Abstract
Purpose: This study evaluated the HIFU treatment time reductions attainable for several scan paths when optimising the heating approach used (single, discrete pulses versus volumetric scanning) and the paths’ focal zone heating locations’; number (NFZL), spacings, sequencing order, number of heating cycles (NCYCLES), and heating times. Also evaluated were the effects of focal zone size, increased tissue absorptivity due to heating, and optimisation technique.
Materials and methods: Treatments of homogeneous constant property tumours were simulated for several simple generic tumour shapes and sizes. The concentrated heating approach (which delivered the desired thermal dose to each location in one discrete heating pulse (NCYCLES = 1)) was compared to the fractionated heating approach (which dosed the tumour using multiple, shorter pulses repeatedly scanned around the heating path (i.e. ‘volumetric scanning’ with NCYCLES > 1)). Treatment times were minimised using both simultaneous, collective pulse optimisation (which used full a priori knowledge of the interacting effects of all pulses) and sequential, single pulse optimisation (which used only the information from previous pulses and cooling of the current pulse).
Results: Optimised concentrated heating always had shorter treatment times than optimised fractionated heating, and concentrated heating resulted in less normal tissue heating. When large, rapid tissue absorptivity changes were present (doubled or quadrupled immediately after heating) the optimal ordering of the scan path's sequence of focal zone locations changed.
Conclusions: Concentrated heating yields significant treatment time reductions and less normal tissue heating when compared to all fractionated scanning approaches, e.g. volumetric scanning.
Introduction
HIFU has been shown to be a promising treatment modality for several types of cancer and uterine fibroids Citation[1–8]. While many factors will affect its ultimate clinical acceptance, long treatment times Citation[9], Citation[10], sometimes several hours Citation[5], can present a serious obstacle to wider clinical implementation. This limitation will become increasingly important when larger malignant tumours (whose irregularly shaped volumes must be completely dosed) in critical locations are treated. Reductions in treatment time are possible through user selection of the HIFU treatment operating parameters, including: the transducer power; the pulse heating times; the focal zone (FZ) location(s) in the tumour; the order in which these locations are treated; the number of times they are each heated; and the focal zone size and shape. Finding an optimised set of treatment parameters that will make a given treatment short enough to be clinically viable presents a complicated problem. Although many studies have looked at treatment time as a factor when studying HIFU treatments, including studies on dose homogeneity inside of the tumour and new transducer systems Citation[11–22], only a few studies have focused solely on the development of a set of optimal treatment scanning parameters to specifically reduce treatment time Citation[23–26]. The problem is made especially difficult because the large dimensionality of the problem combined with long computational times makes a ‘brute force’ search through all treatment parameter space unfeasible Citation[25]. Because of this difficulty with exhaustive searches for optimal treatment parameters, previous research has optimised each user-selectable parameter independently Citation[23], Citation[25]. So far, optimisation research specifically focused on minimising treatment time has been done on the transducer power levels Citation[23], heating and cooling times at each position Citation[23], and the path of the focal zone through the tumour Citation[25].
This study expands that research to compare treatment times of a concentrated and a fractionated pulse heating approach (defined below). It does so while also studying the effects of an increasing number of focal zone heating locations used to heat a tumour of a fixed size (focal zone packing density), and for a range of focal zone spacings, both axial and transverse, for a fixed number of focal zones heating a tumour of a fixed size. Additionally, this study investigates the effects of heating-induced absorptivity changes on the optimal path for treatments using focal zones in an axial stack.
Concentrated versus fractionated focal zone heating
The question of whether to use a single heating pulse to completely deliver a desired total therapeutic thermal dose to each successive focal zone location individually before moving to another location (herein called concentrated heating), versus an approach that successively passes the focal zone over a fixed set of positions in a cyclical fashion that only delivers a fraction of the desired thermal dose to each location during each such pass (herein called fractionated heating) has been the subject of much speculation. These two approaches have different advantages and disadvantages. This paper concentrates on comparing the speed with which they can treat tumours under comparable conditions in order to determine which approach has the potential to be clinically faster. Although several speculative claims have been made regarding treatment speed, no systematic studies have been performed to evaluate their relative heating times under comparable conditions. Many HIFU applications have used concentrated heating in simulations, animal experiments and clinical treatments Citation[4], Citation[16], Citation[17], Citation[27–37]. The concentrated HIFU method was developed, in part, to overcome the long treatment times present in standard hyperthermia Citation[30], Citation[38] by using small, concentrated, high power density focal zones that produced high temperatures in short times. By heating tissues rapidly, that approach both reduced the time available for cooling to occur and took advantage of the non-linear temperature versus thermal dose relationship Citation[30], Citation[39]. Concentrated heating was also introduced to reduce the cooling effects of the (unknown) tumour blood flow Citation[30], Citation[31] by inducing more dependence on thermal conduction. Unknown blood flow effects are now less of a concern since the temperatures present during treatments can be measured with magnetic resonance imaging (MRI) Citation[4], Citation[40–42]. One way to obtain more concentrated treatments is to use a more highly focused beam with a tight focus (with dimensions of approximately 1 mm by 5 mm, e.g. Citation[4]). However, such smaller focal zones require treatment of a larger number of focal zone locations to cover a given tumour, potentially resulting in more heating in the normal tissue due to build up from the larger number of points heated Citation[43], Citation[44].
Other research Citation[21], Citation[45–50] has subsequently modified the concentrated heating approach in one of two ways. First, investigators have studied the use of a larger focal zone (produced either electronically or mechanically) that is then scanned discretely through a reduced number of locations Citation[46], Citation[47], Citation[50]. Focal zones as large as 1 × 1 × 2 cm3 Citation[47] have been used, and one simulation study investigated the use of a large, single focal zone that was optimally shaped to yield a uniform thermal dose in the tumour, thus potentially minimising the total energy needed to treat the tumour Citation[51]. Second, other investigators have proposed using a single focal zone (or multiple foci) to rapidly scan a large volume by using repetitive heating pulses that cyclically heat a sequence of focal zone locations (called volumetric scanning), including extensive research on animal models Citation[21], Citation[45], Citation[49]. These volumetric heating approaches use rapid electronic switching to repeatedly progress through successive treatment locations, and have the potential advantage of giving a more uniform temperature distribution in the tumour. However, problems exist with this approach as well. Due to the smaller power density ratio between the tumour and that in the normal tissue, more near-field heating may occur in these treatments, a trend noted by Damianou and Hynynen Citation[31]. Also, when trying to minimise treatment times, research has indicated Citation[23], Citation[25] that it is always desirable to have the maximum possible power density at each treatment location to take advantage of both the reduced time available for cooling and the non-linear rate of thermal dose deposition, factors whose effects are reduced by the dilution of power present in the larger, or repeatedly scanned, focal zone approaches. Finally, some researchers have investigated a mix of concentrated and diluted focal zones in simulation studies Citation[52].
Though strong opinions exist regarding which treatment strategy (concentrated or fractionated) will achieve optimal results when considering a treatment time metric, little work has been done to directly compare the two methods. This paper directly compares these methods with the end goals of both gathering quantitative evidence on, and explaining the underlying physics of both heating approaches, in order to help resolve this timing question.
Focal zone packing, spacing and scanning path
Most studies using a discrete scanning approach use conservative spacings between focal zone locations to avoid thermal dose ‘holes’ of untreated tissue inside the tumour, with a spacing of about 3 mm laterally Citation[4], Citation[25] and 5 mm or less axially between treatment planes Citation[4], Citation[25], Citation[28] being typical. However, little work has been done on treatments that employ a more aggressive axial and transverse spacing, and little work has been done on the trade-offs involved between the optimal spacing of a smaller number of focal zones versus increasing the focal zone packing density. This need reflects a similar need for systematic studies of different focal zone sizes, which have only been studied for a few isolated cases, often as part of a larger study whose main focus is another phenomenon Citation[11], Citation[17], Citation[20], Citation[22], Citation[53], Citation[54]. Thus, the effect of focal zone size on treatment time remains unclear.
Several previous studies Citation[11], Citation[13], Citation[23], Citation[25], Citation[45], Citation[49], Citation[50] have examined the effect of scanning path in magnetic resonance guided high intensity focused ultrasound (MRgHIFU) treatments. However, much work remains to be done in this area, including an examination of the path when other parameters, including the spacing between focal zone locations in the tumour, have been optimised. The results of previous research strongly suggest that, even in non-optimal scanning conditions, repeated use of ‘axial stacking’ of the focal zones (e.g. where an initial focal zone is placed in the centre of the tumour and subsequent focal zones are placed proximal/distal to this focal zone in a ‘stack’ along the axis of the transducer) can dramatically reduce treatment times.
Tissue absorptivity changes during treatment
Increases in the ultrasound absorptivity coefficient as a result of heating have been observed both experimentally Citation[55–57] and when matching simulation data to experimental results Citation[58]. Previous ex vivo studies have shown that both the magnitude and the rate of increase in the tissue ultrasound absorptivity coefficient due to heating depend on the temperature to which the tissue is heated Citation[59–63], the frequency of the ultrasound beam Citation[57], Citation[60], Citation[61], Citation[63], and both the rate of dose delivery and the maximum thermal dose delivered Citation[59]. However, little research has been performed on the quantitative effects of these changes on HIFU treatments, including whether the presence of absorptivity changes will increase or decrease treatment times, or alter the optimal path for a given treatment. The only guideline present for such choices is the general observation that if very large absorptivity changes are present during a treatment, that treating the proximal tumour locations first should be avoided since the subsequent ultrasound pulses will possibly not be able to then penetrate to the more distal tumour locations Citation[58]. However, though this conclusion is logical given its assumptions, the definition of ‘very large’ still remains to be quantified, as does the temporal speed with which absorptivity changes occur relative to a treatment's scan times. It has, however, been shown that tissue absorptivity changes more after it has been heated and then cooled versus when it is kept in a heated state Citation[62]. That study showed the absorptivity increasing up to, and plateauing at, values of up to twice the unheated values.
Optimisation technique
Several previous studies have examined the problem of optimising HIFU treatments using a variety of techniques and objectives Citation[13], Citation[25], Citation[26], Citation[64–68]. The objectives used for HIFU optimisation generally have the primary goal of either shaping the thermal dose delivered to the tumour and the surrounding tissue Citation[13], Citation[26], Citation[64–68] or of reducing treatment time through parameter optimisation while treating a fixed volume to a desired dose Citation[25], Citation[26]. The optimisation techniques previously used include an adjoint approach (in one dimension) Citation[64], a series of simulated treatments that step through the parameter space at fixed values Citation[65], use of a cost function-based algorithm to minimise tumour overdosing Citation[13], Citation[67], an analytical solution for the thermal dose distribution in a single pulse treatment Citation[66], and a sequential optimisation technique that optimises treatment times at each location without considering future dose Citation[25] beyond the current pulse's effects. This research expands on previous research by comparing two techniques for optimising HIFU treatments to minimise treatment times: the sequential optimisation technique used in previous research Citation[25] and a method of ‘simultaneous, collective pulse optimisation’ that uses all available knowledge for a given treatment, including the a priori knowledge of the heating due to and thermal dose deposited by all pulses during a treatment, to optimise treatments. That latter technique is similar to that used in previous research Citation[13].
Methods
Treatment time and tissue constraints
The total time needed to administer a treatment (which served as the objective function for the optimisation routine) was calculated as the sum of its focal zone location pulse heating times plus (if necessary to prevent normal tissue damage) the related inter-pulse cooling times:where
is the total treatment time,
is the heating time at position n and
is the subsequent inter-pulse cooling time during which power is off before initiating the next heating pulse. In the current studies the normal tissue temperature constraint (see below) was never violated (except for a small subset of non-optimal runs), so inter-pulse cooling was never invoked, and thus the sum of the heating times always equalled the total treatment time. Additionally, a computational constraint that no individual heating pulse could be longer than 300 s was imposed on the solver for almost all treatments.
To ensure efficacy, a thermal dose constraint (which was a constraint function for the optimisation routine) ensured that all voxels in the tumour were treated to a minimum of CEM240 by the end of the treatment (a value commonly used in previous research Citation[69], Citation[70]), where CEM is the ‘cumulative equivalent minutes’ of dose at 43°C Citation[39]. This included the dose accumulated during the cool down period following the last heating pulse of each treatment. To ensure safety, a strict temperature limit (which was an additional constraint function for the optimisation routine) was imposed in two normal tissue constraint planes located 1 cm proximal and distal to the tumour. That requirement waswhere Max(
) is the maximum normal tissue temperature in the constraint planes. This temperature limit replicates the conservative temperature limit used in previous research Citation[23], Citation[25], Citation[64] that triggers normal tissue cooling before significant dose is deposited in the normal tissue due to thermal build up. The location of the CEM30 dose surface in the tissue was also monitored and is reported for the cases where it is of interest (previous research has shown this to be an approximate limit above which irreversible tissue damage occurs Citation[69], Citation[70]).
Additionally, several computational tolerance constraints were imposed on the optimisation routine for the purpose of ensuring convergence. A tolerance for the evaluation of the objective function and each of the individual heating times (generally <10−3 for both) was imposed on the solver for all simulations, and the simulations were terminated if this tolerance was met (i.e. the change of any value from one iteration to the next was less than the tolerance). Also, a maximum allowable number of 3000 iterations per optimisation was imposed on the solver, and the optimisation routine was halted if the maximum number of iterations was reached. In cases where the optimisation routine halted after the maximum number of steps without reaching a solution, a new starting guess for each treatment time (in the range (0, 300)) was input, and the solver was restarted until a feasible solution was found.
Pulse heating approaches
Two pulse heating approaches were used, concentrated and fractionated. There were NFZL focal zone locations along the scan path in any given treatment, where NFZL ranged from 1 to 25, depending on the tumour being treated. Each such location was heated NCYCLES times.
Concentrated heating used a single pulse to heat each focal zone position to the desired thermal dose, and the focal zone did not return to heat any position a second time, i.e. NCYCLES = 1 for this approach. The important independent variable for this approach is thus the pulse heating time at each location.
In fractionated heating, each focal zone position was heated with multiple shorter pulses as the focal zone cyclically scanned the NFZL focal zone locations multiple (NCYCLES > 1) times. Each fractionated pulse heating period was a fraction of the single pulse heating period, and delivered only a fraction of the desired thermal dose to that location. Important user-defined variables are the number of cycles used (NCYCLES) and the time taken to execute the individual pulses within each cycle. For the fractionated heating approach two methods of implementation were investigated. First, since there are a total of NFZL × NCYCLES individual heating periods for each treatment, each of those times could be optimised, herein called the fully optimised, fractionated heating approach. Second, a simpler, more clinically practical version of fractionated heating involves the same fixed heating/dwell time at each location (equivalent to a constant scanning speed), with the cyclic scanning continuing until the complete tumour volume reaches the desired thermal dose, a fractionated heating approach previously characterised as ‘volumetric’ scanning Citation[45], Citation[49]. For high velocity volumetric scanning there is not enough time for the tissue to cool between cycles, and thus the high velocity fractionated heating approach becomes the equivalent of a single, large effective focal zone with the transducer's focal zone power diluted over the complete scanning volume. Conversely, as the scanning speed decreases the heating/dwell time at each position increases, the number of cycles needed to treat each position decreases, and the fractionated approach becomes closer and closer to the concentrated approach, with NCYCLES = 1 converging to the concentrated heating approach.
Optimisation techniques 
For a given treatment with all other treatment parameters fixed (e.g. scan path, number of focal zone locations heated) the optimal values for the individual heating times at each focal zone location were found using a gradient search routine. Two different optimisation techniques were used to determine those times: simultaneous, collective pulse optimisation, and sequential, single pulse optimisation.
Simultaneous, collective pulse optimisation was the primary technique used. It represents a ‘best case’ limit since it assumed, and utilised, complete, a priori knowledge of all of the thermal interactions among all heating pulses at all locations at all times during a treatment to find the set of pulse heating times that minimised the total treatment time. This technique minimised all heating times by reducing overdosing in the tumour by accounting for the SAR overlap and thermal conduction interactions among all pulses in a treatment. It worked by selecting initial guesses for the heating and cooling times at every focal zone heating location during the treatment, and then finding the optimal values of those times that minimised the total treatment time. This involved simultaneously optimising NFZL heating times for the concentrated heating approach and NFZL × NCYCLES heating times for the fully optimised, fractionated heating approach. The fmincon algorithm was run from several random guesses (i.e. in a Monte Carlo fashion) for starting times at each focal zone location to ensure convergence to a global minimum. Either the interior-point method or sequential quadratic programming method was used to generate iteratively updated times Citation[71].
Since the simultaneous optimisation technique requires full a priori knowledge of the thermal effects of all treatment pulses, and such knowledge would be very difficult to realise clinically, it was important to determine whether its resulting treatment time gains would be worth the effort of trying to obtain that knowledge when compared to a more clinically practical technique. Thus, when concentrated heating was used, the collective pulse optimisation technique's treatment time predictions were compared to those of sequential, single pulse optimisation, which required very little a priori knowledge. That technique optimised each successive pulse's heating time individually, on a pulse-by-pulse basis. It used knowledge of the thermal history at each location arising from prior heatings, i.e. knowledge of the prior temperatures and thermal doses in the volume to be heated. The only future effect it anticipated was the dose that would be delivered during that pulse's subsequent (post-heating) cooling period. Thus, in contrast to the simultaneous optimisation technique, the sequential optimisation technique did not anticipate/compensate for any dose delivered by future heating pulses.
Thermal simulations
All thermal simulations were done using a finite difference approximation to the spatial derivatives in the bio-heat equation Citation[72]:that converts the Pennes equation into a set of simultaneous ordinary differential equations that were solved using the Matlab™ function ODE45, which uses a Runge Kutta approach. Here, T is the temperature (°C) at the point being analysed, ρ is the tissue density (kg/m3), C is the specific heat of tissue (J/kg/°C), k is the thermal conductivity of the tissue (W/m-°C), Qap is the applied power density deposited by the external applicator (W/m3), W is the Pennes perfusion parameter (kg/m3/s), Cb is the specific heat of blood and Tb is the arterial blood temperature. All simulations used a 1-mm isotropic spatial grid and a time step of 0.1 s or less. Thermal dose values were calculated using the approach of Sapareto and Dewey Citation[39] using a 1-s time step.
Power deposition
The power deposition distribution was determined as in previous research Citation[23], Citation[25] using the hybrid angular spectrum (HAS) method Citation[73] to simulate a 256-element phased array (Imasonic, Paris, France) with a diameter of 14.5 cm, a geometric focus of 13 cm, and a central frequency of 1 MHz. Resolution of the ultrasound field was 1 mm isotropic. The transducer (which was used in the associated phantom experiments (see below) was simulated to produce the smallest possible focal zone consistent with the diffraction limit, yielding a full width at half maximum (FWHM) focal zone specific absorption rate (SAR) volume of 1 × 1 × 12 mm3. The total power from the transducer was set at a fixed value for all treatments (with a few exceptions as noted). The maximum power density used occurred when the focal zone was centred at the back face of the larger (1 × 1 × 30 mm3) axial tumour, and was 0.6 × 107 W/m3. The associated fixed transducer power magnitude was used in all studies since it has been shown in previous studies Citation[23], Citation[25] that a ‘knee’ occurs near this power density, below which the treatment times become significantly longer, and above which only minimal time gains are possible. This peak power density was similar to that used in previous work Citation[23], Citation[25] and well below limits set by the US Food and Drug Administration (FDA) Citation[74]. The beam was steered electronically along the z-axis (which has been reported to increase near-field heating Citation[75]) for all simulations, and mechanically (simulating the use of piezoelectric motors) for the transverse scans except for the 25-position simulations matched to phantom data, which used electronic steering.
Simulation region
Treatments were simulated in a 7 × 7 × 7 cm3 treatment region, with the exception of the phantom experiment simulations which used a 10 × 10 × 10 cm3 region. The geometric focus of the transducer and the centre of the simulated tumours coincided at 13 cm from the transducer face for all simulations. The simulation geometry is shown in along with a magnification of the ‘axial tumours’ used for the axial focal zone spacing and packing density optimisation studies. The acoustic and thermal properties of the 7 × 7 × 7 mm3 region are listed in . The 10 × 10 × 10 mm3 phantom matching studies had an iteratively calculated conduction coefficient of 0.4 (W/m/°C), zero perfusion, and a transducer power that was a factor of three larger than in the other simulations (to match the experiments). All other property values were identical to those in .
Figure 1. Model used for axial focal zone spacing and packing optimisation studies. The simulation region (left) and a close-up (right) of an “axial tumour” are shown. The black dot at the centre of the tumour (right) marks the location of both the centre of the tumour and the geometric focus of the transducer. Both axial tumours (1.6 and 3.0 cm long) had at least 2.0 cm (slightly more for the smaller tumour) of normal tissue between the skin/water interface and the proximal tumour face to allow for a significant space in which normal tissue heating could occur.
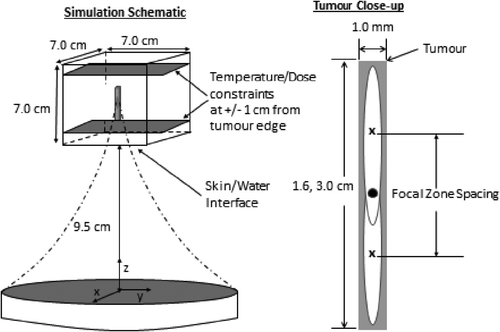
Table I. Tissue and acoustic properties held constant in all simulations except for the changing absorptivity study.
Tumours and scan paths studied
Three types of generic tumours that increased in complexity/extent were studied, each with associated scan paths chosen to study particular optimisation problems: tumours treated by an axial stack of focal zone locations; pairs of adjacent axial stacks to investigate transverse thermal effects, and 25-position transverse scans for which phantom experiments had been performed. All three tumour types were used to compare the two pulse heating approaches and the two optimisation techniques. The need to proceed sequentially in complexity/extent was motivated by the large scale of the problem being solved as noted in the introduction. That is, with over 300 000 finite difference points (1 000 000 in the case of the 10-cm3 region) being modelled over long time periods with small time steps, and with each possible treatment scenario being simulated many times over in the iterative optimisation process, the computational times involved were very large, with over 250 000 h of parallelised computer time being used for the simulations in this study.
Axial tumours
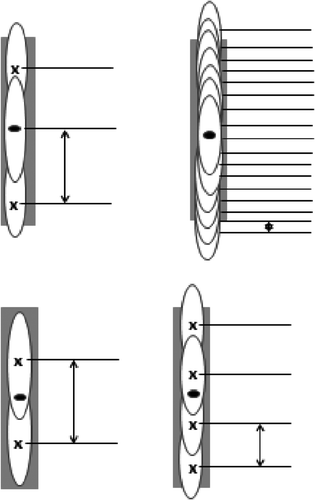
Previous research Citation[25] has shown that a series of axially stacked focal zones that are sequentially applied to a 3D tumour yields the fastest treatments, and that while there were some additional gains attained by optimising stack adjacency, each axial stack was relatively independent of other stacks for most cases. Thus, studying single stacks was a good place to start since it provided useful insights into the main characteristics of the basic physics of the scanning process. Those insights were then useful when studying more complicated problems. Two simple, axial tumours were studied, a 1 × 1 × 16 mm3 (smaller axial tumour) and a 1 × 1 × 30 mm3 (larger axial tumour). These tumours were used to investigate the effects of optimal spacing, axial focal zone packing (in which the number of focal zone locations used to treat a tumour ranged from 1–17) and scan path order (see ). The axial tumours were also used to study the effects of increased tissue absorptivity following heating.
Figure 2. Schematic of treatments on the axial tumour using two, three, four and 17 positions. The black dot at the centre shows the tumour centre and the location of the geometric focus of the transducer. The arrows show the distances used to define the focal zone spacing for every treatment. All focal zones were equally spaced within any given treatment.
Adjacent focal zone stacks
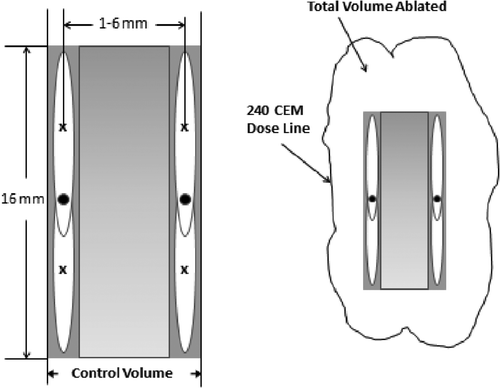
To expand to cases with more focal zones and spatial dimensions, larger tumour regions heated by two adjacent stacks were used to optimise the transverse spacing for both pulse heating approaches. Two adjacent axial stacks, each with two axial focal zone positions, were modelled (see ). The axial spacing between focal zones in each stack was fixed at the optimal value found in the axial tumour study. The transverse distance between the axial stacks’ centre lines was varied from 1 to 6 mm in 1-mm steps.
Figure 3. Schematic of the four-position transverse spacing study using two axial stacks of two positions each. The black dot at the centre of each axial stack of focal zones coincides with the geometric centre of the transducer. The axial spacing between focal zones in each stack was fixed at the optimal value found for the two-position treatment of a single axial stack. The shaded area between the stacks shows the inter-axial tumour volume (left) where the dose is guaranteed to reach the target dose (CEM240) by the end of the treatment. This ‘control volume’ is smaller than the ‘total volume ablated’ by the treatment (right) because thermal dose is also deposited exterior to the inter-axial tumour space.
Planar scanning of a larger tumour
Finally, simulation studies were performed that replicated the experimental conditions used to heat a phantom with 25 focal zone positions in a plane perpendicular to the transducer's axis Citation[76]. Replication allowed the power levels used in the simulations to be made equivalent to those used experimentally, the thermal conductivity of the phantom to be estimated, and the simulation process to be validated Citation[76]. Experimentally, the focal zone was electronically scanned with the 25 focal zone positions arranged in either concentric circles or a square raster pattern at a constant depth in the tissue. The simulated treatment was then iteratively matched to the experimental data by comparing the 240 and CEM30 dose lines of the two treatments through a least-squares fit in three orthogonal planes containing the focal zone's peak power density. The circular scans had a central point plus two concentric circles with radii of 2.25 and 4.5 mm, and 8 and 16 focal zone locations, respectively. The focal points were evenly spaced on the circles, which were scanned in an ‘in-to-out’ pattern with the central point heated first. The square tumour's dimensions were 10 × 10 mm with a 2-mm transverse separation of focal zones, and it was scanned in a 5 × 5 position raster scan pattern starting in one corner. The experimental and simulated concentrated heating approach used fixed heating pulse times of 15 s at each of the 25 locations yielding a heating time of 375 s. In the fractionated heating approach, each point was repeatedly heated 150 times for 0.1 s during each heating, yielding the same total heating time as the concentrated approach. Having equivalent total heating times and applied power levels for both the fractionated and concentrated scans allowed a comparison of their ablation rates by determining which technique first reached the desired dose everywhere in the matched treatment planes. In these (non-optimised) experiments the concentrated heating approach had higher ablation rates than fractionated heating for both the circular and square patterns. This was also true in the associated, matched treatment simulations, which gave results that were very close to those of the experiments Citation[76]. Having performed this initial verification test for the simulations, further simulation studies were performed with the validated model to investigate additional treatment scenarios.
Model of absorptivity changes
To examine the effects of absorptivity changes, a ‘worst-case’ scenario approach was studied for the three-position axial tumour. The smaller axial tumour was divided into three equal sub-volumes, each to be treated by a focal zone position centred on that sub-volume. Absorptivity was either doubled or quadrupled from its initial value at every position in each sub-volume immediately after it had been treated, and before the next position in the treatment path was heated. The same absorptivity increase was made at all points in the sub-volume, regardless of the dose they had received (all of which reached at least CEM240 by the end of the treatment). This model of absorptivity change is much faster (i.e. instantaneous) and much larger than measured in ex vivo dog tissue heated in a water bath Citation[59], which reached a maximum absorptivity of only twice the baseline value. These studies were performed using the concentrated heating approach and the collective optimisation technique.
Results
Results are presented in order of evolving complexity/extent starting with the axial tumour model and ending with the 25-position phantom treatment simulations. For all cases the desired minimum thermal dose, or a higher dose, was delivered to all tumour locations. Results are presented in terms of the total heating times since for all cases of interest the total cooling time was zero. All results are for a constant perfusion of W = 0.5 (kg/m3/s) and a middle/front/back scan path, which is the axial focal zone sequence previously shown to give the shortest heating times Citation[25], unless otherwise indicated.
Axial spacing and packing optimisation
Optimal spacing and packing studies were performed for heating tumours with one, two, three, four or 17 axial focal zone locations. These studies were also used to investigate the effects of heating approach (concentrated and fractionated), optimisation technique (collective and sequential) and focal zone size.
One focal zone position
The simplest case used one focal zone position to heat the small axial tumour, with a search done in 1-mm steps to find the optimal offset of the focal zone position from the centre of the tumour. The offset that produced the shortest treatment times was with the focal zone centred at the tumour centre (zero offset) or slightly distal to it, with the difference in heating times between the two locations being less than 5 s. The resulting treatment time for the central location is shown in (where this single pulse case is equivalent to zero separation of two focal zones). As expected, using only one position of this small focal zone to heat this tumour resulted in long treatments (the focal zone's FWHM axial length is 12 mm, which only covered three quarters of the tumour's length).
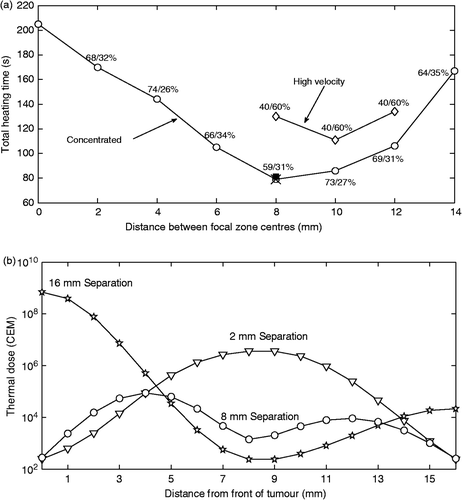
Figure 4. (a) Total heating time versus axial separation of the centres of two focal zones (NFZL = 2) heating the smaller axial tumour using a front–back scan path with the focal zones located symmetrically around the tumour centre. Zero separation indicates complete overlap of the two focal zones (i.e. it is equivalent to using only one focal zone). Results are shown using collective optimisation for both the concentrated heating approach (circles) and the high velocity fractionated heating approach (diamonds). The percentage of the optimised times spent in the front/back locations are shown for all separations. Also shown (at 8 mm) are the total heating times (×) for a fully optimised, fractionated scan for NCYCLES = 2 in which four times were optimised, and for a concentrated scan using sequential optimisation (solid square) whose optimised front/back heating times were similar to the fully optimised times. (b) Thermal dose versus distance from the front of the tumour for the treatments of the small axial tumour using 2 mm (diamonds), 8 mm (squares), and 16 mm (stars) separations with the concentrated heating approach and collective optimisation.
Two focal zone positions
shows the optimised treatment times for treating the smaller axial tumour versus the axial separation of the two focal zones for the collectively optimised, concentrated scanning approach (circles) and the fractionated heating approach (diamonds), for which high velocity scanning was simulated. The percentage of the optimised time spent in the front/back positions is shown for the concentrated case, and the corresponding duty cycle percentages are shown for the high velocity, fractionated scanning approach. For the high velocity scanning approach, optimising the duty cycle is equivalent to optimising a large, effective focal zone's shape. These results show firstly that when both heating approaches were optimised for both focal zone spacing and for front/back heating times, the concentrated heating approach gave shorter treatment times than the high velocity, fractionated heating approach.
Secondly, also shown in (by the ×) is the total treatment time for the fully optimised, fractionated heating approach case with two cycles treating the two positions (NFZL × NCYCLES = 4) at the 8-mm spacing. This result shows that when complete freedom was given to the four individual heating times of the fractionated approach, that approach reduced to the concentrated heating approach – that is, its four times reduced to twice identical to the two (front/back) optimal concentrated heating approach times, plus two zero heating times.
Thirdly, for the concentrated heating approach the optimal spacing corresponds to both an overlap of the two focal zones’ FWHM lengths of 2 mm for each focal zone (17% of the axial FWHM), which prevents a thermal dose ‘hole’ in the centre of the tumour, and an extension of the FWHM SAR to 2 mm beyond the tumour's front and back boundaries. This distribution heats the ends of the tumour, which generally require more energy deposition Citation[25], without spacing the focal zones too far apart. Also as expected, the optimal spacing smoothed out the thermal dose within the tumour. shows the dose distribution in the tumour for the 2 mm, 8 mm and 16 mm spacings.
Fourthly, the results show that the time gains obtained by the more practical, sequential optimisation technique applied to the concentrated heating approach (square symbol) were quite similar to those from the difficult to implement, complete knowledge, collective optimisation strategy that assumed full a priori knowledge of all treatment information.
Finally, studies with the perfusion value increased to 5 kg/m3/s were also performed using both the concentrated and high velocity fractionated approaches at all the focal zone spacings shown in . The results of those studies show that while, as expected, the treatment times were increased versus a lower perfusion, the value of the optimal focal zone spacing and the general ‘U’ shape of the graph were unchanged at the higher perfusion values.
Three focal zone positions
shows the treatment time versus the distance of the proximal and distal focal zone centres from the tumour centre (where the middle focal zone is centred) for the smaller axial tumour. Results are shown for the concentrated heating approach using the collective optimisation technique (open circles) at all spacings, and for the sequential optimisation technique (closed squares) at selected spacings. Also shown at the optimal spacing (6 mm) are 1) the optimised treatment time for the fully optimised, fractionated heating approach for two cycles, for which the six optimised pulse heating times were found to be the same three times as for the collectively optimised concentrated heating approach plus three zero pulse heating times (×), so that the fully optimised fractionated approach became equivalent to the concentrated heating approach, and 2) the optimal heating time for the collectively optimised, high velocity fractionated scan (diamond).
Figure 5. Treatment time versus distance of the proximal and distal focal zones from the tumour centre in a three-position, middle/front/back treatment of the smaller axial tumour for the concentrated heating approach using the collective (open circles) and sequential (solid squares) optimisation techniques. The percentage of time spent in the middle/front/back focal zone locations is shown for the collective optimisation technique. Also shown at the optimal spacing are the result for the fully optimised fractionated heating approach (×) with two cycles (NCYCLES = 2, for which six times were optimised) and for the high velocity, collectively optimised fractionated scan (diamond), i.e. the single, larger effective focal zone case.
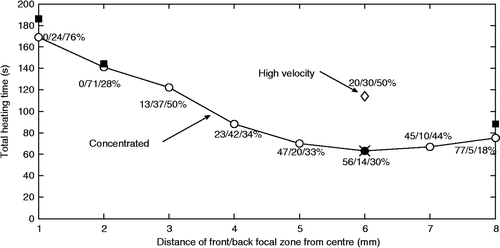
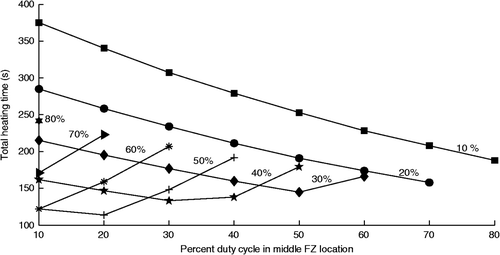
To further investigate the details of the three-position high velocity fractionated heating approach with collective optimisation, the treatment times for all possible duty cycles (in steps of 10%) using three positions were studied with the optimal 6-mm spacing. The results () show that optimising the duty cycles (which, at high scan velocities is equivalent to optimally shaping a fixed, effective focal zone) gives a minimum heating time of 114 s for the optimal distribution (20/30/50% for the middle/front/back focal zones respectively). This time can be compared to the more clinically practical fractionated approach of a fixed heating time at each focal zone location (i.e. a uniform duty cycle of 33/33/33%) which has a total treatment heating time of 168 s, which shows that spatially optimising (shaping) a focal zone of fixed size can yield significant treatment time gains. However, the total heating times for both of those fractionated approaches are still much longer that the concentrated heating approaches’ times for both collective optimisation (63 s, ) and sequential optimisation (64 s, ).
Figure 6. Treatment time versus middle position duty cycle for a high velocity, three-position treatment of the small axial tumour using the optimal spacing of . The duty cycle percentage spent at the back focal zone position is shown above each line, while the front position percentages can be obtained by subtraction.
Focal zone packing
Since the above results showed that increasing the number of focal zones used to heat a given tumour yielded faster treatments, additional optimisation studies were performed with more positions and for treatments of the larger axial tumour, resulting in studies of one, two, three and 17, and three, four and 17 positions for the smaller and larger axial tumours, respectively. The resulting treatment times are shown in for collective optimisation of both the concentrated heating approach and the high velocity fractionated heating approach. The focal zones’ spacings and duty cycles for these treatments were optimised as described below. Due to the fact that the concentrated treatments had shorter treatment times than the high velocity treatments for every treatment configuration examined so far, concentrated treatments were examined in more depth, while still continuing to study a smaller set of fractionated treatments.
Figure 7. Treatment time versus the number of focal zone locations for concentrated heating using the collective optimisation technique (circles) and for the high velocity, fractionated approach that creates a single, large effective focal zone (diamonds). Results are shown for both the smaller and larger axial tumours. Results are also shown for the 17-position case for the concentrated treatment of the smaller axial tumour using sequential optimisation (filled square).
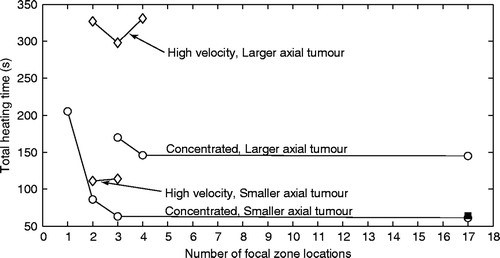
Concentrated heating approach: The treatment times shown in for the two- and three-position treatments of the smaller axial tumour using full optimisation and concentrated scanning are taken from and respectively. These points represent the fastest treatment times found in each of those figures. The treatment time in for the four-position treatment of the smaller axial tumour was obtained as part of a larger study. In that study a complete range of uniform spacings between focal zones was simulated. Those spacings () began at 1 mm and increased to a limit where the most proximal/distal focal zones’ centres were touching the edges of the tumour. The four heating times were optimised at each such spacing. The fastest treatment time for the optimal spacing is shown in . That optimal spacing was 7 mm, resulting in focal zones with centres at 4 mm, 11 mm, 18 mm, and 25 mm from the front face of the tumour.
To study an even denser focal zone packing, treatments with 17 locations were studied with those locations uniformly spaced 1 mm and 2 mm apart for the smaller and larger tumours, respectively. The heating times were optimised at all 17 positions for both tumours using the collective optimisation technique. The results in are shown for the middle/front/back path, which was the fastest path. (Also studied with the collective optimisation approach were a front/middle/back path and a back/middle/front path for the small axial tumour, both of which had treatment times that were a few seconds longer than the middle/front/back path.) The small tumour was also treated with the concentrated heating approach using sequential optimisation and the resulting minimised treatment time was essentially the same as for the collective optimisation technique.
High velocity fractionated heating approach: The data points in for the two- and three-position treatments of the smaller axial tumour are the fastest treatment times for high velocity scans from and respectively. Studies were also performed for the larger axial tumour using high velocity scanning for two, three and four positions. The duty cycles that minimised the treatment times were iteratively optimised for each of those three treatments. In that optimisation a search was conducted that minimised the treatment time for a given duty cycle, and then a new duty cycle was generated via a heuristic algorithm that moved heating time away from overdosed focal zone locations to focal zone locations with minimal dosing. Also, treatments of the larger tumour were performed for three, four and 17 focal zones with a more clinically practical uniform duty cycle at all positions (with a two-position treatment not attempted because of insufficient coverage of the tumour, which would lead to long heating times). In the three-, four-, and 17-position uniform duty cycle cases the treatment times were also quite long, being 525, 602, and 625 s respectively. The treatment times for the uniform duty cycle treatments increase monotonically with the number of positions because the increased number of positions further dilutes the power from the treatments with fewer positions. However, a point is eventually reached where the power cannot be diluted further because it is distributed as uniformly as possible (given the dimensions and shape of the focal zone) throughout the treatment region, which is why the treatment times are seen to plateau between four and 17 positions.
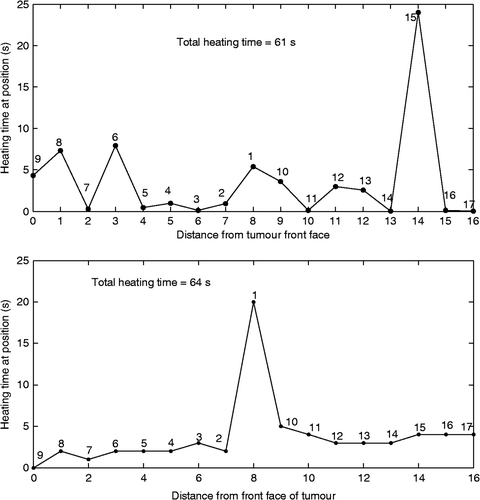
To illustrate how the collectively optimised pulse heating times vary with position for concentrated heating, shows the heating times as a function of distance from the proximal tumour face using 17 positions to heat the smaller axial tumour. shows a similar graph for the sequentially optimised concentrated heating approach case. The general shapes of these curves were the same for the larger tumour.
Figure 8. Heating time at each position versus distance from the front tumour face for the 17-position treatment of the smaller axial tumour using a middle/front–/back treatment path and the concentrated heating approach. Results are shown for both the collectively optimised (top) and sequentially optimised (bottom) techniques. The number by each data point indicates the relative order in which that focal zone location was heated.
Optimal transverse spacing
To investigate a scan path with both axial and transverse components, a study of two adjacent axial stacks was performed, with each stack having two axial heating locations (). Both stacks used the optimal axial spacing for the high velocity fractionated treatments from the two-position study (10 mm, ) – a spacing that slightly favoured the fractionated heating approach. Results are shown in for the ablation rate of the internal tumour volume (the two axial stacks’ voxels plus all voxels between the two stacks) versus transverse spacing for both the collectively optimised concentrated heating approach and the high velocity, fractionated approach at a uniform, 25% duty cycle. The four positions of the optimal heating times of the concentrated approach are shown as the percentage of the total treatment time at each spacing. The 23 other possible paths for the four-position treatments were also investigated for the concentrated heating approach with collective optimisation, and the total treatment times were found to be relatively insensitive to permutations in scan path order.
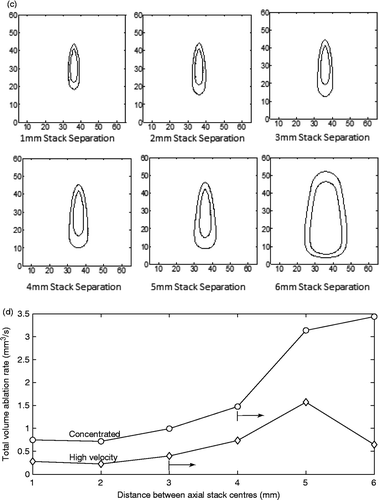
Figure 9. (a) Ablation rate in the control volume (the voxels in the control volume as defined in ) versus centreline separation for two side-by-side, two-position axial stacks for the collectively optimised, concentrated heating approach (open circles) and the optimised high velocity fractionated heating approach with a uniform duty cycle of 25% at each position (diamonds). The percentage of time spent at each of the four positions (front/back for stack 1 then front/back for stack 2) is shown for the concentrated heating approach. The axial spacing between focal zones in each axial stack was 10 mm. The cross (× at 3 mm) shows the result for the fully optimised, fractionated case with two cycles (and hence eight pulse heating times) which reduced to the concentrated heating times plus four zero heating times. Also shown is the result for the sequential optimisation of the concentrated heating approach at the optimal spacing (filled square). The vertical lines indicate the spacing at which each treatment approach first violated the normal tissue constraints, with the arrow indicating that all larger spacings also violated the safety constraint. (b and c) Dose maps of treatments using two adjacent axial stacks (of two positions each) for spacings between the centres of the two stacks of 1–6 mm for the collectively optimised, concentrated focal zones (b) and the high velocity fractionated heating (c). The interior and exterior dose lines for each map are the 240 and 30 CEM lines respectively. The slice contains the two stacks’ axes. Dimensions are in mm. (d) The ablation rate for the total volume (control volume + exterior voxels) treated using two axial stacks with four positions each with all parameters identical to those listed in for treatments using concentrated heating (open circles) and high velocity fractionated (closed circles) heating. The spacings where the normal tissue and dose constraint are first violated are indicated by arrows.
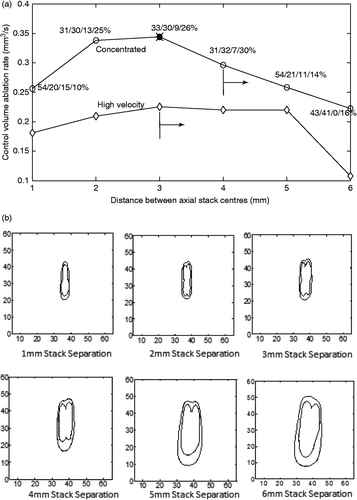
At the optimal transverse spacing (3 mm) of the concentrated approach, this graph also shows the treatment time needed for the fully optimised, fractionated heating approach for two cycles, which requires optimising eight pulse heating times. Again, as in the two- and three-position axial studies, the fully optimised fractionated case resulted in optimal pulse heating times that were the same as the times of the collectively optimised concentrated approach (i.e. Ncycles = 1 with the four concentrated approach heating times plus four zero heating times). Finally, at that optimal 3-mm spacing the total treatment time is also shown for the sequentially optimised concentrated approach, with the times spent at each focal zone location noted (as a percentage of the total treatment time).
show the final dose contours in the plane containing the two stacks’ axes for the collectively optimised, concentrated () and high velocity, fractionated () cases of . For the fastest collectively optimised, concentrated scan spacing (at 3 mm) the CEM30 contour line remains within the required 10 mm proximal and distal normal tissue constraint boundaries, so that heating approach does not violate the normal tissue constraint either for the lenient normal tissue constraint of a CEM30 dose or for the much stricter 6°C temperature constraint. However, for larger spacings, not only do those scans have lower ablation rates, but they also violate both the dose and temperature constraints. By comparison, the collectively optimised high velocity fractionated case violates the CEM30 dose and 6°C temperature constraints even for the 3-mm spacing. Activating constraints would, clinically, require additional cooling times, thus further lengthening treatments and lowering the ablation rate.
Finally, shows the results for the same scans as , but the ablation rate now includes the tissue volume exterior to the axial stacks which was treated above CEM240. Since no fixed volume was being heated to CEM240, in order to fairly compare different cases it was necessary for comparison to have a different standard than treatment time. To do so, the results in are given in terms of the ablation rate present at the time at which the control volume was treated to CEM240 by the concentrated heating approach in . This allows fair comparisons of the two approaches by using the same heating time for both approaches, and then comparing ablation rates at that time. Also shown are the spacings at which each of the approaches first violates the normal tissue constraint for temperature and dose. This violation continued for all treatments to the right of these lines.
Twenty-five-position transverse scans
To further investigate the effects of heating approach and optimisation technique, several cases were run using the 25-position model which was calibrated against the phantom experiments Citation[76]. Three concentrated and three fractionated approach treatments were evaluated for the square tumour.
In the first simulation study of the concentrated approach the heating time was the same for all focal zone locations (as in the experiments), but now that uniform heating time was systematically lowered (until a minimal focal zone heating time was found that treated the complete tumour (9.4 s per location, 236 s total time)). A second concentrated approach simulation used the collective, simultaneous optimisation technique, yielding a set of 25 optimised focal zone heating times, giving an average heating time per location of 8.5 s (213 s total time). A third concentrated approach treatment used the sequential optimisation technique. It had a total treatment time of 219 s.
Three fractionated heating approach cases were simulated that varied the scanning speed (and the related number of traverses through the tumour, NCYCLES) using the uniform heating time approach. The heating time per position was set to either a very rapid switching time between scanning path trajectory points (i.e. fast fractionated heating which created a large, effective focal zone), or 0.1 s, or 1.0 s per position. The treatment time for the large, effective focal zone case was optimised by finding the smallest heating time that treated every voxel of the tumour to at least CEM240. For the treatments using a 0.1 or 1.0 s heating time per position, the number of passes through the trajectory (NCYCLES) was set an initially high value and was sequentially reduced until a minimum number was found that would still treat the entire tumour to CEM240 (114 and 11 cycles, respectively) with no partial passes through the scanning path trajectory.
Absorptivity property changes
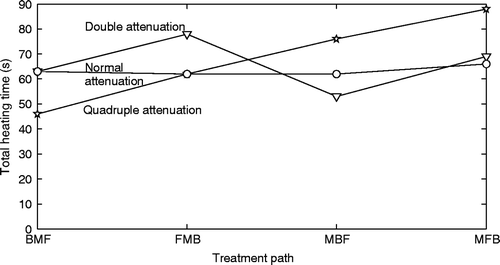
To investigate the effects of increases in tissue absorptivity due to heating of tissue, a study was performed using the small axial tumour and a three-position treatment with the collectively optimised, concentrated heating approach. The optimisation technique took into account the future changes in attenuation due to heating. The absorptivity was either doubled or quadrupled (from an initial value of 5 Np/m at 1 MHz) immediately after each position (and its sub-volume) was heated. shows the resulting treatment times for the four axial treatment paths investigated. All treatments were run from at least 12 randomly selected starting points to check for the presence of local minima. In each case, local minima were found and the lowest treatment time found is shown. A 6°C temperature rise constraint was used in the normal tissue, but was not activated by any of the treatments.
Figure 10. Heating time versus scanning approach for six different treatments on a square treatment region with transducer and tissue parameters matched to data from an agar phantom. The diamonds show the results of the fractionated heating approach with different fixed heating times per pulse. The circles show the concentrated approach results for a fixed (non-optimised) heating time per pulse and a collectively optimised scan, while the solid square shows the sequential optimisation result.
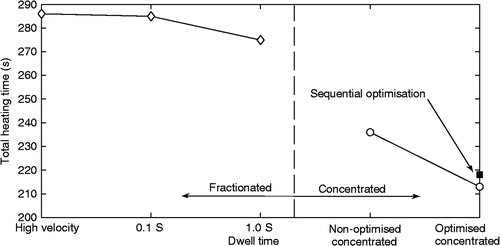
Figure 11. Collectively optimised concentrated heating approach heating times versus treatment path sequence for the smaller axial tumour using three positions with normal absorptivity (circles) immediately doubled absorptivity (triangles) and immediately quadrupled absorptivity (stars). Spacing between focal zones is the optimal value found in the three-position axial spacing study. The abbreviations F, M, and B are used for the front, middle, and back treatment focal zone locations, respectively, and the order in which the letters appear is the order in which the focal zones were heated.
Discussion
Four important conclusions can be drawn from these results. First, all focal zone heating approaches can be considered as lying along a spectrum with the number of cyclic heating passes (NCYCLES) through the tumours’ NFZL heating positions as an independent variable. At one end of the spectrum is the optimised concentrated heating approach (NCYCLES = 1) which always gives the shortest treatment times, while at the other end is the optimised high velocity fractionated scanning (with Ncycle >> 1) which gives the longest treatment times. As the scanning speed of the fractionated heating approach increases (i.e. the dwell time per position decreases and the number of cycles increases), the high velocity fractionated heating approach becomes the equivalent of a single large effective focal zone and it has the longest treatment times. Second, for a given number of focal zone locations used to treat a tumour, both heating approaches have optimal axial and transverse spacings that, when used, can significantly reduce treatment times. For the concentrated heating approach the treatment time decreases monotonically with the number of heating positions, but a point of diminishing returns is reached at a relatively low focal zone packing density. Third, the sequential, pulse by pulse optimisation technique gives almost the same treatment time gains as the much more difficult to implement complete knowledge, simultaneous pulse optimisation technique. Finally, large, rapid changes in tissue absorptivity (i.e. immediately increasing by a factor of four) can affect the optimal choice of scan path, but whether such large, rapid changes will actually occur during treatments remains to be seen.
First, all cases studied showed that using concentrated heating pulses reduced treatment times when compared to the fractionated (‘volumetric scanning’) heating approach. This is illustrated by two consistent trends. First, the collectively optimised concentrated heating approach always had the shorter heating times, and second, the fractionated approach always reduced to the concentrated approach when it was ‘fully optimised’, i.e. when each of its Ncycle × NFZL heating pulses could be independently optimised. The fractionated approach has longer heating times since after it heats one position it then moves away, allowing that position to cool, thus requiring later reheating (and more time). Trying to rapidly switch the focal zone between several points to create a large, effective focal zone to avoid such cooling makes treatments even slower because the single focal zone's concentrated power is then diluted over a wider volume, causing the temperatures to rise more slowly everywhere. This approach thus both takes longer to reach high temperatures where the non-linear thermal dose effects occur, and allows more time for thermal losses by conduction and blood flow. Because long treatment times are likely to be a major barrier to practical clinical implementation of HIFU treatments, the results of this study suggest, from the treatment time viewpoint, that implementing concentrated, rather than fractionated, heating would be advantageous. That is, while other considerations might make volumetric scanning clinically attractive, reducing treatment time is clearly not one of them. This conclusion is reinforced by the current results which show that the fractionated heating approach is also associated with more normal tissue heating than is present in a comparable concentrated heating approach. Thus, in larger, more complicated tumour treatments the fractionated approach would likely also require even more tissue cooling than the concentrated approach, resulting in even larger differences in total treatment time between the two heating approaches.
Also, although they are always slower than the comparable concentrated scans, the treatment times for high velocity fractionated scans are decreased greatly when the duty cycle and focal zone spacing are optimised – when compared to the more typical, and practical, approach of using a uniform duty cycle and a conservative spacing. The optimised shapes of the effective focal zones created in this study by high velocity fractionated scans roughly mimic the ‘volcano’ shape found to be optimal in previous research Citation[51] with a simple effective focal zone power deposition model.
Second, when using the concentrated heating approach it is clear that increasing the number of focal zone locations used to treat a given tumour monotonically decreases the heating time needed. The mechanism for this treatment time reduction is that heating more locations along a given focal zone trajectory makes it easier to both avoid low dose, ‘untreated holes’ in the tumour, and also to reduce the amount of overdosing at all locations. However, the treatment time gains so obtained seem to reach a point of diminishing returns at quite low focal zone packing densities. In the limit (NFZL = ∞), this method can be considered as a continuously scanning method with an optimised velocity distribution. However, the results from the 17-position study indicate that extrapolating out to large numbers of positions by such scanning will likely yield only minimal additional time gains compared to simpler treatments with a smaller number of focal zone locations, e.g. NFZL = 4 in the current simple generic tumour model heated by this transducer. Finally, the concentrated approach may be optimised even further by reducing the current requirement that each FZ heating location be associated with dosing the focal zone's complete FWHM volume. That is, requiring it to dose a smaller volume (with one MR voxel centred in the FZ being the limit) would give more ability to optimally shape the dose contours by utilising controlled heating at even more FZ positions. Clearly such an approach would decrease heating times, but by how much remains unclear, especially given the small gains when going from four to 17 FZ positions in the current study.
Third, the results show that treatments at optimal spacings reduce treatment time, with larger reductions coming from the use of optimal spacings in the axial direction than in the transverse direction. This greater reduction comes as a result of the axial overlap of the sequential focal zone's SAR patterns, and thus the greater level of preheating of the current focal zone due to the previous focal zones’ heating versus heating focal zones in a transverse raster fashion within a single axial plane. This greater level of interaction between the heating of pulses in an axial stack means not only that the dose is deposited at an accelerated rate when points are heated along an axial stack, but that there is more of an opportunity to shape the final profile of the dose deposited in the tumour along this axial stack than there is in transverse scanning paths, where less interaction occurs.
Given the results presented here and in previous studies Citation[23], Citation[25], it seems quite clear that the shortest treatment times will occur when using axially stacked, collectively optimised, concentrated heating of tumours with a large number of small focal zones at optimal spacings and with high powers. An important treatment time question then is whether such an approach can be implemented in a clinically practical manner. There are several results in the current paper that indicate that such practically can be achieved. First, there appear to be only marginal time gains from using the (very difficult, if not impossible to achieve) full knowledge, collective optimisation technique when compared to sequential, single pulse optimisation. This seems to be a result of the fact that such knowledge would only be of major value when there is a significant amount of thermal interaction among a large number of pulses. However, for the optimal scan paths Citation[25] such interactions only occur between a small number of pulses (primarily those in any given axial stack, and to a lesser extent the adjacent stacks). This makes sequential optimisation practical, and gives almost the same treatment times as collective optimisation. Second, the sequential optimisation approach will likely be easy to implement practically in MRgHIFU, with MR temperature measurements allowing one to account for prior heating and dose delivery to each site, and to develop adaptive models to continually improve predictions of the doses delivered during tumour cooling. Since MRgHIFU can measure temperatures rapidly enough (a few seconds between measurements for a high spatial resolution Citation[77]), real-time control of individual pulses is possible so that close to (sequentially) optimal heating times can be realised clinically, a process that will be achieved more accurately as MRI temperature imaging improves. Third, given the significantly diminishing returns obtained with high focal zone packing densities, the current study indicates that a relatively small number of focal zones (per stack) will be adequate to rapidly treat tumours, making the treatment planning and control more practical. As larger and more complicated tumours are treated with more extensive and complicated paths (compared to the initial simple generic tumours and paths studied herein) there may be more interactions among heating pulses. Such interactions could yield further opportunities to reduce treatment time through the anticipation of future heating effects by either simultaneous collective optimisation of the complete treatment, or sequential optimisation that anticipates the effects of a small number of future heating pulses, e.g. all of the pulses in a stack, but not the complete treatment.
Another factor that can have a significant impact on treatment times and ablation rates is tissue perfusion. For the current study, the low perfusion value of 0.5 kg/m3/s was used for almost all studies (with the exception of the two-position axial spacing optimisation study where it was found that a 5.0 kg/m3/s tissue perfusion value left the optimal spacing and shape of the graph unchanged). Previous studies Citation[78–80] have shown that HIFU treatments can be sensitive to changes in the tissue perfusion network, with differences in simulated ablation volumes up to 70% being reported due to the choice of perfusion model Citation[78]. The results in the current paper indicate that while treatment times will increase as perfusion increases, the optimal heating approach will likely remain the concentrated approach. Similarly, as perfusion increases the individual pulses, effects will have less interaction, thus bringing the results from the collective optimisation techniques closer to the sequential optimisation results. Future studies should investigate tissue-specific scenarios using organ-specific blood perfusions and tumour models in order to expand upon the more generic results presented herein which provide guidelines for future research.
Finally, the tissue absorptivity coefficient changes had complex effects on the optimal heating times of the focal zone sequence in an axial stack. While a back/middle/front treatment path could always be used to avoid the problem of passing ultrasound through regions in which the absorptivity coefficient has increased, it is unclear whether this would always be time optimal. In particular, such a path leads to longer treatment times under some variable absorptivity models, such as doubling the absorptivity immediately after treating each position. Further in vivo experimental determinations of how absorptivity changes with heating parameters are needed to further quantify these effects, particularly on a more clinically realistic model than the simplified model used in this study that begins to quantify the effects of absorbtivity changes.
Conclusion
This study examined the treatment of tumours using concentrated and fractionated heating approaches. The results show that a concentrated heating approach produced faster treatments for all tumours studied, both for simplified treatments on axial stacks and for treatments on larger areas with simulation data matched to phantom experiments. Additionally, this study showed that optimal focal zone spacings exist in both the transverse and axial directions, and that although treatment time decreases monotonically as the number of focal zone locations is increased, the resulting treatment time gains reach a point of diminishing returns at low focal zone packing densities. Also, this study showed that, at least for the simple generic tumours studied herein, sequential optimisation of individual heating pulses gives almost the same treatment times as the much more difficult to implement simultaneous, collective pulse optimisation technique that requires complete a priori knowledge of the entire treatment. Finally, this study shows that absorptivity changes have complex effects on treatment times and on the selection of treatment path, and that further experimental data is needed to determine the magnitudes of such changes, as well as for blood perfusion, that occur clinically in tumours.
Acknowledgements
We greatly appreciate the help of Dennis Parker, Urvi Vyas, Martin Cuma, Doug Christensen, Chris Dillon, Allison Payne, and Josh de Bever, and use of the UCAIR facilities for this work. A generous allocation of extensive computer time from the Center for High Performance Computing at the University of Utah is also gratefully acknowledged.
Declaration of interest: This work was partially supported by grants from the US National Institutes of Health (R01-CA134599), Siemens Medical Solutions, the Focused Ultrasound Foundation, a University of Utah Synergy Grant and the Ben B. and Iris M. Margolis Foundation. The authors alone are responsible for the content and writing of the paper.
References
- Fennessy FM, Tempany CM. MRI-guided focused ultrasound surgery of uterine leiomyomas. Acad Radiol 2005; 12: 1158–1166
- Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynnen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: Early results. Am J Roentgenol 2004; 183: 1713–1719
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005; 93: 890–895
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study. Radiology 2001; 219: 176–185
- Hesley GK, Felmlee JP, Gebhart JB, Dunagan KT, Gorny KR, Kesler JB, et al. Noninvasive treatment of uterine fibroids: Early Mayo Clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clin Proc 2006; 81: 936–942
- Daum DR, Smith NB, King R, Hynynen K. In vivo demonstration of noninvasive thermal surgery of the liver and kidney using an ultrasonic phased array – Comparison of strategies using phased array systems. Ultrasound Med Biol 1999; 25: 1087–1098
- Poissonnier L, Chapelon J-Y, Rouvière O, Curiel L, Bouvier R, Martin X, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol 2007; 51: 381–387
- Blana A, Murat FJ, Walter B, Thuroff S, Wieland WF, Chaussy C, et al. First analysis of the long-term results with transrectal HIFU in patients with localised prostate cancer. Eur Urol 2008; 53: 1194–1203
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005; 5: 321–327
- Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasiv Ther 2006; 15: 26–35
- Fan X, Hynynen K. Ultrasound surgery using multiple sonications – Treatment time considerations. Ultrasound Med Biol 1996; 22: 471–482
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005; 235: 659–667
- Malinen M, Huttunen T, Kaipio JP, Hynynen K. Scanning path optimization for ultrasound surgery. Phys Med Biol 2005; 50: 3473–3490
- Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, et al. High-intensity focused ultrasound (HIFU): Effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol 2009; 19: 437–445
- Melodelima D, N'Djin WA, Parmentier H, Chesnais S, Rivoire M, Chapelon J-Y. Ultrasound surgery with a toric transducer allows the treatment of large volumes over short periods of time. Appl Phys Lett 2007; 91: 193901–193903
- Zhou Y, Kargl SG, Hwang JH. The effect of the scanning pathway in high-intensity focused ultrasound therapy on lesion production. Ultrasound Med Biol 2011; 37: 1457–1468
- Hariharan P, Myers MR, Banerjee RK. HIFU procedures at moderate intensities – Effect of large blood vessels. Phys Med Biol 2007; 52: 3493
- Malinen M, Huttunen T, Kaipio JP, Hynynen K. Scanning path optimization for ultrasound surgery. Phys Med Biol 2005; 50: 3473–3490
- Liu H-L, Chen Y-Y, Yen J-Y, Lin W-L. Treatment time reduction for large thermal lesions by using a multiple 1D ultrasound phased array system. Phys Med Biol 2003; 48: 1173–1190
- Sasaki K, Azuma T, Kawabata K-I, Shimoda M, Kokue E-I, Umemura S-I. Effect of split-focus approach on producing larger coagulation in swine liver. Ultrasound Med Biol 2003; 29: 591–599
- Charles Mougenot RS, Jean Palussière, Nicolas Grenier, Chrit TW. Moonen, . Automatic spatial and temporal temperature control for MR-guided focused ultrasound using fast 3D MR thermometry and multispiral trajectory of the focal point. Magn Reson Med 2004; 52: 1005–1015
- Seip R, Sanghvi NT, Uchida T, Umemura SI. Comparison of split-beam transducer geometries and excitation configurations for transrectal prostate HIFU treatments. Proc IEEE Ultrason Symp 2001; 2: 1343–1346
- Payne A, Vyas U, Blankespoor A, Christensen D, Roemer R. Minimisation of HIFU pulse heating and interpulse cooling times. Int J Hyperthermia 2010; 26: 198–208
- Roemer R, Payne AH, Minimization of HIFU dose delivery time. Paper presented at the International Society of Therapeutic Ultrasound, 2007, Seoul, Korea, 12–15 June
- Coon J, Payne A, Roemer R. HIFU treatment time reduction in superficial tumours through focal zone path selection. Int J Hyperthermia 2011; 27: 465–481
- Huttunen T, Kaipio JP, Malinen M. Optimal control in high intensity focused ultrasound surgery. Optimization in Medicine, CJS Alves, PM Pardalos, LN Vicente. Springer, New York 2008; 169–195
- Hynynen K, Roemer R, Anhalt D, Johnson C, Xu ZX, Swindell W, et al. A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. Int J Hyperthermia 1987; 3: 21–35
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004; 42: 931–935
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1099–1106
- Billard BE, Hynynen K, Roemer RB. Effects of physical paramaters on high temperature ultrasound hyperthermia. Ultrasound Med Biol 1990; 16: 409–420
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am 1994; 95: 1641–1649
- Gianfelice D, Khiat A, Boulanger Y, Amara M, Belblidia A. Feasibility of magnetic resonance imaging–guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J Vasc Interv Radiol 2003; 14: 1275–1282
- McDannold N, Moss M, Killiany R, Rosene DL, King RL, Jolesz FA, et al. MRI-guided focused ultrasound surgery in the brain: Tests in a primate model. Magn Reson Med 2003; 49: 1188–1191
- McDannold NJ, Jolesz FA, Hynynen KH. Determination of the optimal delay between sonications during focused ultrasound surgery in rabbits by using MR imaging to monitor thermal buildup in vivo. Radiology 1999; 211: 419–426
- Palussiere J, Salomir R, Le Bail B, Fawaz R, Quesson B, Grenier N, et al. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med 2003; 49: 89–98
- McDannold N, Hynynen K, Wolf D, Wolf G, Jolesz F. MRI evaluation of thermal ablation of tumours with focused ultrasound. J Magn Reson Imaging 1998; 8: 91–100
- McDannold N, Hynynen K, Jolesz F. MRI monitoring of the thermal ablation of tissue: Effects of long exposure times. J Magn Reson Imaging 2001; 13: 421–427
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Todd N, Payne A, Parker D, 3-D MR temperature imaging with model predictive filtering reconstruction. Paper presented at the International Society for Magnetic Resonance in Medicine Annual Meeting; 17–24 April 2009, Honolulu, Hawaii
- Poorter JD, Wagter CD, Deene YD, Thomsen C, Ståhlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: In vivo results in human muscle. Magn Reson Med 1995; 33: 74–81
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995; 34: 814–823
- Damianou C, Hynynen K. Focal spacing and near-field heating during pulsed high temperature ultrasound therapy. Ultrasound Med Biol 1993; 19: 777–787
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am 1994; 95: 1641–1649
- Kohler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009; 36: 3521–535
- Wu X, Sherar M. Theoretical evaluation of moderately focused spherical transducers and multi-focus acoustic lens/transducer systems for ultrasound thermal therapy. Phys Med Biol 2002; 47: 1603–1621
- Cheng T-Y, Ju K-C, Ho C-S, Chen Y-Y, Chang H, Lin W-L. Split-focused ultrasound transducer with multidirectional heating for breast tumor thermal surgery. Med Phys 2008; 35: 1387–1397
- Gorny KR, Hangiandreou NJ, Hesley GK, Gostout BS, McGee KP, Felmlee JP. MR guided focused ultrasound: Technical acceptance measures for a clinical system. Phys Med Biol 2006; 51: 3155–3173
- Enholm JK, Kohler MO, Quesson B, Mougenot C, Moonen CTW, Sokka SD. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng 2010; 57: 103–113
- Liu H-L, Lin W-L, Chen Y-Y. A fast and conformal heating scheme for producing large thermal lesions using a 2D ultrasound phased array. Int J Hyperthermia 2007; 23: 69–82
- Cheng KS, Roemer RB. Closed-form solution for the thermal dose delivered during single pulse thermal therapies. Int J Hyperthermia 2005; 21: 215–230
- Fan X, Hynynen K. A study of various parameters of spherically curved phased arrays for noninvasive ultrasound surgery. Phys Med Biol 1996; 41: 591–608
- Cheng K, Roemer R. Blood perfusion and thermal conduction effects in Gaussian beam, minimum time single-pulse thermal therapies. Med Phys 2005; 32: 311–317
- Patel PR, Luk A, Durrani A, Dromi S, Cuesta J, Angstadt M, et al. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int J Hyperthermia 2008; 24: 537–549
- Zderic V, Keshavarzi A, Andrew MA, Vaezy S, Martin RW. Attenuation of porcine tissues in vivo after high-intensity ultrasound treatment. Ultrasound Med Biol 2004; 30: 61–66
- Keshavarzi A, Vaezy S, Kaczkowski PJ, Keilman G, Martin R, Chi EY, et al. Attenuation coefficient and sound speed in human myometrium and uterine fibroid tumors. J Ultrasound Med 2001; 20: 473–480
- Worthington AE, Trachtenberg J, Sherar MD. Ultrasound properties of human prostate tissue during heating. Ultrasound Med Biol 2002; 28: 1311–1318
- Kolios M, Sherar M, Hunt J. Temperature dependent tissue properties and ultrasonic lesion formation. Adv Heat Mass Transfer Biotech 1999; 44: 113–118
- Damianou CA, Sanghvi NT, Fry FJ, Maass-Moreno R. Dependence of ultrasonic attenuation and absorption in dog soft tissues on temperature and thermal dose. J Acoust Soc Am 1997; 102: 628–634
- Clarke RL, Bush NL, Ter Haar GR. The changes in acoustic attenuation due to in vitro heating. Ultrasound Med Biol 2003; 29: 127–135
- Gertner MR, Wilson BC, Sherar MD. Ultrasound properties of liver tissue during heating. Ultrasound Med Biol 1997; 23: 1395–1403
- Techavipoo U, Varghese T, Chen Q, Stiles TA, Zagzebski JA, Frank GR. Temperature dependence of ultrasonic propagation speed and attenuation in excised canine liver tissue measured using transmitted and reflected pulses. J Acoust Soc Am 2004; 115(6)2859–2865
- Worthington AE, Sherar MD. Changes in ultrasound properties of porcine kidney tissue during heating. Ultrasound Med Biol 2001; 27: 673–682
- Loulou T, Scott EP. Thermal dose optimization in hyperthermia treatments by using the conjugate gradient method. Numer Heat Transfer A 2002; 42: 661–683
- Lin WL, Liang TC, Yen JY, Liu HL, Chen YY. Optimization of power deposition and a heating strategy for external ultrasound thermal therapy. Med Phys 2001; 28: 2172–2181
- Cheng KS, Roemer RB. Optimal power deposition patterns for ideal high temperature therapy/hyperthermia treatments. Int J Hyperthermia 2004; 20: 57–72
- Hong W, Aarsvold J, O'Donnell M, Cain C. Thermal dose optimization for ultrasound tissue ablation. IEEE Trans Ultrason Ferr 1999; 46: 913–928
- Daum DR, Hynynen K. Optimization of thermal dose using switching mode patterns of a spherically shaped square element phased array. Proc IEEE Ultrason Symp 1996; 2: 1309–1312
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011; 27: 320–343
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Mathworks. Constrained nonlinear optimization algorithms, 2011. Available at: http://www.mathworks.com/products/optimization/description3.html: Mathworks Inc
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Vyas U, Christensen D, Ultrasound beam propagation using the hybrid angular spectrum method. Paper presented at the Engineering in Medicine and Biology Society 30th Annual International Conference of the IEEE; 20–25 August, 2008, Vancouver, Canada
- FDA. US, Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers, 2009. Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm089001.htm]
- Payne A, Vyas U, Todd N, de Bever J, Christensen DA, Parker DL. The effect of electronically steering a phased array ultrasound transducer on near-field tissue heating. Med Phys 2011; 38(9)4971–81
- Coon J, Treatment time reduction through parameter optimization in magnetic resonance guided high intensity focused ultrasound treatments. Doctoral dissertation, University of Utah, 2012
- Todd N, Payne A, Parker DL. Model predictive filtering for improved temporal resolution in MRI temperature imaging. Magn Reson Med 2010; 63: 1269–1279
- Schutt DJ, Haemmerich D. Effects of variation in perfusion rates and of perfusion models in computational models of radio frequency tumor ablation. Med Phys 2008; 35: 3462–3470
- Prakash P, Diederich CJ. Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: Implications for treatment planning, monitoring and control. Int J Hyperthermia 2012; 28: 69–86
- He X, McGee S, Coad JE, Schmidlin F, Iaizzo PA, Swanlund DJ, et al. Investigation of the thermal and tissue injury behaviour in microwave thermal therapy using a porcine kidney model. Int J Hyperthermia 2004; 20: 567–593