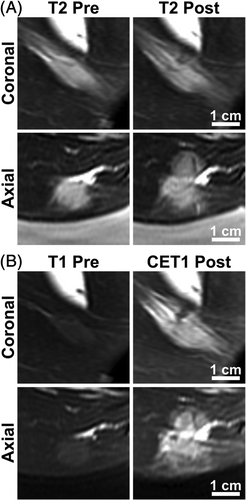
MRI-controlled focused ultrasound achieved spatially uniform tumour heating, with temperature elevations localised to the target region () and maintained over the heating duration (). MR temperature measurements in 12 tumour-bearing rabbits are summarised in . The mean temperature of 42.8°C achieved in the target region closely matched the goal of 43°C, with 10th and 90th percentile temperatures of 41.4°C and 44.2°C. Drug infusion began after target region temperatures reached 42°C, which was achieved after a mean of 6.5 ± 1 min. Mean temperatures high enough to achieve drug release from thermosensitive liposomes (41°C) were localised to an 11.2 ± 1.6 mm diameter region, and maintained above 41°C for 20.3 ± 4.5 min in the 10 mm diameter targeted region. The median thermal dose in the target region was 12.3 CEM43. In five rabbits varying degrees of thermal damage were observed on post-treatment imaging. presents T2-weighted and contrast-enhanced T1-weighted images for the rabbit that received T10 temperatures of 45.8°C and an accumulated thermal dose of 26.5 CEM43. In four of the reported experiments, temperature control and ultrasound exposure were interrupted after less than 20 min at the plateau temperature due to operator error or sudden rabbit motion. Results for six additional rabbits are not reported because temperature control was interrupted prior to drug infusion.
Figure 2. MRI detection of tissue damage in a tumour-bearing rabbit treated with hyperthermia and thermosensitive liposomal doxorubicin. The rabbit that received a thermal dose of 26.5 equivalent min at 43°C is shown. (A) T2-weighted images in coronal (top) and axial (bottom) planes through the tumour, acquired prior to treatment (left) and following treatment (right). (B) T1-weighted images in the same imaging planes. Gadolinium contrast agent was injected immediately before acquiring post-treatment T1-weighted images.
Drug deposition in VX2 tumours
Plasma doxorubicin concentrations in rabbits treated with hyperthermia during infusion of either thermosensitive liposomes or free drug are shown in . In two early experiments blood samples were not collected, and in some animals, blood samples at the latest time point could not be collected due to clotting in the arterial catheter. For LTLD, an estimated 47% of the total injected doxorubicin remained in the bloodstream at 30 min after the start of infusion (assuming a total plasma volume of 3.6 mL per 100 g body mass Citation[35]), falling to 15% after 2 h with a terminal half-life of 88.1 ± 22.8 min (SEM). For free doxorubicin, which has an initial half-life of less than 5 min Citation[36], rapid plasma clearance left very little drug in the bloodstream by the time the first sample was collected.
Figure 3. Plasma and tissue doxorubicin concentrations in tumour-bearing rabbits. (A) Blood samples collected prior to and at 30-min intervals after intravenous administration of lyso-thermosensitive liposomal doxorubicin (LTLD, n = 5) or free doxorubicin (n = 3). Peak concentrations, likely to have occurred at the end of the 6–7 min infusion (dark shading), could not be measured as this was during MRI-controlled focused ultrasound heating (light shading). Monoexponential fit is shown with 95% confidence interval. (B) Biodistribution of doxorubicin 2 h after intravenous injection of LTLD (n = 7) or free doxorubicin (n = 3). For both formulations rabbits were administered 2.5 mg doxorubicin/kg over 6–7 min during MRI-controlled focused ultrasound hyperthermia localised to one tumour and its surrounding muscle. Mean ± standard deviation of doxorubicin concentration shown.
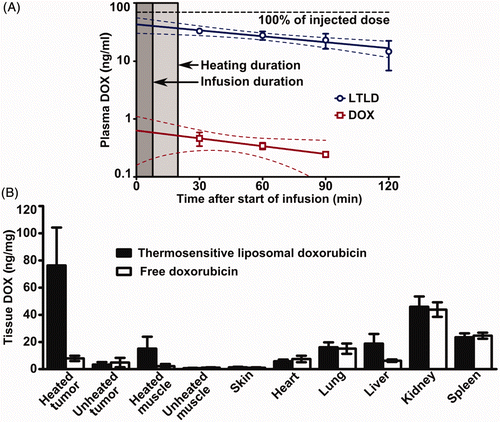
summarises overall drug concentrations in tissue homogenates from rabbits administered LTLD or free doxorubicin, sampled two hours after the start of drug infusion. The two drug formulations had similar biodistributions, except for an increased doxorubicin concentration for LTLD in the liver, where liposomes are known to accumulate Citation[5]. Rabbits treated with thermosensitive liposomes showed a moderate 4.7-fold enhancement in doxorubicin deposition in unheated tumours with respect to the surrounding unheated muscle (3.4 ± 1.8 versus 0.7 ± 0.2 ng/mg, p = 0.016). In rabbits treated with free doxorubicin no significant difference was observed in unheated tumours over unheated muscle (4.9 ± 3.5 versus 1.1 ± 0.2 ng/mg, p = 0.5), nor for heated over unheated tumours (7.9 ± 1.9 versus 4.9 ± 3.5 ng/mg, p = 0.25) or muscle (2.2 ± 1.6 versus 1.1 ± 0.2 ng/mg, p = 0.25). Following treatment with hyperthermia and thermosensitive liposomes, doxorubicin concentrations were 26.7 ± 16.2 times higher in heated tumours than in unheated tumours (76.3 ± 27.9 versus 3.4 ± 1.8 ng/mg, p = 0.016), and the thermal enhancement ratio for thermosensitive liposomal doxorubicin was significantly higher than for free drug (26.7 ± 16.2 versus 2.6 ± 2.3 times, p = 0.017). For LTLD, doxorubicin concentrations in heated muscle surrounding targeted tumours were 22.2 ± 12.3 times higher than in unheated muscle (15.1 ± 8.8 versus 0.7 ± 0.2, p = 0.016), but heated tumours had 4.9 ± 1.2 times higher drug concentrations than heated muscle (76.3 ± 27.9 versus 15.1 ± 8.8 ng/mg, p = 0.016).
Increased delivery of bioavailable drug in tumour cells
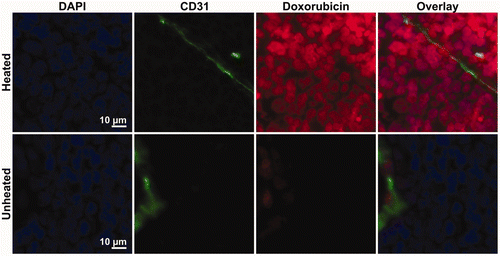
provides representative composite images of the microregional distribution of doxorubicin fluorescence (red) in relation to DAPI-stained cell nuclei (blue) and CD31-stained blood vessel endothelial cells (green) in heated and unheated VX2 tumours. Both tumours are from the same animal, harvested 2 h after intravenous infusion of 8.3 mg/kg doxorubicin in thermosensitive liposomes. In contrast to the rabbits used for biodistribution measurements in homogenised samples, no saline perfusion was performed prior to sacrifice. The overall doxorubicin fluorescence intensity in the heated tumour was much higher than in the unheated tumour, despite having somewhat lower vessel density. Within individual tumours, blood vessel density was heterogeneous; in heated tumours, doxorubicin fluorescence was higher in regions with increased vessel density, while in unheated tumours the doxorubicin signal was uniformly low and indistinguishable from background autofluorescence. includes characteristic 0.5 × 0.5 mm2 sections from both highly vascularised (, microvessel density approximately 200 vessels/mm2) and poorly vascularised (, vessel density 50 vessels/mm2) regions of the heated and unheated tumours. The doxorubicin fluorescence in the heated tumour demonstrates a punctate pattern of increased drug accumulation co-localised with DAPI staining of DNA in the cell, displayed at 40× magnification in . In heated tumours, nucleus-specific accumulation of fluorescence from released doxorubicin was consistent even for cells situated many cell layers from the nearest tumour vessel; in unheated tumours, low levels of doxorubicin fluorescence were indistinguishable from the background cellular autofluorescence. This suggests that thermally triggered release from thermosensitive liposomes increased the accumulation of bioavailable doxorubicin in the nuclei of tumour cells.
Figure 4. Microregional distribution of doxorubicin in heated and unheated tumours. Rabbits with VX2 tumours implanted in both thighs were treated with MRI-controlled focused ultrasound hyperthermia in one tumour during intravenous infusion of thermosensitive liposomal doxorubicin. Tumours were harvested 2 h after infusion and then sectioned, stained and tiled at 10× magnification. Composite images of heated and unheated tumours from one animal display DAPI staining of cell nuclei in blue and background-subtracted doxorubicin fluorescence in red with CD31-stained endothelial cells identifying tumour vessels in green.
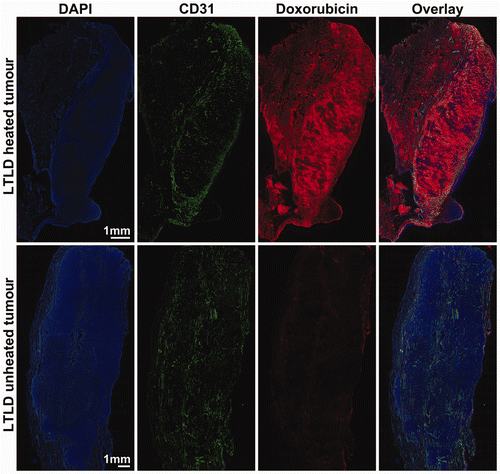
Figure 5. Heterogeneity of microregional distribution of doxorubicin in regions with varying tumour vascularity. Representative 0.5 mm × 0.5 mm regions of composite images from heated and unheated tumours showing doxorubicin fluorescence (red), DAPI staining (blue), and CD31 staining of vessel endothelial cells (green) from areas where vessel density was relatively high (A) and low (B). The number of vessels per mm2 was measured by counting distinct regions of CD31-stained endothelial cells.
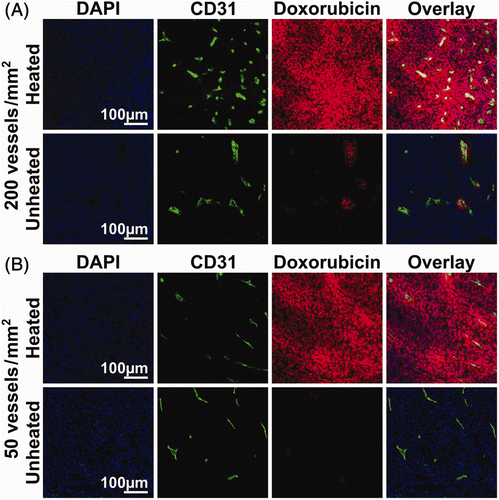
Figure 6. Accumulation of doxorubicin in the cells of heated and unheated tumours following administration of thermosensitive liposomal doxorubicin. In 40× images of the heated tumour, doxorubicin fluorescence (red) demonstrates co-localisation with DAPI staining of cell nuclei (blue) outside of the CD31-stained blood vessels (green). In the unheated tumour, doxorubicin fluorescence was limited to perivascular regions; low levels of doxorubicin fluorescence in the tumour interstitium could not be distinguished from the background.
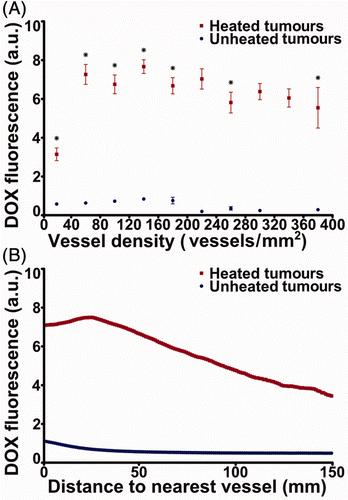
The effect of thermally triggered release on drug deposition in the microenvironment of heated and unheated tumours from two rabbits treated with thermosensitive liposomal doxorubicin is quantified in . In 0.5 × 0.5 mm2 regions with matched microvessel densities of 0 to 400 vessels/mm2, the average doxorubicin fluorescence in sections of heated tumours was 3.8 to 9.2 times greater than in unheated sections (p < 0.05, two-sided Wilcoxon rank-sum test). This consistent increase in doxorubicin fluorescence provided by heat-triggered release, even in regions with low vascular density, suggests improved drug distribution within the tumour microenvironment. In , the uniformity of doxorubicin distribution in the tumour microenvironment is quantified by the mean ± standard error of doxorubicin fluorescence intensity in relation to distance to the nearest vessel, averaged across regions containing a majority of tumour cells in sections from both rabbits. The fluorescence intensity of doxorubicin in heated tumours decays with distance the way it does with free doxorubicin in mouse tumours Citation[34], but remains higher than in unheated tumours.
Figure 7. Spatial distribution of doxorubicin fluorescence with respect to vessel density and vessel location in heated and unheated tumours of rabbits administered thermosensitive liposomal doxorubicin. (A) Background-subtracted fluorescence intensity of doxorubicin in 500 µm × 500 µm regions of 6 µm frozen sections from two rabbits, classified by the vessel density in those regions. Mean ± SEM is representative of intra-tumour variability based on the aggregate of 675 regions across tumours from two rabbits. *p < 0.05, two-sided Wilcoxon rank-sum test. (B) Background-subtracted doxorubicin fluorescence intensity (mean ± SEM of aggregated data from two rabbit tumours) averaged by distance to the nearest CD31-stained pixel across all imaged sections of heated and unheated VX2 tumours from two rabbits administered thermosensitive liposomal doxorubicin. Note: a.u., arbitrary units.
Discussion
In this study, thermally mediated drug release resulted in enhanced local drug deposition with specific accumulation of bioavailable drug in the nuclei of tumour cells. These results were achieved using a commercial formulation of thermosensitive liposomal doxorubicin and non-invasive MRI-controlled focused ultrasound hyperthermia in a large animal tumour model. The large size and heterogeneous vasculature of rabbit VX2 tumours Citation[37], Citation[38] permits testing of the robustness of heating techniques and provides insights on drug delivery in solid tumours that have distinct regions of high and low vessel density and perfusion Citation[38], similar to tumours encountered in the clinic.
Our results demonstrate that hyperthermia-mediated drug delivery increases drug deposition in targeted tissue for a given systemic dose. Administration of thermosensitive liposomal doxorubicin resulted in a 4.7-fold increase in drug deposition in unheated tumours versus surrounding muscle. Localised hyperthermia increased the doxorubicin concentration in heated tumours and surrounding muscle over unheated tumours and muscle by 26.7 and 22.2 times, respectively. A 4.9-fold increase was observed in heated tumours over heated muscle. Significant enhancements were not observed in heated tumours and normal tissue when rabbits were administered free doxorubicin. With thermosensitive liposomes, enhancements in tumours over muscle suggest tumour-specific liposome extravasation, while the enhancement in heated over unheated muscle stresses the importance of localised hyperthermia to prevent unwanted drug release in normal tissues.
Measurements of plasma doxorubicin concentrations demonstrate an increased circulation time for doxorubicin in thermosensitive liposomes over free drug Citation[36], suggesting that prolonged hyperthermia should increase local drug deposition as intact liposomes continually circulate into heated regions and release their payload.
Similar increases in drug concentration were observed in heated tumours and normal muscle, despite previous findings demonstrating that the hyperthermia-mediated extravasation of 100 nm liposomes exploited in tumours Citation[39] does not occur in healthy muscle Citation[4]. Furthermore, liposomes were administered during heating, without allowing sufficient time for passive liposome extravasation and accumulation in targeted tumours. These observations support the hypothesis proposed by other recent studies Citation[27], Citation[40] that the primary mechanism for hyperthermia-enhanced drug deposition using thermosensitive liposomes is the triggered release of bioavailable drug in the vasculature, followed by the subsequent extravasation of active drug into the tumour interstitium.
The 26.7-fold enhancement in drug delivery observed with thermosensitive liposomal doxorubicin in heated tumours is somewhat higher than the 5 - to 15-fold enhancements reported previously Citation[7–9], Citation[11], Citation[12], Citation[26], Citation[41], Citation[42]; however, most of those studies used open-loop, regional heating techniques in small rodent tumours, with a larger heated volume to body weight ratio. The recent study of Ranjan et al. Citation[27] used a clinical MRI-guided focused ultrasound device and the same liposome formulation, but reported only a 3.4-fold increase in doxorubicin concentration between heated and unheated rabbit VX2 tumours. One reason for this discrepancy is that they heated 4 mm diameter tumour regions to 40–41°C, while we used multi-point temperature control to heat 10 mm diameter regions to 43°C, with hyperthermic temperatures extending beyond the tumour along the ultrasound beam. By achieving sufficient temperatures for triggered drug release (>41°C) in smaller regions, they observed reduced enhancements in whole tumour homogenates but more specific tumour targeting with respect to adjacent unheated muscle. In our study, heating to 43°C resulted in thermal damage in some rabbits, as expected for thermal dose between 5 and 30 CEM43 Citation[30]. However, these higher exposures are still not likely to cause damage in human tissue Citation[43], and may provide important clinical benefit, as heat-induced doxorubicin chemosensitisation occurs at a threshold of 43°C Citation[44]. Achieving large increases in tumour drug concentration may be clinically important, as previous in vitro studies suggest intracellular doxorubicin concentrations of approximately 60 ng/mg are required for greater than 99% cell kill Citation[45].
Our assessment of the effect of triggered release on the microregional distribution of doxorubicin by fluorescence microscopy permitted confirmation that enhanced drug concentrations in heated tumours corresponds to intracellular uptake of bioavailable drug. Doxorubicin fluorescence co-localised with DAPI staining of cell nuclei throughout the heated tumour, demonstrating generally increased doxorubicin deposition in comparison with the unheated tumour. Discrepancies in the degree of doxorubicin fluorescence enhancement in heated over unheated tumours between homogenised tissues and microscopy are likely related to the effects of slide preparation on doxorubicin fluorescence and differences in the excitation wavelength used for microscopy, as well as the inability to completely isolate doxorubicin fluorescence from background in unheated tumours. Not performing saline perfusion prior to tumour harvest for microscopy may also have contributed to an under-estimated enhancement ratio, although doxorubicin fluorescence may be lower in intact liposomes due to quenching Citation[13]. These results extend previous qualitative observations of overall doxorubicin distribution in VX2 tumours following heat-triggered release Citation[27] and quantitative analysis in rabbit thigh muscle Citation[40].
In agreement with the results of Ranjan et al. Citation[27], our measurements of overall doxorubicin accumulation in homogenised tissue samples from untargeted organs showed a similar biodistribution in rabbits receiving either free or liposomal drug, with the exception of increased concentrations in the liver for LTLD, where liposomes are expected to accumulate. Additional experiments using fluorescence microscopy in untargeted organs would be useful in determining whether drug accumulation in those tissues resulted from the extravasation of circulating liposomes or from intravascular drug release.
MR thermometry data demonstrated that focused ultrasound, controlled by quantitative MR images of tissue temperature, can be used to achieve temporally and spatially uniform mild hyperthermia in heterogeneously perfused tumours. The mechanically steered system used in this study is a cost-effective preclinical approximation of electronically steered clinical MRI-guided focused ultrasound systems Citation[46], providing flexibility in customisable scanning trajectories and multi-point temperature control algorithms in a research setting. However, to reduce the effects of transducer motion on MR thermometry, we used a single-element transducer with a relatively high f-number that could heat the tumour while being located far from the imaging plane Citation[25]. The use of an f-number 2 transducer resulted in heating being spread out along the axis of the ultrasound beam, causing drug release in muscle along the beam path. Our control algorithm could easily be applied with existing clinical devices, in which phased array transducers would allow better control of the ultrasound field, while multi-plane MR thermometry could improve safety and temperature control Citation[47], Citation[48].
Conclusion
Our study contributes to a growing body of literature that demonstrates localised, image-guided drug deposition in implanted tumours, using MRI-controlled focused ultrasound hyperthermia to achieve non-invasive thermally mediated drug release from thermosensitive liposomes Citation[8], Citation[9], Citation[27], Citation[40], Citation[48]. Heat-triggered release of doxorubicin from liposomes in the tumour vasculature allowed bioavailable drug to accumulate in the tumour and localise at its site of lethal activity in the nuclei of tumour cells. Our study complements recent work by achieving large increases in doxorubicin concentration in tumours for a given systemic dose, and providing quantitative analysis of drug distribution in the tumour microenvironment. Clinically, enhancing the accumulation and penetration of anticancer drugs in solid tumours could increase cell kill in each chemotherapy treatment cycle and limit the opportunity for tumours to repopulate and gain drug resistance between cycles, thus providing patients with locally advanced solid tumours a better chance of relapse-free survival.
Acknowledgements
The authors thank Alexandra Garces and Shawna Rideout for providing their expertise during rabbit experiments, as well as Anthony Chau and Adam Waspe for assistance with the positioning system, and Jasdeep Saggar for assistance with fluorescence microscopy.
Declaration of interest: Thermosensitive liposomal doxorubicin was provided by Celsion. This work was supported by funding from a Terry Fox Foundation New Frontiers Program project grant from the National Cancer Institute of Canada (17005), by an Ontario Research Fund grant from the Government of Ontario (RE-02-32), and by the Canada Research Chairs Program. Robert Staruch is supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Alexander Graham Bell Canada Graduate Scholarship. Kullervo Hynynen and Rajiv Chopra are founders and have shares in FUS Instruments, a company that is commercialising the preclinical FUS system described in these experiments. The authors alone are responsible for the content and writing of the paper.
References
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6: 583–592
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010; 7: 653–664
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007; 99: 1441–1454
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and In Vivo Drug Release Rates in Liposomal Nanocarrier Development. J Pharm Sci 2008; 97: 4696–4740
- Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 2001; 53: 285–305
- Li L, ten Hagen TL, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AM, et al. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J Control Release 2010; 143: 274–279
- de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J Control Release 2011; 150: 102–110
- Tagami T, Ernsting MJ, Li S. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release 2011; 152: 303–309
- Chen Q, Krol A, Wright A, Needham D, Dewhirst MW, Yuan F. Tumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposome. Int J Hyperthermia 2008; 24: 475–482
- Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia 2010; 26: 485–498
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Gasselhuber A, Dreher MR, Negussie A, Wood BJ, Rattay F, Haemmerich D. Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperthermia 2010; 26: 499–513
- Poon RTP, Borys N. Lyso-thermosensitive liposomal doxorubicin: An adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol 2011; 7: 937–945
- Celsion. Phase 1/2 study of ThermoDox with approved hyperthermia in treatment of breast cancer recurrence at the chest wall (DIGNITY). Available at: http://www.clinicaltrials.gov/ct2/show/NCT00826085 (accessed 19 March 2011)
- Van Der Zee J, De Bruijne M, Mens JWM, Ameziane A, Broekmeyer-Reurink MP, Drizdal T, et al. Reirradiation combined with hyperthermia in breast cancer recurrences: Overview of experience in Erasmus MC. Int J Hyperthermia 2010; 26: 638–648
- Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011; 258: 351–369
- Hynynen K. MRIgHIFU: A tool for image-guided therapeutics. J Magn Reson Imaging 2011; 34: 482–493
- Salomir R, Vimeux FC, de Zwart JA, Grenier N, Moonen CTW. Hyperthermia by MR-guided focused ultrasound: Accurate temperature control based on fast MRI and a physical model of local energy deposition and heat conduction. Magn Reson Med 2000; 43: 342–347
- Smith NB, Merrilees NK, Dahleh M, Hynynen K. Control system for an MRI compatible intracavitary ultrasound array for thermal treatment of prostate disease. Int J Hyperthermia 2001; 17: 271–282
- Arora D, Cooley D, Perry T, Guo J, Richardson A, Moellmer J, et al. MR thermometry-based feedback control of efficacy and safety in minimum-time thermal therapies: Phantom and in-vivo evaluations. Int J Hyperthermia 2006; 22: 29–42
- Enholm JK, Kohler MO, Quesson B, Mougenot C, Moonen CT, Sokka SD. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng 2010; 57: 103–113
- Negussie AH, Yarmolenko PS, Partanen A, Ranjan A, Jacobs G, Woods D, et al. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int J Hyperthermia 2011; 27: 140–155
- Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP, et al. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials 2011; 32: 6570–6578
- Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia 2011; 27: 156–171
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res 2007; 13: 2722–2727
- Ranjan A, Jacobs G, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit VX2 tumor model. J Control Release 2011; 158: 487–494
- Chopra R, Curiel L, Staruch R, Morrison L, Hynynen K. An MRI-compatible system for focused ultrasound experiments in small animal models. Med Phys 2009; 36: 1867–1874
- Dudar TE, Jain RK. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res 1984; 44: 605–612
- McDannold NJ, King RL, Jolesz FA, Hynynen KH. Usefulness of MR imaging-derived thermometry and dosimetry in determining the threshold for tissue damage induced by thermal surgery in rabbits. Radiology 2000; 216: 517–523
- Staruch R, Chopra R, Hynynen K. MRI-controlled focused ultrasound hyperthermia for targeted drug delivery in bone: In vivo results. Radiology 2012; 263: 117–127
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Bachur NR, Moore AL, Bernstein JG, Liu A. Tissue distribution and disposition of daunomycin (NCS-82151) in mice: Fluorometric and isotopic methods. Cancer Chemother Rep 1970; 54: 89–94
- Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res 2005; 11: 8782–8788
- Little RA. Changes in blood volume of rabbit with age. J Physiol 1970; 208: 485–497
- Robert J, Illiadis A, Hoerni B, Cano JP, Durand M, Lagarde C. Pharmacokinetics of Adriamycin in patients with breast cancer – Correlation between pharmacokinetic parameters and clinical short-term response. Eur J Cancer Clin Oncol 1982; 18: 739–745
- Kidd JG, Rous P. A transplantable rabbit carcinoma originating in a virus-induced papilloma and containing the virus in masked or altered form. J Exp Med 1940; 71: 813–U22
- Purdie TG, Henderson E, Lee TY. Functional CT imaging of angiogenesis in rabbit VX2 soft-tissue tumour. Phys Med Biol 2001; 46: 3161–3175
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Gasselhuber A, Dreher MR, Partanen A, Yarmolenko PS, Woods D, Wood BJ, et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: Computational modelling and preliminary in vivo validation. Int J Hyperthermia 2012; 28: 337–348
- Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, et al. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. J Natl Cancer Inst 2007; 99: 53–63
- Patel P, Luk A, Durrani A, Dromi S, Cuesta J, Angstadt M, et al. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int J Hyperthermia 2008; 24: 537–549
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011; 27: 320–343
- Marmor JB. Interactions of hyperthermia and chemotherapy in animals. Cancer Res 1979; 39: 2269–2276
- Kerr DJ, Kerr AM, Freshney RI, Kaye SB. Comparative intracellular uptake of Adriamycin and 4'-deoxydoxorubicin by non-small cell lung-tumor cells in culture and its relationship to cell-survival. Biochem Pharmacol 1986; 35: 2817–2823
- Kohler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009; 36: 3521–3535
- Mougenot C, Quesson B, de Senneville BD, de Oliveira PL, Sprinkhuizen S, Palussiere J, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU). Magn Reson Med 2009; 61: 603–614
- Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012; 28: 320–336
