Abstract
Purpose: Control of therapeutic gene expression in tumours is a major goal of gene therapy research, as it can restrict cytotoxic gene expression in cancer cells. In addition, the combination of hyperthermia with gene therapy through the application of heat-inducible vectors can result in considerable improvements in therapeutic efficiency. In this study, to combine heat-inducibility with high-level transgene expression, we developed a heat-inducible transgene expression system with transcriptional amplification mediated by a tetracycline-responsive transactivator.
Materials and methods: A hybrid promoter was generated by placing the heat shock protein (HSP) 70B′ promoter under the tetracycline-repressor responsive element sequence, and a reporter/therapeutic gene expression plasmid was constructed by placing a reporter/therapeutic gene under the control of this hybrid promoter.
Results: When the transactivator expression plasmid harbouring an expression cassette of the tetracycline-responsive transactivator gene was co-transfected with a reporter gene expression plasmid, the reporter gene expression was controlled by heat treatment. With this system, high levels of heat-induced transgene expression were observed compared to that from the HSP promoter alone without the transactivator. Evaluation of in vitro therapeutic effects using cancer cell lines revealed that therapeutic gene expression effectively caused cell death in a greater percentage of the cells.
Conclusion: These findings indicate that this strategy improves the efficacy of cancer gene therapy.
Introduction
Gene therapy represents an innovative strategy for the treatment of cancer and other diseases. Efficient control of therapeutic gene expression is a major goal in the field of gene transfer and therapy. Inducible vectors are useful for spatial and temporal expression of therapeutic genes in gene therapy, as they can restrict cytotoxic gene expression to target cells. One of the strategies using inducible vectors is based on the control of therapeutic gene expression by conventional treatment modalities, which hold particular promise for cancer therapy. An additive or synergistic effect can be achieved by combining cancer therapies, such as chemotherapy Citation[1], radiation Citation[2] or hyperthermia Citation[3], with gene therapy using a corresponding inducible vector. These combination approaches have been successfully employed for the regulated expression of tumour-toxic genes for cytokines such as tumour necrosis factor (TNF)-α, or for enzymes such as herpes simplex virus thymidine kinase (HSV-tk) followed by ganciclovir (GCV) administration.
Hyperthermia is a promising approach to cancer therapy, and has been used for many years to treat a wide variety of tumours in both experimental animals and human patients. Moreover, hyperthermia has often been used in multimodality strategies as it can enhance the effects of chemotherapy Citation[4], radiotherapy Citation[5] and immunotherapy Citation[6]. Therefore, the combination of hyperthermia with heat-induced therapeutic gene expression is an attractive strategy. To achieve this, several promoters have been used for the construction of viral and non-viral vectors with heat-inducible gene expression in numerous gene therapy studies Citation[7], Citation[8]. The major target proteins induced by heating are the heat shock proteins (HSPs). HSPs are molecular chaperones involved in prevention of misfolding and aggregation of denatured proteins after cellular damage, including thermal stress. One of the most extensively studied groups of HSPs is the Hsp70 family. Among these, hsp70B′ is a human Hsp70 chaperone that is reported to be strictly inducible by heat treatment, exhibiting poor basal expression levels in most cells. The Hsp70 promoters are regulated by heat shock transcription factor (HSF), which undergoes trimerisation, phosphorylation and nuclear translocation by stress stimuli Citation[9]. In the nucleus it binds to a heat shock element (HSE) sequence of the Hsp70 promoter, thus, regulation of heat-inducible gene expression is achieved. However, Hsp70 promoters are not as strong as constitutively active viral promoters Citation[10]. Therefore, it is essential to find ways to enhance the transcriptional activity of Hsp70 promoters.
Tetracycline (Tet)-responsive expression systems using fusion proteins between Tet-repressors and viral transcriptional activators (Tet-responsive transactivators) have been successfully employed in many cell types and in transgenic animals Citation[11], Citation[12], providing highly efficient regulation of transgene expression with low background. The transgene expression in the so-called Tet-Off system is induced in the absence of a regulating drug, Tet, or its derivatives such as doxycycline (Dox). The Tet-Off system consists of a combination of two vectors, one for constitutively expressing the Tet-responsive transactivator protein (tTA), and the other for expressing a gene of interest under the control of the minimal cytomegalovirus (CMV) promoter (PCMVmin) containing a binding site (Tet-repressor responsive element, TRE) for tTA. Thus, if cells are transfected with the two vectors, transgene transcription is highly activated by tTA. To combine heat-inducibility with high-level transgene expression, in the present study we developed a heat-inducible transgene expression system with transcriptional amplification mediated by tTA. A hybrid promoter, TRE-HSP, was generated by placing the hsp70B′ promoter downstream of the TRE sequence, and a reporter or therapeutic gene expression plasmid was constructed by placing a reporter gene (LacZ) or a therapeutic gene (TNF-α or HSV-tk) under the control of the hybrid promoter. The transactivator expression plasmid encoding an expression cassette of tTA gene was co-transfected with the reporter/therapeutic gene expression plasmid. Using these plasmids we investigated whether heat-inducible expression by the TRE-HSP hybrid promoter was enhanced by tTA. Furthermore, the in vitro therapeutic effects were evaluated by heat-inducible expression of therapeutic genes in cultured carcinoma cell lines.
Materials and methods
Plasmid construction
Plasmid vectors used in this study are shown in . The pTet-Off plasmid (Clontech, Mountain View, CA, USA) was digested with XhoI to remove the neomycin-resistant gene expression cassette, and then self-ligated to generate the transactivator expression plasmid, pCMV/tTA (). A LacZ gene fragment derived from the pMSCV/NΔAβ Citation[13] was ligated into NotI-digested pTRE2hyg (Clontech) to generate pTRE-CMVmin/Z (), which contains the β-galactosidase gene under the control of PCMVmin downstream of a TRE sequence.
Figure 1. Construction of the transgene expression unit. (A) Transactivator expression plasmid. (B) Reporter gene expression plasmids. (C) Therapeutic gene expression plasmids.
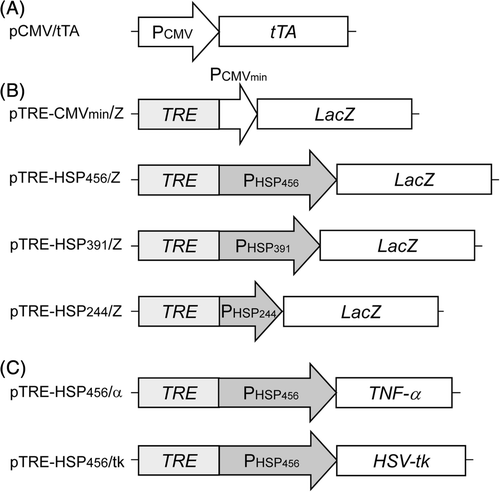
A DNA fragment for human hsp70B′ promoter sequence was obtained from HEK293 cells (Riken BioResource Center, Tsukuba, Japan) by polymerase chain reaction (PCR). Genomic DNA from the cells was prepared using a genomic DNA extraction kit (MagExtractor Genome; Toyobo, Osaka, Japan). The following sequences were used as forward primers for PHSP456, 5′-GGG GTA CCG CCT CTA AAG TTG CTG CTT TTG-3′; for PHSP391, 5′-GGG GTA CCA GCT AGA ACC TTC CCC GCA T-3′; and for PHSP244, 5′-GGG GTA CCC CCG GGC GGG CGA GAG GCT CTC A-3′. The oligonucleotide 5′-GGA ATT CAA GCT TCT TGT CGG ATG CTG-3′ was commonly used as the reverse primer. The underlined bases represent restriction enzyme sites (KpnI and EcoRI sites) for the forward and reverse primers, respectively. PCR was initiated using KOD-plus DNA polymerase (Toyobo) at 94°C for 2 min, followed by 30 cycles of amplification at 94°C for 15 s, 62°C for 30 s and 68°C for 15 s. The amplified hsp70B′ promoter gene fragments were digested with KpnI and EcoRI, and ligated into KpnI-digested and EcoRI-digested pTRE-CMVmin/Z to generate the reporter gene expression plasmids (), pTRE-HSP456/Z, pTRE-HSP391/Z and pTRE-HSP244/Z. The DNA sequences of the promoter regions were confirmed using a DNA sequencer (Prism 3130 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA) and compared with their sequences in the GenBank database (ID: AL590385).
Two types of the therapeutic gene expression plasmids were constructed (). A DNA fragment encoding a human TNF-α gene was amplified by PCR from pDrive/hTNF-α (IHS1380-97432513, Open Biosystems, Huntsville, AL, USA) using the following primers, 5′-ATT TGC GGC CGC ACC ATG AGC ACT GAA AGC ATG AT-3′ and 5′-ATT TGC GGC CGC TCA CAG GGC AAT GAT CCC-3′, to insert NotI digestion sites (underlined) onto either end of the PCR product. The PCR was initiated using KOD-plus polymerase at 94°C for 2 min, followed by 30 cycles of amplification at 94°C for 15 s, 57°C for 30 s and 68°C for 45 s. The amplified PCR product was digested with the respective restriction enzymes and ligated into NotI-digested pTRE-HSP456/Z to generate pTRE-HSP456/α. A DNA fragment encoding a HSV-tk gene prepared from the pQMSCV/PTNIG Citation[14] digested with SacII was ligated into the blunt-ended pTRE-HSP456/Z after digesting with NotI to generate pTRE-HSP456/tk. The DNA sequences for the therapeutic genes were confirmed using a DNA sequencer.
Cell culture
Human cancer cell lines, cervical cancer HeLa, hepatoblastoma HepG2 and lung carcinoma A549, were obtained from Riken BioResource Center. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin G potassium and 0.1 mg/mL streptomycin sulfate. Cells were cultured at 37°C in a 5% CO2 incubator.
Reporter gene assays using reporter gene expression plasmids
HeLa cells were seeded in 60 mm tissue culture dishes at a concentration of 1.0 × 106 cells/dish. The next day, cells were co-transfected with the reporter gene expression plasmid (4 µg/dish) and the transactivator expression plasmid (pCMV/tTA, 4 µg/dish) using a lipofection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA, USA). As a control experiment without the transactivator expression plasmid, pETBlue-2 (4 µg/dish; Novagen, Madison, WI, USA) was used as a mock plasmid and co-transfected with the reporter gene expression plasmid. Heat shock treatment was performed 24 h after transfection. The cell culture dishes were sealed and directly immersed into a calibrated water bath at 41°C, 43°C or 45°C for 1 h. After the heat treatment, the cell culture dishes were immediately returned to the 37°C 5% CO2 incubator, and the cells were harvested periodically to quantify the β-galactosidase activity Citation[13].
Cytotoxic assay using TNF-α gene expression plasmid
HeLa, HepG2 or A549 cells were seeded in 100 mm tissue culture dishes at a concentration of 1.0 × 106 cells/dish. The next day, cells were co-transfected with both pCMV/tTA (0.4, 1.0 and 2.0 µg/dish for HeLa, A549 and HepG2 cells, respectively) and pTRE-HSP456/α (0.4, 1.0 and 2.0 µg/dish for HeLa, A549 and HepG2 cells, respectively) using Lipofectamine 2000. As a control experiment, pETBlue-2 was used as the mock plasmid. Heat shock treatment was performed 24 h post transfection. After the heat treatment, cells were collected and reseeded in 6-well cell culture plates at 1.0 × 105 cells/well, and incubated for 24 h at 37°C 5% CO2. Cells and culture medium were then collected for the cytotoxicity assays and enzyme-linked immunosorbent assays (ELISA) for TNF-α, respectively. To evaluate the cytotoxicity, the number of viable cells was counted by the trypan blue dye exclusion method using a haemocytometer. The cell viability was determined as follows: cell viability [%] = (number of viable cells in the tested dish)/(number of viable cells in the mock-transfected control dish at 37°C) × 100. The TNF-α concentration in the cell culture medium was determined by Quantikine human TNF-α ELISA kit (R&D Systems, Minneapolis, MN, USA) and normalised against the number of viable cells. Statistical comparisons were performed using the Mann-Whitney rank sum test. Differences in statistical comparisons were considered significant at P values of <0.05.
Cytotoxic assays using HSV-tk gene expression plasmid
HeLa, HepG2 or A549 cells were seeded in 6-well tissue culture plates at 1.6 × 105 cells/well 24 h prior to transfection. The next day cells were co-transfected with both pCMV/tTA (0.8 µg/well) and pTRE-HSP456/tk (0.8 µg/well) using Lipofectamine 2000. As a control experiment, pETBlue-2 was used as the mock plasmid. Heat shock treatment was performed 24 h after transfection. Cells were then collected and reseeded in 96-well cell culture plates at 5.0 × 103 cells/well in 150 µL fresh culture medium containing GCV (0.1 µg/mL for HeLa and A549 cells, and 1.0 µg/mL for HepG2 cells), and incubated for 48 h at 37°C 5% CO2. Cytotoxicity was evaluated with a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) based on the WST-8 method according to the manufacturer's protocol. The cell viability was determined as follows: Cell viability [%] = (number of viable cells in the tested dish)/(number of viable cells in the mock-transfected control dish at 37°C) × 100. Statistical comparisons were performed using the Mann-Whitney rank sum test. Differences in statistical comparisons were considered significant at P values of <0.05. To detect apoptotic cells after the heat-induced HSV-tk/GCV treatment, the TdT-mediated dUTP nick end labelling (TUNEL) assay was performed by using an in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Briefly, cells were treated with TUNEL reaction mixture including fluorescein isothiocyanate (FITC)-conjugated dUTP and incubated in the dark for 1 h at 37°C. The cells were observed under a BZ-9000 fluorescence microscope (Keyence, Tokyo, Japan). The numbers of FITC-stained cells in three non-overlapping fields were counted using Photoshop software (Adobe Systems, San Jose, CA, USA).
Results and discussion
Expression system design
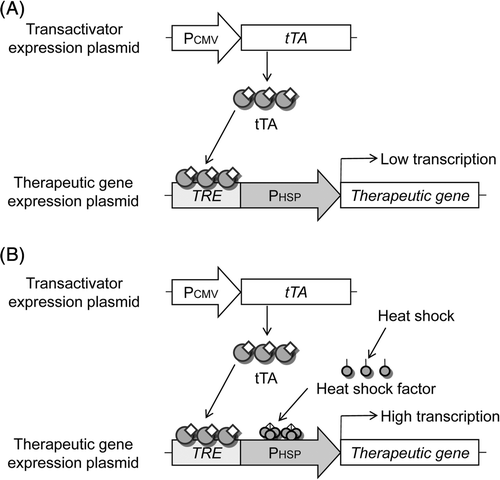
In order to enhance the heat-induced activity of the hsp70B′ promoter, we have developed a novel hybrid promoter system incorporating the Tet-responsive transactivator system into the hsp70B′ promoter. A schematic diagram of heat-inducible transgene expression with transcriptional amplification mediated by tTA is shown in . The first step involves the constitutive expression of tTA driven by the CMV promoter. tTA then binds to the TRE sequence of the hybrid promoter. Without heating, the TRE-HSP promoter cannot be highly activated because of the regulation of the hsp70B′ promoter, although an additional leak of expression may be induced by tTA (). When cells are exposed to heat treatment, HSF is activated and translocates to the nucleus where it binds to the HSE sequence and drives a therapeutic gene. Upon the activation of hsp70B′ promoter, tTA bound to TRE sequence also potentially activates transcription of the therapeutic gene (), thus leading to the transcriptional amplification of the TRE-HSP hybrid promoter, mediated by tTA.
Figure 2. Schematic of the strategy for heat-inducible gene expression of TRE-HSP hybrid promoter. (A) Mechanism of action in non-heated cells. We hypothesise that the following sequence of events occurs: (1) tTA is constitutively expressed under CMV promoter; (2) tTA can bind to the TRE within the TRE-HSP hybrid promoter upstream of a therapeutic gene; (3) but the therapeutic gene cannot be induced because the hsp70B′ promoter is not induced in non-heated cells. (B) Mechanism of action in heated cells. In this case, HSF is activated and translocates to the cell nucleus, where it binds to the HSE, and the hsp70B′ promoter drives the therapeutic gene. Once the hsp70B′ promoter is activated, the tTA potentially activates transcription of the therapeutic gene, thus leading to transcriptional amplification of the TRE-HSP hybrid promoter mediated by tTA.
Heat-inducible reporter gene expression mediated by transactivator
The system was first tested by co-transfection of the transactivator expression plasmid and the reporter gene expression plasmid into HeLa cells. shows the time course of β-galactosidase activity of the transfected cells after heat treatment. Without heat treatment (37°C), only low levels of β-galactosidase activity was detected in the cells transfected with pTRE-HSP456/Z alone or pTRE-HSP456/Z and pCMV/tTA, indicating that the hybrid promoter system retained a low basal level of expression from hsp70B′ promoter even in the presence of tTA. Without transfection of the transactivator expression plasmid (pTRE-HSP456/Z alone), heat-induced activation of the TRE-HSP promoter was observed by heat treatment at 43°C for 1 h. β-galactosidase activity increased and peaked at 6 h after the heat treatment (). The expression level driven by TRE-HSP promoter was comparable to that driven by native hsp70B′ promoter without TRE sequence (Figure S1), indicating that the TRE sequence upstream hsp70B′ promoter had no effect on the hsp70B′ promoter activity in the absence of tTA. In contrast, higher levels of heat-induced gene expression were observed in the cells co-transfected with the plasmids (pTRE-HSP456/Z + pCMV/tTA) compared with heat-induced gene expression without tTA (pTRE-HSP456/Z alone). In the cells co-transfected with pTRE-HSP456/Z and pCMV/tTA, the time-course profile of reporter gene expression was similar to that without pCMV/tTA (pTRE-HSP456/Z alone), suggesting that the hybrid promoter maintained the heat-regulation property of the hsp70B′ promoter.
Figure 3. Reporter gene expression driven by the hybrid promoter. (A) HeLa cells were transfected with pTRE-HSP456/Z alone (open symbols) or pTRE-HSP456/Z plus pCMV/tTA (closed symbols), and incubated at 37°C (circles) or heated at 43°C for 1 h (triangles). As a control, HeLa cells were co-transfected with pTRE-CMVmin/Z and pCMV/tTA (cross symbols). The data are expressed as mean ± SD (n = 3). (B) HeLa cells were transfected with pTRE-HSP456/Z alone (open symbols) or pTRE-HSP456/Z plus pCMV/tTA (closed symbols), and incubated at 37°C (circles), 41°C (squares), 43°C (triangles) or 45°C (diamonds) for 1 h. Relative β-galactosidase activity is defined as the relative value of the β-galactosidase activity of the cells transfected with pTRE-HSP456/Z alone without heating (37°C) at 0 h. The data are expressed as mean ± SD (n = 3).
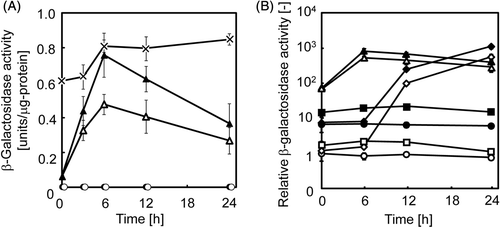
In order to evaluate the levels of heat-induced gene expression, the original Tet system (pTRE-CMVmin/Z + pCMV/tTA), in which tTA-mediated reporter gene expression is strongly induced from the TRE-CMVmin promoter, was used as the control. As shown in , without the tTA expression plasmid (pTRE-HSP456/Z alone), the heat-induced β-galactosidase activity was lower than that of the original Tet system throughout the culture period, and the maximum expression level was 59% of that of the original Tet system. However, when cells were co-transfected with pTRE-HSP456/Z and pCMV/tTA, the maximum expression reached similar levels of the original Tet system, which is known to be a high-level gene expression system Citation[15]. These results demonstrated that the hybrid promoter system possessed both the heat-inducible property of hsp70B′ promoter, and the high-level expression property of the Tet system.
Time course profile of reporter gene expression under various temperatures
shows the relative β-galactosidase activities after heat treatment with different temperatures. Without heat treatment (37°C), when cells were co-transfected with pTRE-HSP456/Z and pCMV/tTA, the β-galactosidase activity was seven times higher than without the transactivator expression plasmid (pTRE-HSP456/Z alone) (). When the cells were heated at 41°C, 43°C or 45°C, higher levels of heat-induced gene expression were observed by co-transfection with the plasmids. Interestingly, the time course profile of reporter gene expression was quite different, especially between the cells that underwent heat treatment at 43°C and 45°C. For cells heated to 45°C, the reporter gene expression was delayed; the expression was not induced at 6 h, but was then drastically induced and reached similar expression levels to that of the cells heated to 43°C at 24h (). A similar result was observed using another stress-inducible GADD153 promoter system, in which the reporter gene expression patterns varied with temperature (43°C and 45°C) Citation[16]. In the present study, the β-galactosidase activity in the cells co-transfected with the transactivator expression plasmid was comparable to the basal level (37°C) during 0–6 h after heat treatment at 45°C for 1 h (). These results suggest that the activity of hsp70B′ promoter was inhibited until 6 h after the heat treatment at 45°C for 1 h because of severe stress and cellular damage, and the promoter activity was subsequently regained as the cells recovered from the damage.
Effect of deletion of hsp70B′ promoter region on the hybrid promoter activity
Six putative HSE motifs in the hsp70B′ promoter sequence (NNGAANNTTCNN, Citation[17]) can be found, according to the GenBank database (ID: AL590385); two individual HSEs, −274 to −263 bp and −181 to −170 bp, and four overlapping HSEs between −100 and −59 bp, which are minimally required for essential stress-inducible expression Citation[17]. In order to explore the functional region in the hybrid promoter we constructed three reporter gene plasmids containing different sized regions of the hsp70B′ promoter (): pTRE-HSP456/Z and pTRE-HSP391/Z contained both the individual and overlapping HSEs, while pTRE-HSP244/Z contained only the overlapping HSEs region (, left). When cells were transfected with pTRE-HSP456/Z or pTRE-HSP391/Z and pCMV/tTA and underwent heat treatment at 43°C for 1 h, β-galactosidase activity increased above twice that of the cells transfected with pTRE-HSP456/Z alone or pTRE-HSP391/Z alone. For pTRE-HSP244/Z, the heat-induced activity of the TRE-HSP hybrid promoter decreased, possibly because of the deletion of two individual HSEs. Interestingly, when cells were co-transfected with the transactivator expression plasmid (pTRE-HSP244/Z + pCMV/tTA) and underwent heat treatment at 43°C for 1 h, the cells were able to induce a five-fold increase in β-galactosidase activity, as compared with that of the cells transfected with pTRE-HSP244/Z alone (, right), suggesting a reduced restriction of the hsp70B′ promoter due to the deletion of HSEs, which in turn enhanced the tTA-mediated transcriptional activation of the reporter gene.
Figure 4. Deletion analysis of hsp70B′ promoter region in the hybrid promoter. The structures of the hsp70B′ promoter region of pTRE-HSP456/Z and the 5′-deletion mutants, pTRE-HSP391/Z and pTRE-HSP244/Z are shown in the left panel. Arrows indicate the position of putative HSEs. The β-galactosidase activities of each construct at 24 h after heat treatment at 43°C for 1 h are shown in the right panel. Open and closed columns indicate that cells were transfected with the reporter gene expression plasmid alone, and the reporter gene expression plasmid plus transactivator expression plasmid, respectively. Relative β-galactosidase activity is defined as the relative value to the β-galactosidase activity of the cells transfected with pTRE-HSP244/Z alone. The data are expressed as mean ± SD (n = 3).
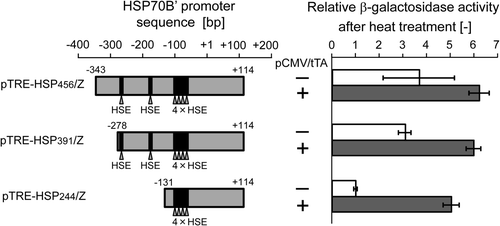
In synthetic biology, the development of engineering-based approaches that enable the construction of gene regulatory systems is a defining goal Citation[18]. To achieve this, synthetic transactivator/repressor elements in the Tet systems have been incorporated as components of the gene regulatory systems Citation[19]. In the present study, three hybrid promoter systems (pTRE-HSP456/Z, pTRE-HSP391/Z and TRE-pHSP244/Z) were constructed. Among them, pTRE-HSP244/Z showed the greatest dynamic induction range after heat shock treatment, when combined with the transactivator expression plasmid (), suggesting that this system provides a component of gene regulatory systems for synthetic biology. Furthermore, live cell-based biosensors utilising the Hsp70 promoter have been proposed Citation[20] because the induction of HSP genes is a potential marker of general cytotoxicity. Wada et al. Citation[17] reported that a 397 bp fragment of human hsp70B′ promoter showed sensitive induction by cadmium chloride. The hybrid promoter system developed in this study may provide more sensitive live cell biosensors because of the incorporation of transcriptional amplification. Taken together, the enhanced hsp70B′ promoter constitutes a promising tool for both synthetic biology and live cell-based biosensors.
Effect of enhanced heat-inducible TNF-α gene expression on cancer cell lines
Since the hybrid promoter developed in this study exhibited heat-inducible and high-level transgene expression using the reporter gene, the applicability of the system to cancer gene therapy was examined using therapeutic genes. For this purpose, the hybrid promoter containing a 456 bp fragment of hsp70B′ promoter was used because of its high activity upon heat shock induction ().
Heat inducible promoter systems may enable spatial and temporal targeting of therapy. This is critical for the use of potent but cytotoxic cytokines limited for practical utility due to systemic toxicity concerns. TNF-α is a good model of such cytokines, because it was first identified because of its ability to induce rapid necrosis of experimental cancers Citation[21] and, subsequently, its clinical use in humans has been greatly limited by systemic toxicity Citation[22]. As an in vitro model of heat-inducible gene therapy, we constructed the TNF-α gene expression plasmid pTRE-HSP456/α (), and both the cytotoxic effects and TNF-α production levels were examined using three human cancer cell lines. In our preliminary experiments, in contrast to the results of the reporter gene assays (), significant cytotoxicity was observed in the cells without heat treatment when the cells were co-transfected with both pTRE-HSP456/α (4 µg/dish) and pCMV/tTA (4 µg/dish). TNF-α induces the production of reactive oxygen species (ROS), which causes cell damage Citation[23]. ROS can also induce Hsp70 expression via the JAK/STAT pathway Citation[24]. This suggests that TNF-α is induced by heat shock, which in turn may further activate the hsp70B′ promoter. Similarly, the stress-inducible GADD153 promoter was induced by TNF-α as well as by heat shock Citation[25]. However, the detailed mechanism still remains to be elucidated. Thus, the basal expression level of TNF-α should be kept low by reducing the usage of both the transactivator and TNF-α gene expression plasmids.
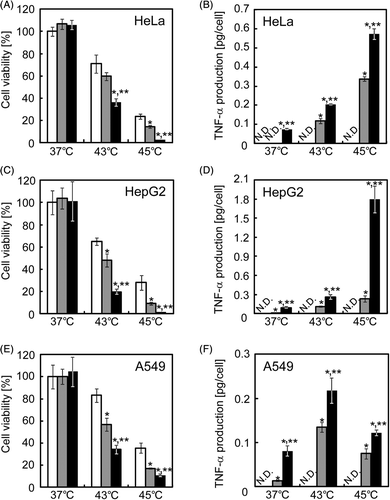
When cells were co-transfected with pCMV/tTA (0.4, 2.0 and 1.0 µg/dish for HeLa, HepG2 and A549 cells, respectively) and pTRE-HSP456/α (0.4, 2.0 and 1.0 µg/dish for HeLa, HepG2 and A549 cells, respectively), the basal production of TNF-α was kept at low levels (∼0.1 pg/cell, ), and no significant reduction of cell viability was observed (). With the heat treatment at 43°C for 1 h, the cell viabilities of the mock-transfected HeLa, HepG2 and A549 cells were 71.0 ± 8.1%, 64.9 ± 3.2% and 83.3 ± 6.1%, respectively. The cell viability was drastically decreased by heat treatment at 45°C for 1 h, and the cell viabilities of HeLa, HepG2 and A549 cells were 23.5 ± 2.1%, 27.3 ± 7.2% and 34.1 ± 3.9%, respectively. When the cells were transfected with pTRE-HSP456/α and underwent heat treatment at 43°C for 1 h, the heat-inducible TNF-α production was observed () and the cell viabilities decreased to 59.7 ± 3.7%, 48.2 ± 4.1% and 58.0 ± 4.8% for HeLa, HepG2 and A549 cells, respectively (). The levels of heat-inducible TNF-α production driven by TRE-HSP promoter and the resultant cell viability of HeLa cells were comparable to native hsp70B′ promoter without TRE sequence (Figure S2). TNF-α production was significantly enhanced by the co-transfection with the transactivator expression plasmid (pTRE-HSP456/α + pCMV/tTA), and the TNF-α production of the cancer cell lines at 1 day after heating at 43°C was about twice as high as that without pCMV/tTA transfection (pTRE-HSP456/α alone; ). Following co-transfection (pTRE-HSP456/α + pCMV/tTA), the cell viabilities after the heat treatment significantly decreased to 36.5 ± 3.3%, 19.5 ± 2.1% and 34.1 ± 3.9% for HeLa, HepG2 and A549 cells, respectively (). With the heat treatment at 45°C for 1 h, TNF-α production was significantly enhanced by the co-transfection (pTRE-HSP456/α + pCMV/tTA), and the cell viabilities drastically decreased to 2.4 ± 0.2%, 1.7 ± 0.2% and 10.6 ± 1.1% for HeLa, HepG2 and A549 cells, respectively ().
Figure 5. In vitro cytotoxicity of heat-inducible TNF-α gene expression. HeLa (A and B), HepG2 (C and D) and A549 (E and F) cells were transfected with the mock plasmid (white columns), pTRE-HSP456/α alone (grey columns) or pTRE-HSP456/α plus pCMV/tTA (black columns). The cells were incubated at 37°C or underwent heat treatment at 43°C or 45°C for 1 h. At 24 h after heat treatment, cells and culture medium were collected for the cell viability assay (A, C and E) and ELISA for TNF-α (B, D and F), respectively. The data are expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.05 versus the mock plasmid and pTRE-HSP456/α alone at each temperature, respectively. ND, not detected, where the minimum detectable range of TNF-α using the ELISA kit is > 1.6 pg/mL, according to the manufacturer's instruction.
Effect of enhanced heat-inducible suicide gene therapy on cancer cell lines
One of the most intensively studied strategies in cancer gene therapy is the so-called suicide gene therapy, with HSV-tk gene expression followed by GCV administration Citation[26]. The enzyme product of HSV-tk gene can convert the non-toxic antiviral drug, GCV, into a toxic form that interferes with DNA replication and induces apoptotic cell death. As another in vitro model of heat-inducible gene therapy, we generated the heat-inducible HSV-tk gene expression plasmid, pTRE-HSP456/tk (), and the cytotoxic effects of HSV-tk/GCV system on human cancer cell lines were examined.
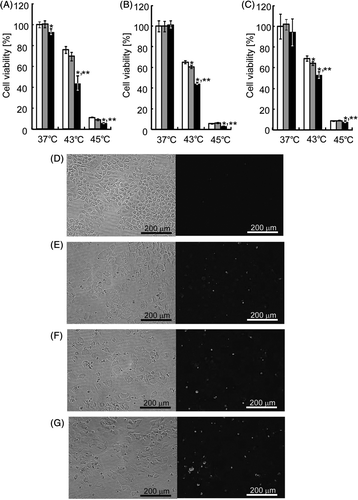
Unlike the TNF-α gene therapy, the cytotoxic effects of HSV-tk gene therapy can be regulated by two factors: the HSV-tk gene expression level and GCV concentration. Three cell lines were transfected with the same amount of pTRE-HSP456/tk (0.8 µg/dish), followed by GCV exposure at different concentrations. One of the criteria in the present study was low cytotoxicity caused by basal expression level of therapeutic gene under culture conditions at 37°C. Also, in order to achieve a good treatment efficacy, another criterion was a significantly greater percentage of cell death under clinically acceptable GCV concentrations (∼5 µg/mL, which is comparable to the standard human dose of 5 mg/kg/day Citation[27]). As shown in , when cells were co-transfected with pTRE-HSP456/tk and pCMV/tTA followed by GCV exposure at 0.1 µg/mL (HeLa and A549 cells) or 1.0 µg/mL (HepG2 cells), no significant (HepG2 and A549) or slight (HeLa) cytotoxicity was observed at 37°C. With heat treatment at 43°C for 1 h, when cells were transfected with pTRE-HSP456/tk alone followed by GCV exposure, the cell viabilities were 69.4 ± 3.9%, 60.9 ± 1.6% and 64.4 ± 1.7% for HeLa, HepG2 and A549 cells, respectively (). The effect of heat-inducible suicide gene therapy driven by the TRE-HSP promoter alone on cell viability of HeLa cells was comparable to native hsp70B′ promoter without TRE sequence (Figure S3). On the other hand, with the heat treatment at 43°C for 1 h, when cells were co-transfected with pTRE-HSP456/tk and pCMV/tTA followed by GCV exposure, the cell viabilities significantly decreased to 43.6 ± 7.1%, 44.6 ± 1.4% and 53.2 ± 3.2% for HeLa, HepG2 and A549 cells, respectively (). With heat treatment at 45°C for 1 h, the cytotoxic effect on the co-transfected cells was significantly enhanced, and the cell viabilities drastically decreased to 8.7 ± 0.8%, 3.3 ± 0.2% and 7.5 ± 1.0% for HeLa, HepG2 and A549 cells, respectively (). To evaluate the mechanism of the decrease in cell viability, TUNEL assays were performed on HeLa cells. As shown in , only few TUNEL-positive cells (1.7 ± 1.5 cells/mm2) were observed in the mock-transfected control cells cultured at 37°C. When the mock-transfected cells were heat-treated at 43°C for 1 h, heat-induced apoptotic cells (35.4 ± 4.6 cells/mm2) were observed (). When HeLa cells were treated with in vitro heat-induced suicide gene therapy using pTRE-HSP456/tk alone, a substantial number (59.1 ± 11.6 cells/mm2) of apoptotic cells was observed (). The greatest number of apoptotic cells (83.3 ± 10.7 cells/mm2) was observed when the cells were treated with in vitro heat-induced suicide gene therapy using the both plasmids (pTRE-HSP456/tk + pCMV/tTA) (). These results suggest that the apoptosis-inducing effect of heat-induced suicide gene therapy was enhanced using the hybrid promoter system.
Figure 6. In vitro cytotoxicity of heat-inducible HSV-tk gene therapy. HeLa (A), HepG2 (B) and A549 (C) cells were transfected with the mock plasmid (white columns), pTRE-HSP456/tk alone (grey columns) or pTRE-HSP456/tk plus pCMV/tTA (black columns). Cells were incubated at 37°C or underwent heat treatment at 43°C or 45°C for 1 h. At 24 h after heat treatment, cell viability was evaluated by the WST-8 method. The data are expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.05 versus the mock plasmid and pTRE-HSP456/tk alone at each temperature, respectively. (D–G) Microscopic observation of HeLa cells after gene delivery and heat treatment. Cells were transfected with the mock plasmid (D and E), pTRE-HSP456/tk alone (F) or pTRE-HSP456/tk plus pCMV/tTA (G), and incubated at 37°C (D) or heated at 43°C for 1 h (E–G). Apoptotic cells were detected by fluorescence microscopy based on the TUNEL assay. (Left) Bright field microscopic images. (Right) Fluorescence microscopic images.
Conclusion
We have developed and characterised a novel heat-inducible transcriptional amplification system, demonstrating that high-level transgene expression mediated by the transactivator is induced by heat treatment without a loss in expression from the hsp70B′ promoter. Such an inducible gene expression system could be potentially altered to include any desired transgene, which is also ideal for gene therapeutic applications. This transcriptional regulation can be combined with localised hyperthermia systems for heat-inducible cancer gene therapy. Local hyperthermia, for example, can be induced by magnetic nanoparticles exposed to an alternating magnetic field (AMF). Since magnetic nanoparticles produce heat under AMF, heat is only generated in the area where the AMF is applied. For cell and gene therapy, Ortner et al. Citation[28] reported magnetic field-controlled gene expression in encapsulated cells, combining the Hsp70 promoter with the magnetite nanoparticle-mediated heating system. Cancer hyperthermia using magnetic nanoparticles has largely been an experimental modality, but in recent years there have been remarkable advances in magnetite nanoparticle-mediated hyperthermia due to the efforts of many researchers Citation[8], Citation[29]. For heat-inducible gene therapy in a hyperthermia system using magnetite nanoparticles, hyperthermia is directly applied to the targeted tumour cells, while also acting as the activator of the gene therapy. In the present study, the Tet-responsive transactivator was employed as the ‘second activator’ for heat-inducible gene therapy, and an enhanced heat-inducible gene expression system was developed. These findings indicate that this strategy may improve the efficacy of cancer gene therapy.
Declaration of interest: This work was supported in part by Grant-in-Aid for Scientific Research on Innovative Areas (24119512) of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT). The authors alone are responsible for the content and writing of the paper.
References
- Walther W, Wendt J, Stein U. Employment of the mdr1 promoter for the chemotherapy-inducible expression of therapeutic genes in cancer gene therapy. Gene Ther 1997; 4: 544–552
- Hallahan DE, Mauceri HJ, Seung LP, Dunphy EJ, Wayne JD, Hanna NN, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med 1995; 1: 786–791
- Blackburn RV, Galoforo SS, Corry PM, Lee YJ. Adenoviral-mediated transfer of a heat-inducible double suicide gene into prostate carcinoma cells. Cancer Res 1998; 58: 1358–1362
- Lee H, Kim S, Choi BH, Park MT, Lee J, Jeong SY, et al. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int J Hyperthermia 2011; 27: 698–707
- Wang H, Li X, Xi X, Hu B, Zhao L, Liao Y, et al. Effects of magnetic induction hyperthermia and radiotherapy alone or combined on a murine 4T1 metastatic breast cancer model. Int J Hyperthermia 2011; 27: 563–572
- Knippertz I, Stein MF, Dörrie J, Schaft N, Müller I, Deinzer A, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011; 27: 591–603
- Walther W, Stein U. Heat-responsive gene expression for gene therapy. Adv Drug Deliv Rev 2009; 61: 641–649
- Ito A, Shinkai M, Honda H, Kobayashi T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther 2001; 8: 649–654
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem 1996; 271: 3355–3358
- Wada K, Taniguchi A, Okano T. Highly sensitive detection of cytotoxicity using a modified HSP70B′ promoter. Biotechnol Bioeng 2007; 97: 871–876
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551
- Kodama D, Nishimiya D, Nishijima K, Okino Y, Inayoshi Y, Kojima Y, et al. Chicken oviduct-specific expression of transgene by a hybrid ovalbumin enhancer and the Tet expression system. J Biosci Bioeng 2012; 113: 146–153
- Kamihira M, Ono K, Esaka K, Nishijima K, Kigaku R, Komatsu H, et al. High-level expression of single-chain Fv-Fc fusion protein in serum and egg white of genetically manipulated chickens by using a retroviral vector. J Virol 2005; 79: 10864–10874
- Huang S, Kawabe Y, Ito A, Kamihira M. Adeno-associated virus Rep-mediated targeting of integrase-defective retroviral vector DNA circles into human chromosome 19. Biochem Biophys Res Commun 2012; 417: 78–83
- Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA 2001; 98: 14595–14600
- Ito A, Shinkai M, Bouhon IA, Honda H, Kobayashi T. Induction of TNF-α gene expression by heat inducible promoter GADD153. Jpn J Hyperthermic Oncol 2000; 16: 91–97
- Wada K, Taniguchi A, Xu L, Okano T. Rapid and highly sensitive detection of cadmium chloride induced cytotoxicity using the HSP70B′ promoter in live cells. Biotechnol Bioeng 2005; 92: 410–415
- McMillen D, Kopell N, Hasty J, Collins JJ. Synchronizing genetic relaxation oscillators by intercell signaling. Proc Natl Acad Sci USA 2002; 99: 679–684
- Tigges M, Dénervaud N, Greber D, Stelling J, Fussenegger M. A synthetic low-frequency mammalian oscillator. Nucleic Acids Res 2010; 38: 2702–2711
- Taniguchi A. Live cell-based sensor cells. Biomaterials, 2010; 31: 5911–5915
- Old LJ. Tumor necrosis factor (TNF). Science 1985; 230: 630–632
- Balkwill F. Tumor necrosis factor and cancer. Nat Rev Cancer 2009; 9: 361–371
- Yamauchi N, Kuriyama H, Watanabe N, Neda H, Maeda M, Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res 1989; 49: 1671–1675
- Madamanchi NR, Li S, Patterson C, Runge MS. Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler Thromb Vasc Biol 2001; 21: 321–326
- Ito A, Shinkai M, Bouhon IA, Honda H, Kobayashi T. Bystander-killing effect and cyclic induction of TNF-alpha gene under heat-inducible promoter gadd153. J Biosci Bioeng 2000; 90: 437–441
- Fillat C, Carrió M, Cascante A, Sangro B. Suicide gene therapy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: Fifteen years of application. Curr Gene Ther 2003; 3: 13–26
- Loimas S, Toppinen MR, Visakorpi T, Jänne J, Wahlfors J. Human prostate carcinoma cells as targets for herpes simplex virus thymidine kinase-mediated suicide gene therapy. Cancer Gene Ther 2001; 8: 137–144
- Ortner V, Kaspar C, Halter C, Töllner L, Mykhaylyk O, Walzer J, et al. Magnetic field-controlled gene expression in encapsulated cells. J Control Release 2012; 158: 424–432
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 2005; 100: 1–11
