Abstract
Purpose: The aim of this study was to evaluate inhibitory effects of L-ascorbic acid-2-O-phosphate-Na2 (APS), a pro-vitamin C, combined with hyperthermia on adipogenic differentiation of mouse stromal cells, OP9.
Materials and methods: OP9 preadipocytes were differentiated with serum replacement, administered with APS, and simultaneously treated with hyperthermia using a capacitive-resistive electric transfer (CRet) apparatus, which was conducted repeatedly twice a day. After 2 days, intracellular lipid droplets were stained with Oil Red O, then observed by microscopy and assessed spectrophotometrically.
Results: After stimulation by serum replacement for 2 days, lipid droplets were accumulated surrounding nucleus of OP9 cells. When APS of 0.15–0.6 mM was administered without hyperthermia, the amount of lipid droplets was markedly suppressed to 50.5%∼−11.3% versus the undifferentiated control, and diminished huge aggregates of lipid droplets. In OP9 cells treated by hyperthermia at 42°C for 0.5 min, 1 min or 3 min in the absence of APS, adipogenesis was suppressed abruptly in a time-dependent manner to 95.4%, 18.7% or −5.5%, respectively. Whereas, the percentage of adipogenesis was 96.8% in OP9 cells treated by mild hyperthermia alone at 41°C for 1 min. The simultaneous application of APS and hyperthermia at 41°C for 1 min markedly suppressed the accumulation of lipid droplets to 25.7%∼−66.2%. By scanning electron microscopy (SEM) observation, the surface of OP9 cells treated with APS and hyperthermia appeared to have the morphological property of undifferentiated OP9 cells.
Conclusion: Combined treatment of APS and mild hyperthermia suppresses adipogenesis in OP9 cells, particularly in lipid droplets accumulation during spontaneous differentiation of OP9 preadipocytes.
Introduction
For the basis of therapies against obesity-associated metabolic syndrome, physiological conditions with oxidative stress are considered as a crucial cause of adipogenesis Citation[1], Citation[2]. Recent investigations have indicated that reactive oxygen species (ROS) are increased during adipocyte differentiation, whereas antioxidants such as coenzyme Q, synthesised intrinsically, inhibit it Citation[3], Citation[4]. It is also indicated that NADPH oxidase is the major source for ROS in adipocytes, and that augmented NADPH oxidase seems to contribute to increased ROS production in adipose tissue in obese mice Citation[5]. And ROS levels are an important trigger for insulin resistance in obese insulin-resistant mice Citation[6]. Accordingly, we have examined whether antioxidants inhibit intracellular adipogenesis. We have shown on mouse stromal preadipocytes OP9 that intracellular ROS increased paralleling with lipid droplet accumulation along with differentiation of OP9 cells, but the antioxidative nano-material, poly-hydroxylated fullerene or squalane-dissolved fullerene significantly suppressed intracellular lipid accumulation together with repression of intracellular ROS and the transcriptional factor PPARγ2 expression Citation[7–10].
Concerning ROS production and hyperthermia, it is reported by Medina-Navarro and Guerrero-Linares that whole-body hyperthermia at 42°C reduces oxidative stress produced by a metabolic toxin 3-NP in the striatum of rats Citation[11]. The combined application of hyperthermia at 43–44°C and oxidative stress of tert-butyl hydroperoxides, for example, induces cell death in cancer cells Citation[12]. Although in vivo hyperthermia at 42–43°C increases production of ROS, especially hydroxyl radicals and superoxide anions, lowers enzymatic antioxidant defences and causes subsequent damage, pretreatment with antioxidants (e.g. mannitol or α-tocopherol) is able to prevent oxidative damage, resulting in increased survival time Citation[13]. Sakamoto et al. suggest in vivo that heat stress by hyperthermia at 35°C for 12 h enhances physiological oxidative stress, but α-tocopherol acetate administration alleviates the hyperthermia-induced death of pre-implanted embryos by reducing physiological oxidative stress Citation[14]; and ‘mild hyperthermia’ has been studied extensively in recent years Citation[15–17]. It is reported that mild hyperthermia at 40–45°C is suitable for applications in drug delivery when combined with radiotherapy and chemotherapy on solid tumours Citation[18]. Our previous study suggested that 6-O-palmitoyl-L-ascorbic acid, a lipophilic derivative of L-ascorbic acid, could easily permeate into human squamous carcinoma HSC-4 cells, and hyperthermia could accelerate its oxidation to ascorbyl radicals, which resulted in anti-proliferative effect and cellular damage on HSC-4 cells Citation[19]. Meanwhile, in the present study we hypothesised that the redox state control by antioxidant and the mild hyperthermia at 41–42°C in adipocytes is a potentially effective strategy against lipid accumulation preceded by adipogenic differentiation. We tested the anti-adipogenic activity of the hydrophilic auto-oxidation-resistant type of vitamin C (pro-vitamin C) Citation[20–22], L-ascorbic acid-2-O-phosphate-Na2 (APS) and electrical hyperthermia in OP9 cells. OP9 cells, a line of bone marrow-derived mouse stromal cells, are a useful model for adipogenesis due to rapid differentiation into adipocytes Citation[23]. The amount of intracellular lipid droplets was determined with Oil Red O stain and observed by microscopy.
Materials and methods
Cell culture
OP9 cells, a line of bone marrow-derived mouse stromal cells were obtained from Riken BioResource Center (RCB1124; Ibaraki, Japan). OP9 cells were cultured in MEM-α medium (Nissui Pharmaceutical, Tokyo) supplemented with 20% heat-inactivated foetal bovine serum (FBS) (Biological Industries, Kibbutz Beit-Haemek, Israel) and 2 mmol L-glutamine (Wako Pure Chemical Industries, Osaka, Japan) at 37°C in 95% humidified air and 5% CO2. OP9 preadipocytes were cultured at a low cell density to adopt a spindly morphology and differentiate into adipocytes poorly without any treatments.
The differentiation of OP9 preadipocytes and combined treatment of APS and hyperthermia
OP9 preadipocytes were stimulated to differentiate to adipocytes by serum replacement containing insulin of 1.7 µmol as a final concentration Citation[8], Citation[9], Citation[21], and Oil Red O staining was employed to determine the amount of intracellular lipid droplets Citation[9], Citation[24–26]. OP9 preadipocytes of 3.0 × 104 cells were seeded in each well of a 24-well plate (Becton Dickinson, Sparks, MD, USA) and grown to confluence for 48 h at 37°C in 95% humidified air and 5% CO2. Then OP9 preadipocytes were differentiated to adipocytes in serum replacement supplemented medium: MEM-α with 15% KnockOut™ SR (Life Technologies, Tokyo) and simultaneously treated with APS (Wako) of 0.15–0.6 mM and/or hyperthermia at 41°C for 1 min. This combined treatment of APS and hyperthermia in conditioned medium supplemented with 15% serum replacement was conducted repeatedly twice a day at intervals of 6 h and 18 h in sequence. To examine the time-dependency, hyperthermia at 42°C for 0.5–3 min was conducted. After 2 days, intracellular lipid droplets in OP9 cells were stained with Oil Red O, then observed using a Hoffman modulation contrast microscope (ECLIPSE Ti, Nikon, Tokyo), and assessed spectrophotometrically. Then surface ultrastructure of OP9 cells was observed using a scanning electron microscope (VE-9800, Keyence, Osaka). Results were expressed as ‘a percentage of adipogenesis’ which is calculated from Abs530 nm:
Electrical hyperthermia treatment with a capacitive-resistive electric transfer system
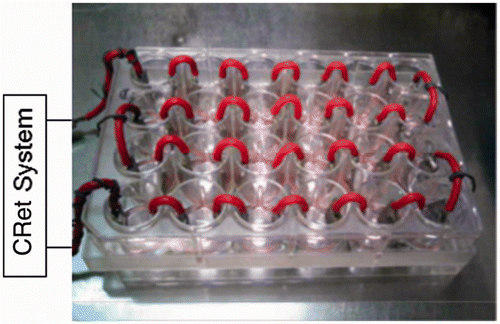
We applied hyperthermia treatment with a capacitive-resistive electric transfer (CRet) system which can produce the electric stimulus of 0.45 MHz and thermal effects synergistically in tissues Citation[27]. The exposure to CRet electric currents was carried out through pairs of stainless steel electrodes designated ad hoc for in vitro stimulation () as previously described Citation[19]. The electrodes were inserted in each well, and connected in series to a CRet signal generator (INDIBA D.H.-308, Barcelona, Spain). The signal parameters of the CRet system were monitored throughout exposure. Temperature of the medium increased to 41°C approximately for 1 min and could be kept for an established period.
Oil Red O staining
OP9 cells in 24-well plates were washed twice with phosphate-buffered saline (Nissui, Tokyo), fixed for 30 min with 4% paraformaldehyde (pH 7.4, Wako), then stained for 30 min with 0.25% Oil Red O (Wako) in 60% aqueous 2-propanol (Wako). After being washed with distilled water, OP9 cells were stained with Mayer's haemalum solution (Wako) for 10 min. Thereafter, Oil red O dye retained in the cell was quantified by elution into 80% 2-propanol, and absorbance at 530 nm was measured with a microplate reader (FLUOstar OPTIMA, BMG Labtech, Offenburg, Germany).
Morphological observation with a scanning electron microscope
OP9 cells were prefixed by immersion in 1% paraformaldehyde/1% glutaraldehyde (Wako) in 0.1 M phosphate buffer solution (pH 7.4, Wako) for 24 h at room temperature and post-fixed in 1% osmium tetraoxide (OsO4, Wako) in 0.1 M phosphate buffer solution (pH 7.2) for 1 h at 4°C. Then, specimens were treated in a graded series of ethylene glycol (Wako) and tert-butyl alcohol (Wako), dried in a vacuum-freeze dryer (ES-3020, Hitachi High-technologies, Tokyo), and gold-coated using an ion coater (IB-2, EIKO Engineering, Ibaraki, Japan), then the surface ultrastructure of OP9 cells was observed with a SEM (VE-9800, Keyence, Osaka).
Statistical analysis
Results of the experiments performed in triplicate were expressed as means ± SD, for n = 3, and evaluated by the student's t-test. The values of p < 0.05 were regarded as being statistically significant.
Results
Inhibitory effects of APS and hyperthermia on adipogenetic differentiation of OP9 cells according to Oil Red O staining and cell morphology
The effects of APS and hyperthermia using a CRet system on adipogenetic differentiation of OP9 cells were evaluated with Oil Red O staining. After the stimulation by serum replacement, OP9 preadipocytes initiated differentiation, and lipid droplets became abundant in differentiated adipocytes. The absorbances at 530 nm of differentiated adipocytes were higher than the undifferentiated control by 12.5–19.3% ( and ), which was consistent with the appearance of the formed lipid droplets accumulated in OP9 cells ( and ). Lipid droplets were generated in OP9 cells surrounding the nucleus (). The treatment with APS and hyperthermia decreased accumulation of intracellular lipid droplets (). The hyperthermia significantly reduced the amount of lipid droplets, that is, percentage of adipogenesis (percentage of the positive control) in OP9 cells treated with hyperthermia at 42°C for 0.5 min, 1 min or 3 min was suppressed abruptly in a time-dependent manner to 95.4%, 18.7% or −5.5%, respectively (). In contrast, when OP9 cells were exposed to mild hyperthermia at 41°C for 1 min, the percentage of adipogenesis was 96.8% (). APS also significantly reduced lipid accumulation. When APS of 0.15–0.6 mM was administered in the medium, the accumulation of lipid droplets was markedly suppressed to 50.5%∼−11.3% versus that of the differentiated control (). And the simultaneous combined treatment of APS and hyperthermia at 41°C for 1 min suppressed the accumulation of lipid droplets to 25.7%∼−66.2% (), together with diminished huge aggregates of lipid droplets, and size and number of lipid droplets were decreased ( and ). Likewise, it was observed by SEM that a thick mat of lipid droplets existed in differentiated OP9 cells, but they were appreciably suppressed in OP9 cells treated with APS of 0.6 mM or simultaneous application of APS of 0.6 mM and hyperthermia at 41°C for 1 min, indicating morphological property of undifferentiated OP9 cells ().
Figure 2. Inhibitory effects of hyperthermia at 42°C using a CRet system on intracellular lipid accumulation in OP9 mouse stromal cells as assessed spectrophotometrically by Oil Red O stain and Hoffman modulation contrast microscopy. A: OP9 cells were induced to differentiation and exposed to hyperthermia at 42°C for 0 min, 0.5 min, 1 min, or 3 min using a CRet system. Then intracellular lipid droplets and nucleus were stained with Oil Red O and Mayer's hemalum solution, respectively. The absorbances of extracts from intracellular lipid droplets stained with Oil Red O were measured at 530 nm and evaluated as % of the positive control which was differentiated but did not undergo the hyperthermia. % of adipogenesis = [(Abs530 nm of test − Abs530 nm of undifferentiated control)/(Abs530 nm of differentiated adipocyte control − Abs530 nm of undifferentiated control)] × 100. Mean ± SD, n = 3, *p < 0.05 (vs. 0 min). B: Scale bars = 50 µm, magnification: ×200.
![Figure 2. Inhibitory effects of hyperthermia at 42°C using a CRet system on intracellular lipid accumulation in OP9 mouse stromal cells as assessed spectrophotometrically by Oil Red O stain and Hoffman modulation contrast microscopy. A: OP9 cells were induced to differentiation and exposed to hyperthermia at 42°C for 0 min, 0.5 min, 1 min, or 3 min using a CRet system. Then intracellular lipid droplets and nucleus were stained with Oil Red O and Mayer's hemalum solution, respectively. The absorbances of extracts from intracellular lipid droplets stained with Oil Red O were measured at 530 nm and evaluated as % of the positive control which was differentiated but did not undergo the hyperthermia. % of adipogenesis = [(Abs530 nm of test − Abs530 nm of undifferentiated control)/(Abs530 nm of differentiated adipocyte control − Abs530 nm of undifferentiated control)] × 100. Mean ± SD, n = 3, *p < 0.05 (vs. 0 min). B: Scale bars = 50 µm, magnification: ×200.](/cms/asset/10802769-cebd-4136-b2eb-efe62acf482e/ihyt_a_750016_f0002_b.gif)
Figure 3. Inhibitory effects of ascorbic acid-2-O-phosphate-Na2 (APS) in combined application of hyperthermia at 41°C for 1 min on intracellular lipid accumulation in OP9 cells as measured spectrophotometrically for Oil Red O stain. OP9 cells were induced to differentiation and exposed to treatment with APS and hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] or sham-manipulated [CRet (−)] then treated as described in . Mean ± SD, n = 3, *p < 0.05 (vs. 0 mM of sham-manipulated), #p < 0.05 (vs. each dose of sham-manipulated).
![Figure 3. Inhibitory effects of ascorbic acid-2-O-phosphate-Na2 (APS) in combined application of hyperthermia at 41°C for 1 min on intracellular lipid accumulation in OP9 cells as measured spectrophotometrically for Oil Red O stain. OP9 cells were induced to differentiation and exposed to treatment with APS and hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] or sham-manipulated [CRet (−)] then treated as described in Figure 3. Mean ± SD, n = 3, *p < 0.05 (vs. 0 mM of sham-manipulated), #p < 0.05 (vs. each dose of sham-manipulated).](/cms/asset/0d22d055-cdbe-4168-8183-942701cf2d37/ihyt_a_750016_f0003_b.gif)
Figure 4. Inhibitory effects of APS in combined application of hyperthermia at 41°C for 1 min on intracellular lipid accumulation in OP9 cells as observed using a Hoffman modulation contrast microscope. OP9 cells were treated as described in . Scale bars = 250 µm, magnification: ×40.
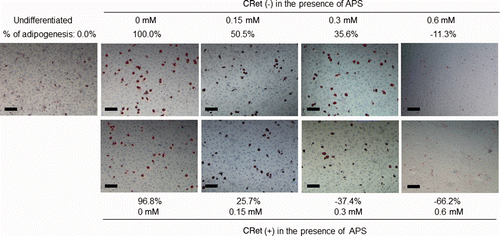
Figure 5. Inhibition of intracellular lipid accumulation by APS in combined application of hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] in OP9 cells with comparing to differentiated control of sham-manipulated [CRet (−)] as observed using a Hoffman modulation contrast microscope. OP9 cells were treated as described in . Scale bars = 50 µm, magnification: ×200.
![Figure 5. Inhibition of intracellular lipid accumulation by APS in combined application of hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] in OP9 cells with comparing to differentiated control of sham-manipulated [CRet (−)] as observed using a Hoffman modulation contrast microscope. OP9 cells were treated as described in Figure 3. Scale bars = 50 µm, magnification: ×200.](/cms/asset/139923e4-d3da-4102-8ee6-acd07ebbaa05/ihyt_a_750016_f0005_b.gif)
Figure 6. Inhibition of intracellular lipid accumulation by APS in combined application of hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] in OP9 cells with comparing to differentiated control of sham-manipulated [CRet (−)] as observed using a Hoffman modulation contrast microscope. OP9 cells were treated as described in . Scale bars = 10 µm, magnification: ×200.
![Figure 6. Inhibition of intracellular lipid accumulation by APS in combined application of hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] in OP9 cells with comparing to differentiated control of sham-manipulated [CRet (−)] as observed using a Hoffman modulation contrast microscope. OP9 cells were treated as described in Figure 3. Scale bars = 10 µm, magnification: ×200.](/cms/asset/59618443-30a9-4493-a7f0-450f9328baa7/ihyt_a_750016_f0006_b.gif)
Figure 7. Surface ultrastructure of OP9 cells treated with 0.6 mM APS combined with hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] or sham-manipulated [CRet (−)], which was observed using a scanning electron microscope. OP9 cells were treated as described in . Scale bars = 10 µm, magnification: ×1500.
![Figure 7. Surface ultrastructure of OP9 cells treated with 0.6 mM APS combined with hyperthermia at 41°C for 1 min using a CRet system [CRet (+)] or sham-manipulated [CRet (−)], which was observed using a scanning electron microscope. OP9 cells were treated as described in Figure 3. Scale bars = 10 µm, magnification: ×1500.](/cms/asset/37eae344-9608-4782-8f2b-c7b5339a2db8/ihyt_a_750016_f0007_b.gif)
Discussion
We examined the inhibitory effect of combined application of APS, a stabilised derivative of L-ascorbic acid, and hyperthermia on adipogenic differentiation of mouse stromal cells, OP9. Post-confluent OP9 preadipocytes were stimulated to differentiate by serum replacement and were exposed to APS and hyperthermia at 41°C for 1 min at the interval of twice a day for two days. Although preliminarily differentiated OP9 cells were not tested, our results indicate that the addition of 0.15–0.6 mM APS inhibited adipogenesis in OP9 cells in a dose-dependent manner (). L-Ascorbic acid that was esterolytically supplied from APS is considered to contribute to the formation of an antioxidative micro-environment around preadipocytes, which is unsuitable for adipogenic-differentiation to adipocytes.
It was found that OP9 cells initiated adipogenic differentiation when grown to confluence Citation[21], and proliferating adipocytes will not accumulate lipid droplets in their cytoplasm even in the presence of hormonal stimulation Citation[3], Citation[28]. Lee et al. Citation[2] demonstrated that preadipocytes treated with antioxidants delayed the progression of the cell cycle, indicating that ROS production is specifically required for progression of the cell cycle. It is also reported that adipocytes are thermo-sensitive, and cell viability dropped drastically with 1 min of hyperthermia at 45–50°C Citation[29]. In accordance with these findings, our results indicate that the exposure of OP9 cells to APS and hyperthermia causes lower levels of intracellular adipogenesis and differentiation (). Cell-growth inhibition was observed in the dose of 0.6 mM APS, which could be attributed to ascorbyl radical generation enhanced by hyperthermia Citation[19], suggesting that a higher dose of APS is undesirable for combined application with hyperthermia (). Hyperthermia at 42°C for 0.5–3 min for OP9 cells drastically decreased accumulation of lipid droplets in a time-dependent manner () in contrast to almost unaltered adipogenesis for 41°C for 1 min, demonstrating a marked difference between 41°C and 42°C; clinical application of hyperthermia for anti-adipogenesis may be preferable at 41°C using a CRet apparatus. We know the accumulation of fat is not necessarily harmful for our health and avoidance of the adipocyte differentiation should theoretically promote the fat deposition in strange tissues or so-called fat redistribution and produce insulin resistance Citation[30–32].
Conventional hyperthermic apparatuses increase the skin surface temperature higher than required, leading to the of application of heat-absorptive implements to prevent skin burn, whereas a CRet apparatus enables realisation of the established temperature at the target depth of the skin located on preadipocytes and adipocyte, without skin-burn-preventing implements. Concerning the mechanism of hyperthermia, it is reported that induction of heat shock protein Hsp72 by mild electrical hyperthermia improves metabolic profiles such as inflammation and insulin resistance in diabetic model mice Citation[33], Citation[34]. Though obesity is a well-known health risk factor for diabetes, our results might be attributed to synergistic effects due to both antioxidant activity of APS against intracellular ROS and metabolic improvement by mild electrical hyperthermia along with adipogenic differentiation. The elucidation of signalling pathways and factors in adipogenic differentiation of OP9 cells when treated with APS and hyperthermia should be investigated in future studies.
Declaration of interest: We thank INDIBA-Japan, Tokyo, for technical assistance. The authors alone are responsible for the content and writing of the paper.
References
- Puri N, Sodhi K, Haarstad M, Kim DH, Bohinc S, Foglio E, et al. Heme induced oxidative stress attenuates sirtuin 1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J Cell Biochem 2012; 113: 1926–1935
- Lee OH, Seo MJ, Choi HS, Lee BY. Pycnogenol® inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother Res 2012; 26: 403–411
- Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009; 284: 10601–10609
- Bour S, Carmona MC, Galinier A, Caspar-Bauguil S, Van Gaal L, Staels B, et al. Coenzyme Q as an antiadipogenic factor. Antioxid Redox Signal 2011; 14: 403–413
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944–948
- Saitoh Y, Xiao L, Mizuno H, Kato S, Aoshima H, Taira H, et al. Novel polyhydroxylated fullerene suppresses intracellular oxidative stress together with repression of intracellular lipid accumulation during the differentiation of OP9 preadipocytes into adipocytes. Free Radic Res 2010; 44: 1072–1081
- Xiao L, Aoshima H, Saitoh Y, Miwa N. The effect of squalane-dissolved fullerene-C60 on adipogenesis-accompanied oxidative stress and macrophage activation in a preadipocyte-monocyte co-culture system. Biomaterials 2010; 31: 5976–5985
- Xiao L, Aoshima H, Saitoh Y, Miwa N. Highly hydroxylated fullerene localizes at the cytoskeleton and inhibits oxidative stress in adipocytes and a subcutaneous adipose-tissue equivalent. Free Radic Biol Med 2011; 51: 1376–1389
- Saitoh Y, Mizuno H, Xiao L, Hyoudou S, Kokubo K, Miwa N. Polyhydroxylated fullerene C60(OH)44 suppresses intracellular lipid accumulation together with repression of intracellular superoxide anion radicals and subsequent PPARγ2 expression during spontaneous differentiation of OP9 preadipocytes into adipocytes. Mol Cell Biochem 2012; 366: 191–200
- Medina-Navarro R, Guerrero-Linares I. Whole body hyperthermia reduces oxidative stress in the striatum of rats in an animal model of mitochondrial toxicity with 3-nitropropionic acid. Int J Hyperthermia 2009; 25: 280–288
- Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol Int 2008; 32: 715–723
- Chang CK, Chang CP, Liu SY, Lin MT. Oxidative stress and ischemic injuries in heat stroke. Prog Brain Res 2007; 162: 525–546
- Sakamoto N, Ozawa M, Yokotani-Tomita K, Morimoto A, Matsuzuka T, Ijiri D, et al. DL-alpha-tocopherol acetate mitigates maternal hyperthermia-induced pre-implantation embryonic death accompanied by a reduction of physiological oxidative stress in mice. Reproduction 2008; 135: 489–496
- Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-γ-production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012; 28: 9–18
- Shah NG, Tulapurkar ME, Damarla M, Singh IS, Goldblum SE, Shapiro P, et al. Febrile-range hyperthermia augments reversible TNF-α-induced hyperpermeability in human microvascular lung endothelial cells. Int J Hyperthermia 2012; 28: 627–635
- Knippertz I, Stein MF, Dörrie J, Schaft N, Müller I, Deinzer A, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011; 27: 591–603
- Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012; 28: 320–336
- Kato S, Asada R, Kageyama K, Saitoh Y, Miwa N. Anticancer effects of 6-O-palmitoyl ascorbate combined with a capacitive-resistive electric transfer hyperthermic apparatus as compared with ascorbate in relation to ascorbyl radical generation. Cytotechnology 2011; 63: 425–435
- Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: Characterization of a useful new model of adipogenesis. J Lipid Res 2006; 47: 450–460
- Eguchi M, Miyazaki T, Masatsuji-Kato E, Tsuzuki T, Oribe T, Miwa N. Cytoprotection against ischemia-induced DNA cleavages and cell injuries in the rat liver by pro-vitamin C via hydrolytic conversion into ascorbate. Mol Cell Biochem 2003; 252: 17–23
- Eguchi M, Monden K, Miwa N. Role of MAPK phosphorylation in cytoprotection by pro-vitamin C against oxidative stress-induced injuries in cultured cardiomyoblasts and perfused rat heart. J Cell Biochem 2003; 90: 219–226
- Fujiwara M, Nagao M, Monden K, Misumi M, Kageyama K, Yamamoto K, et al. Enhanced protection against peroxidation-induced mortality of aortic endothelial cells by ascorbic acid-2-O-phosphate abundantly accumulated in the cell as the dephosphorylated form. Free Radic Res 1997; 27: 97–104
- Greenberger JS. Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro 1979; 15: 823–828
- Hausman GJ. Techniques for studying adipocytes. Stain Technol 1981; 56: 149–154
- Dave S, Kaur NJ, Nanduri R, Dkhar HK, Kumar A, Gupta P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS One 2012; 7: e30831
- Hernández-Bule ML, Trillo MA, Cid MA, Leal J, Ubeda A. In vitro exposure to 0.57-MHz electric currents exerts cytostatic effects in HepG2 human hepatocarcinoma cells. Int J Oncol 2007; 30: 583–592
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 2000; 16: 145–171
- Franco W, Kothare A, Ronan SJ, Grekin RC, McCalmont TH. Hyperthermic injury to adipocyte cells by selective heating of subcutaneous fat with a novel radiofrequency device: Feasibility studies. Lasers Surg Med 2010; 42: 361–370
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78: 783–809
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 2001; 104: 531–543
- Chandalia M, Davila H, Pan W, Szuszkiewicz M, Tuvdendorj D, Livingston EH, et al. Adipose tissue dysfunction in humans: A potential role for the transmembrane protein ENPP1. J Clin Endocrinol Metab 2012 (Epub ahead of print)
- Kondo T, Sasaki K, Matsuyama R, Morino-Koga S, Adachi H, Suico MA, et al. Hyperthermia with mild electrical stimulation protects pancreatic β-cells from cell stresses and apoptosis. Diabetes 2012; 61: 838–847
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HspP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 2008; 105: 1739–1744