Abstract
Purpose: Evaluation of the effectiveness of highly focalised thermotherapy (HFT) in a melanoma mouse model, using a ferrimagnetic cement (FC) and repeated low hyperthermia treatments.
Materials and methods: A melanoma mouse model was induced with B16F10 cells in C57BL6 mice. The FC, injected into the tumour, was used as the magnetic vehicle for HFT. FC location within the tumour was assessed by radiography and its capability to generate heat, when exposed to an external high frequency magnetic field (HFMF), monitored by thermal camera. The HFT treatment consisted of three HFMF exposures, with 48-h intervals, each one lasting 30 min, with a 5–6°C tumour temperature increase. At the end of the experiment, FC samples were characterised by scanning electron microscopy (SEM) and energy dispersion spectroscopy (EDS). The presence of iron contents was analysed in the tumour, lungs, liver and spleen. Histological evaluation and immunohistochemical staining for caspase-3 were performed. Tumour growth was monitored during the experiment.
Results: Surface analysis showed FC stabilisation within the tumour, and iron was absent. The thermal camera confirmed the localised temperature increase in the tumour. HFT treatments inhibited the tumour growth by ∼70% compared to controls. This was due to cell destruction by necrosis and apoptosis.
Conclusions: The HFT, using the FC, proved to be a minimally invasive technique that statistically inhibited tumour growth. Results suggested that this methodology seems to be a promising technique for the treatment of solid tumours, allowing repeated low hyperthermia treatments, which can be easier and less traumatic than other hyperthermia techniques.
Introduction
Hyperthermia is a widely studied method in cancer treatment, operating in the tumour microenvironment and neovascularisation. Its effects may be decisive in tumour cell destruction, and may induce an immunological and apoptotic response in the whole organism Citation[1–6]. The temperature increase of the tumour tissues refers to values between 41°C and 46°C. Higher temperatures, up to 56°C, produce the ‘thermo-ablation’, yielding widespread necrosis, coagulation or carbonisation (depending on the temperature) Citation[7]. It has been reported that the cytotoxic effect of mild temperature hyperthermia (MTH) alone, around 41°C, is decreased due to the development of chronic thermotolerance Citation[8]. Nevertheless, this modality has recently drawn attention in cancer therapy due to findings that most human tumour cell lines are more sensitive to MTH than rodent cells Citation[9], Citation[10], Citation[11]. In addition to its direct cytotoxic effects, MTH is also reported to provide a number of other clinical advantages, including immunostimulatory effects against tumours, improvement of oxygenation, and positive effects on drug delivery via enlargement of vessel pore size in tumour tissue Citation[12], Citation[13], Citation[14]. O’Neill et al. Citation[15] in 1998 found statistically significant levels of apoptosis at a hyperthermia temperature of 41°C, peaking at 43°C, and rapidly dropping with temperature augmentation to 45°C and beyond. Virtually no apoptosis was detectable at or above 46°C, the mode of cell death becoming nearly 100% necrotic. They suggest that there is a narrow apoptotic temperature window between 41° and 45°C Citation[15].
The methods currently available to produce hyperthermia are generally limited by the inability to selectively target the tumour cells, with the subsequent risk of affecting adjacent healthy tissues Citation[16], Citation[17]. A variety of methodologies aim at the localisation and restriction of heat in the tumour, without damaging the healthy neighbouring tissues Citation[18], Citation[19], Citation[20]. To overcome this limitation, and in the context of the magnetically induced hyperthermia Citation[18], Citation[21–24], the highly focalised thermotherapy (HFT) technique was developed. This methodology is based on magnetic mediated hyperthermia (MMH) that consists of the insertion of magnetic particles within the tumour tissue and subsequent exposure to an external high frequency magnetic field (HFMF), which will heat the material's magnetic particles and subsequently the tumour cells. This action causes a localised increase of the temperature due to hysteresis loss, Néel relaxation, or induced eddy currents. The generated heat is then conducted into the surrounding cancerous tissue Citation[18]. There are many approaches to finding the most effective vehicle to place the magnetic particle into the tumour, such as intravascular infusion Citation[25], Citation[26], magnetic fluids Citation[21], Citation[27], Citation[28], magnetite cationic liposomes Citation[29], Citation[30], Citation[31], needle-type magnetite Citation[22], Citation[32], thermosensitive ferromagnetic particles injected in phosphate-buffered saline Citation[33], ferromagnetic ceramics Citation[34], Citation[35], calcium zinc iron silicon oxide composite Citation[36] and polymethylmethacrylate (PMMA)-based bone cements Citation[37].
Considering this, we have previously developed and characterised a ferrimagnetic cement (FC), composed of a silicate cement and magnetite Citation[38]. Assessment of the biological response through the intramuscular implantation in a rat model revealed a transient local inflammatory reaction similar to the control, which gradually decrease over the implantation time (9 weeks). The in vitro studies showed that FC can be injected directly into the tumour and has the ability to generate heat when placed within a magnetic field. This study also showed that the developed FC remained in the tumour after injection, for long enough to perform repeated hyperthermia treatments Citation[38]. In addition, the FC presented bioactive behaviour, reflected by the quick formation of a calcium phosphate surface layer following immersion in a modified simulated body fluid (SBF). This finding suggests that FC has the ability to form a CaP-containing layer when in contact with biological fluids, which is a relevant feature, as the presence of this layer at the cement/tumour interface contributes to the stabilisation of the FC within the tumour, preventing its clearance out of the tumour.
The use of a material that can carry the magnetic particles, be applied by injection into the tumour and remains locally, will prevent the damage of other tissues and allow the entire body to be exposed to a magnetic field. The possibility of repeated HFMF exposures without the need of new injections is also desirable. This is based on Yanase et al.'s studies Citation[39], which reported the effectiveness of multiple HFMF exposures in attaining tumour regression. The goal of the HTF technique using FC is to destroy the tumour in a minor invasive manner, with minimal discomfort to the patient. In this context, the aim of the present study was to evaluate the effectiveness of the HFT using FC in the treatment of a melanoma mouse model by repeated treatments of low hyperthermia.
Materials and methods
Ferrimagnetic cement
FC is a fine powder system, which has a chemical composition in percentage weight, based on oxides, corresponding to 10SiO2, 2Al2O3, 52Fe2O3, 0.6MgO, 33CaO, 2.4(SiO3 + K2O), with a maximum particle size of 5 µm. It has a bioactive behaviour when immersed in a SBF solution and, based on the in vivo studies it can be considered a biocompatible material Citation[38].
The powder/water ratio to obtain the FC paste is 5:1 that, with firm pressure (1 kgf), results in an injectability value corresponding to 90% ± 1% of the total FC paste volume, within 5 min after mixing Citation[38].
Animals and cell line
Inbred female C57BL6 mice 8–10 weeks old and weighing 20–25 g were purchased from the Animal Laboratory at the Institute of Molecular and Cellular Biology (IBMC, Oporto, Portugal). The animals were maintained under conventional conditions (12 h of light per day, a standard laboratory diet and tap water ad libitum). The experiments were carried out according to NP EN ISO 10993-2:2000 guidelines Citation[40], taking into account the animals’ welfare conditions and minimising the number of laboratory animals used.
The B16F10 melanoma cell line (ATCC) was maintained in standard culture conditions, i.e. α-minimal essential medium (α-MEM) containing 10% foetal bovine serum, 2.5 mg mL−1 fungizone, penicillin-streptomycin (100 IU mL−1 and 2.5 µg mL−1, respectively), and incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
Tumour induction
The experimental tumours were induced by subcutaneous inoculation of melanoma cells (2 × 105 cells/80 µL culture medium) in the animals’ dorsal lumbosacral region. Tumours grew freely until they reached approximately 10 mm in diameter (corresponding to ≈520 mm3 in volume), which was observed 15 days after inoculation.
This model was selected because melanoma is a superficial tumour, allowing the easy injection of the cement, the monitoring of the tumour growth rate and the tumour temperature during HFT treatments.
HFT treatment
The animals with the induced tumours were divided into three groups, two control groups and a treatment group. In control group I (n = 5), tumours grew freely, without any treatment. In control group II (n = 5), tumours were injected with FC without being exposed to HFMF. In the treatment group (n = 10), tumours were injected with FC, and 48 h later the animals were exposed to HFMF (frequency 10 kHz) created by a vertical coil (diameter 110 mm, 12 turns), using an induction system high frequency electronic furnace K10/RV (CALAMARI, Milan, Italy). The treatment group and control group II animals were injected with FC in the tumour centre (16-gauge needle), with as much FC as each tumour allowed (between 0.1 mL and 0.2 mL). With this protocol, the intratumoral pressure did not cause the tumours to rupture upon injection. FC distribution within the tumour (group II and treatment group) was assessed by radiography (Trophy CCX digital computer controlled X-ray). To overcome the limitation of a two-dimensional (2D) image to document the distribution of the FC, when the FC localisation was not clear, frontal and lateral images of the tumour were made.
Animals were anaesthetised with a ketamine/xylazine solution before being submitted to HFMF. The treatment consisted of three exposures of the animal model to HFMF, each one for 30 min, at 48-h intervals. Afterwards, animals were maintained under the conditions described above for a further 6 days, when the first animal died (an animal from control group II).
In pilot studies, another control group with the tumour (but without the cement) was exposed to HFMF. The temperature in the tumour did not increase, so this group was not included in the final study, thus reducing the number of animals used.
Temperature monitoring
The animal body temperature was monitored through a thermal camera, FLIR A325 (Wilsonville, OR, USA), and analysed using the software ThermaCAM™ Researcher Professional 2.9 (FLIR). The control of the HFMF strength was manually adjusted, so that the desired temperature of the tumour was kept constant. To allow a more accurate body temperature measurement using thermography, the fur that covers the animal's body was removed in the tumour area. The camera colour palette range was set from 19.5° to 43°C, and the area to be evaluated, defined as a circle within the software, logged the maximum value. The temperature variation on the surface obtained by the 2D infrared surface image was expected to correspond to a similar temperature change throughout the tumour. Because of the high tumour vascularisation, the heat increase in a tumour point was expected to be rapidly dissipated through the tumour tissues.
Tumour growth
After FC injection, the tumour growth of each mouse was monitored every 2 days, for 12 days, by 2D measurements of individual tumours. These measurements were recorded throughout the experiment period until the animals’ sacrifice, enabling the evaluation of the tumour volume change. Tumour volume (mm3) was calculated by the following formula Citation[41].
Statistical analyses were performed to evaluate the time course of tumour growth along the HFT treatment between the study groups. The statistical test used to perform the data analysis was the ANOVA repeated measures with post-hoc Bonferroni test (whose purpose was to see if there were differences in values found in control and treatment groups on the various measurements). In order to apply this parametric strategy, the requirements of normality (the Shapiro-Wilk test – p > 0.05), homogeneity of variances (Levene's test – p > 0.05) and sphericity (Mauchy's test with the Greenhouse-Geisser correction – p > 0.05) were first verified. All the tumour volumes data were represented as mean ± standard error (SE).
FC behaviour within the tumour and iron staining
For the FC behaviour analysis and iron staining, three animals from group II and the treatment group were euthanised at the end of the experiment, and their spleen, liver, lung and tumour were collected and snap frozen.
The surface of the FC was removed from the tumour and analysed under scanning electron microscopy of high resolution (Schottky) with energy dispersion spectroscopy (SEM/EDS) in atmospheric equipment (FEI Quanta 400FEG ESEM/EDAX Genesis X4M - CEMUP, Oporto, Portugal), enabling samples to be directly observed. Staining for the presence of iron in the collected tissues was carried out using Perls’ Prussian blue staining Citation[42].
Histological analyses
Animals were euthanised at different time periods, 4, 24 and 48 hours after the last treatment, and the respective tumours were removed. The collected samples were fixed in 10% buffered formalin and paraffin-embedded. Two consecutive sections, 2 µm thick, were made, one being stained by haematoxylin and eosin (HE) for histopathological evaluation, and the other for the immunohistochemical detection of caspase-3. Both were independently examined by two pathologists (I.A. and F.G.). In case of an opinion divergence, an agreed diagnosis was reached using a multi-head microscope.
For the immunohistochemical staining of caspase-3 (an apoptosis marker), antigen retrieval was performed on deparaffinised sections by boiling in a steamer in 10 mmol/L sodium citrate buffer, pH 6.0 for 3 min. Slices were allowed to cool for 10 min at room temperature, and were rinsed twice in triphosphate buffered saline (TBS) for 5 min. After blocking endogenous peroxidase with hydrogen peroxide 3% in methanol for 10 min, sections were subjected to immunohistochemical staining with a polyclonal antiserum against cleaved caspase-3 (Asp175, Cell Signaling Technology, Boston, MA, USA), diluted 1:100, overnight at 4°C, and visualised with the Novolink™ Max-Polymer detection system (Novocastra, Wetzlar, Germany). Sections were rinsed with TBS between each step of the procedure. Colour was developed using AEC chromogen (DakoCytomation, Glostrup, Denmark) and sections were then lightly counterstained with haematoxylin, and mounted using an aqueous medium. Negative controls were included in which the primary antibody was omitted and replaced by TBS.
To access immunoreactivity of caspase-3, selected tumour sections were analysed, marking the formation of a red-brown product of reaction at the place of the target antigen, regardless of its intensity. Areas of necrosis or inflammatory cell infiltration were avoided. Scores were evaluated by visual assessment of the number of positive cells in ten randomly high power fields of magnification, with the help of a microscope. The percentage of positively stained cells in the total cellular neoplastic population was taken as the immunohistochemical index.
Results
FC distribution within the tumour
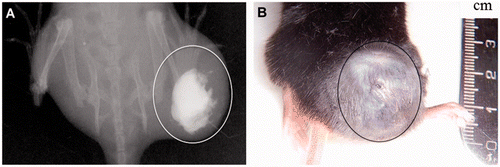
After the injection of the FC, the distribution within the tumour, analysed through the animal's radiographic image, is shown in . FC localisation is mainly centred in the tumour, which is the desirable distribution in order to obtain a uniform dissipation of heat within tumour.
Temperature monitoring during HFMF
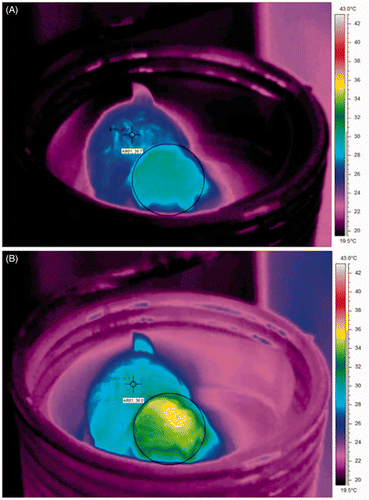
Thermographic images (thermograms) showed that before HFMF exposure the average body temperature of the animals was ±27°C and the temperature in the tumour was ±30°C (). The HFT application in the melanoma mouse model resulted in a temperature increase in the tumour. The initial tumour temperature (33–34°C) increased 5–6°C in the first 5 min, and subsequently, it was maintained constant (38–39°C) through the remaining treatment period, by controlling the magnetic field intensity (). In contrast, the average body temperature showed only a limited increase of 2–3°C (29–30°C to 32–33°C).
Tumour growth
Tumour growth was calculated from day 15 after tumour induction until the first animal death. The tumour growth was exponential in the animals of groups I and II. In the treatment group, a great decrease was observed after the HFMF exposure ().
Figure 3. The time courses of tumour growth over 12 days in the animals of control groups I and II and treatment group: average tumour volume (mm3) at 2-day intervals. The FC injection within the tumour was performed at day 0 (group II and the treatment group). Animals of the treatment group were exposed to HFMF at days 2, 4 and 6. *Significantly different from control group I; #Significantly different from control group II.
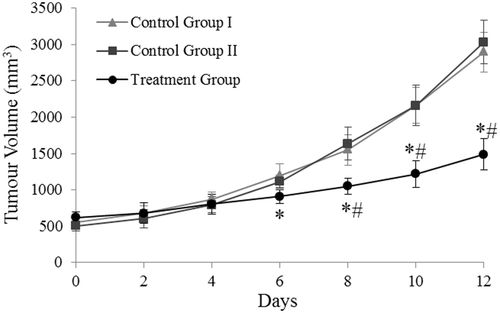
The time course data obtained from the tumour measurements of the three groups studied were statistically treated using the repeated measures ANOVA with a Greenhouse-Geisser correction. The evolution of tumour growth among the three groups differed, as shown in , in a statistically significant manner (F(2.488) = 155.492, p < 0.01) ().
FC behaviour within the tumour and iron measurements
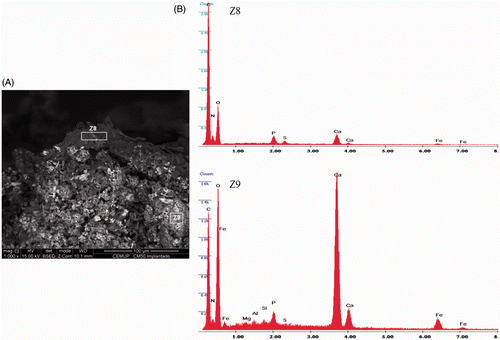
The FC morphology (SEM) and the qualitative and semi-quantitative composition (EDS), after injection within the tumour, were assessed, at the cement/tissue interface and within the cement. The FC showed a rough-type morphology (). The EDS spectrum performed within the FC showed the expected cement composition, mainly the presence of Ca, Fe, Si, Al, Mg associated to P and C (). At the FC surface, EDS spectrum exhibited the formation of a layer involving the cement, composed of calcium and phosphorus precipitates with a peak of carbon and a high decrease of iron, as a result of FC interaction with the surrounding environment ().
Figure 4. (A) Morphological SEM appearance of a FC sample, injected within the tumour. (B) EDS analysis of the FC surface (Z8), which is mainly composed of calcium associated with phosphorus and carbon; EDS analysis within FC (Z9), showing Ca, Fe, Si, Al, and Mg associated to P and C peaks.
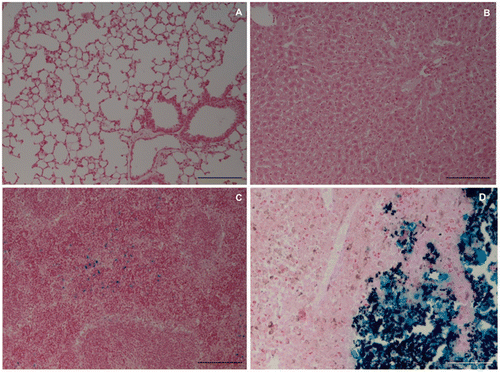
The presence of iron, using the Perls staining, in crucial organs such as lungs and liver, was negative in both control group II and the treatment group. In the spleen, some iron traces were detected. In the tumour injected with FC, a large amount of iron was observed near the FC, as expected due to its composition. Representative images are shown in for the animals of the treatment group.
Histological analyses
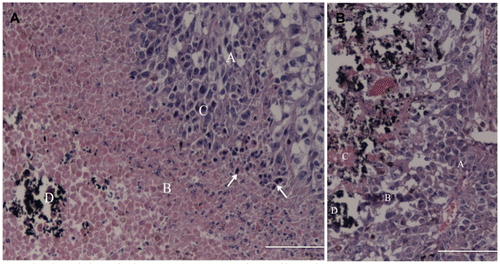
The histological analyses of the tumour samples from the treatment group, obtained 24 h after the last HFT treatment, stained with HE, revealed a proliferation of tumour cells composed of large round to polygonal cells that usually form nests, surrounding multiple areas of necrosis. The tumour cells presented oval to vesicular nuclei with variable pleomorphism, prominent nucleoli and moderate to abundant cytoplasm with highly variable degrees of pigmentation (brown granular appearance) (). Deposition of an amorphous black material was found restricted to the areas of necrosis, consistent with the FC. Also identified were many apoptotic figures characterised by extreme chromatin condensation, fragmented nuclei with apoptotic bodies, cytoplasm shrinking and membrane blebbing among viable tumour cells and far from the areas of necrosis. Comparatively, in the control group II samples the presence of tumour cells was observed near to the FC, with few inflammatory cells in the transition area, and the necrotic cells were observed only inside the FC ().
Figure 6. Histological analysis (HE) of the tumour from the treatment group (left image) and control group II (right image). Treatment group: the upper right corner shows the neoplastic cells (A) with areas of necrosis (B), inflammatory infiltrate (C) composed mainly of lymphocytes and plasma cells (arrows); in the lower left corner, the ferrimagnetic material (D). Control group II: the image's right site shows the tumour cells (A), some inflammatory infiltrate (B) and small necrotic areas (C) associated with FC (D). Bar = 12.5 µm.
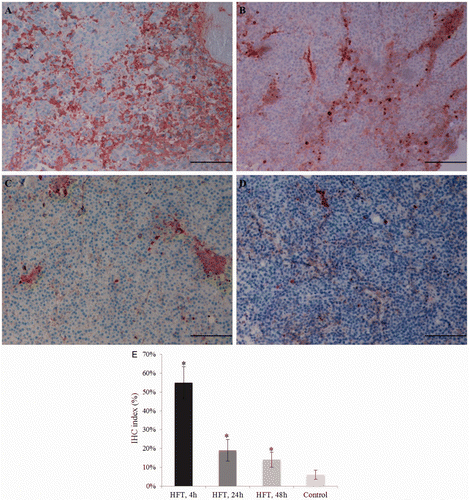
Positive immuno-labelling for caspase-3 in the tumours from the treatment group was identified at 4 h, 24 h and 48 h after the last treatment (). The immunohistochemical index decreased during this time period and was, respectively, =55%, 19% and 14%. Comparatively, the immunohistochemical index in the control group II was 6% at the time, corresponding to the 4 h after the treatment ().
Figure 7. Immunohistochemistry for caspase-3 in the tumour from the treatment group (A–C) and the control group II (D). In the treatment group, moderate cytoplasmic immunostaining in most of the tumour cells, and strong immunostaining in a few scattered cells, at 4 h (A), 24 h (B) and 48 h (C) after the treatment; the decrease in the number of apoptotic cells is evident. Comparatively, samples from control group II showed a lower staining (D). Bar = 12.5 µm. The immunohistochemistry index is also shown (E).
Discussion
Localised hyperthermia is a powerful therapeutic modality in tumour treatment. The HFT technique, using FC, has been developed as an attempt to keep the magnetic particles within the tumour, allowing repeated low hyperthermia treatments. Even in case of recurrence after the treatment, the possibility to repeat injections in adjacent areas of the tumour is desirable. Results of the present study show that the tested FC meets these requirements, making it possible to apply the HFT technique in a melanoma mouse model. Additionally, the evaluation of the effectiveness of FC in tumour inhibition and destruction was also an objective of this study. This was performed by the application of repeated HFMF exposures, with 48-h intervals which appear to be essential to avoid heat shock protein-induced thermoresistance Citation[43], Citation[44].
Results showed that FC can be injected, and remains within the tumour. SEM/EDS analysis revealed that the interaction of FC with the surrounding tumour environment resulted in the formation of a calcium- and phosphorus-containing layer at the cement/tumour interface. FC is a calcium phosphate silicate cement and, as it is a hydraulic cement, it develops its properties in a humid environment. FC is bioactive and biocompatible. Both the bioactivity and biocompatibility result from the release of calcium hydroxide from the cement and its interaction with the phosphorus from the surrounding environment. The calcium phosphate layer stabilises in time, and the Ca(OH)2 stays captive within the cement, lined by the formed layer, as confirmed in previous in vitro studies Citation[38]. Thus, this calcium phosphate interface layer stabilised the FC structure and prevented its clearance out of the tumour. Within the FC, composition analysis showed the main compounds of the FC, confirming previous in vitro studies Citation[38]. The progressive encapsulation of the FC by calcium compounds, after injection in a biological environment, is important to stabilise the material inside the body allowing a repeated treatment with the same material. In addition, the FC remained located within the tumour and, due to the poor tumour vascularisation, their components did not reach other organs, as confirmed in the staining for iron compounds. The iron trace detected in the spleen might be considered as a normal occurrence, since it is a highly vascularised organ in which the iron from the haemoglobin of phagocytised erythrocytes is currently converted to haemosiderin for storage Citation[45]. Nevertheless, the observed trace staining does not have any significance when compared with the amount inside the tumour. Instead, in other studies, by using different ferrimagnetic materials and restricting the HFMF exposure to the tumour, a significant amount of iron was found in lungs and liver Citation[46]. So, the HFT technique described in the present study allows the animal's entire body to be exposed to the HFMF, preventing unwanted hyperthermia effects in other tissues.
In the superficial melanoma model described in this work, the animal's temperature can be monitored using a thermographic camera. With this methodology, it is possible to measure the temperature variation simultaneously in the whole body and in the tumour during the treatment, avoiding cutting the skin for a probe that would cause leakage of the gelatinous melanoma tumour parenchyma, introducing increased potential variability in tumour volumes Citation[46]. In the present work, the tumour temperature was set approximately 5°C higher than the animal's initial temperature. Results showed that the initial tumour temperature rapidly increased and remained constant throughout the treatment period, whereas the average body temperature suffered only a limited increase. This indicates the feasibility of the HFT treatment in heating the tumour specifically, with just a slight increase of the temperature in healthy tissues.
The time course evaluation of tumour growth, comparing control and treatment groups, demonstrated that HFT inhibits normal tumour growth. This finding can be initially explained by the tumour injection of FC, causing damage in the tumour and, secondly, by the tumour temperature increasing during the HFMF exposures. However, the tumour dimension (around 10 mm) when the HFT treatment started was bigger than that used in other studies (5–6 mm) with the same melanoma model treated with hyperthermia Citation[23], Citation[47], Citation[48]. This might explain, at least partially, the incapability to completely destroy the tumour with the protocol used.
Many authors, with different approaches, have already validated the feasibility of hyperthermia in the treatment of tumours. However, the molecular mechanism and the reason why different heat therapies inhibit tumour growth are not well known. The histological analysis performed in the present study is in agreement with tumour growth inhibition enhanced by cell death through necrosis or apoptosis Citation[22]. Although the temperature needed to induce apoptosis or necrosis might vary among cells types, one would expect that higher temperatures would result in necrosis. However, apoptosis-mediated tumour destruction is desirable, since it leads to fewer side effects. Necrosis, instead, is characterised by rapid cytoplasm swelling and it culminates in rupture of the plasma membrane and organelle breakdown as a consequence of extreme physicochemical stress. It can also lead to local inflammation due to release of intracellular factors from dead cells, the so called damage-associated molecular patterns that alert the innate immune system Citation[49]. In the present work, in the HE staining, areas of necrosis associated with mixed inflammatory infiltrate (neutrophils, lymphocytes and some plasma cells) were identified in the treatment group samples around the FC. Compared with control group II, the necrosis was identified just in the cells present within the FC content and some inflammatory cells near to FC. Considering this data, the necrotic area observed in the treatment group was due to the generated heat.
Apoptosis, first described by Kerr et al. in 1972 Citation[50], is defined as a programmed cell death mechanism. Apoptotic cell death acts as part of a quality control and repair mechanism by elimination of unwanted, genetically damaged, or senescent cells, and as such is critically important for the development of organisms. The immunohistochemical analysis used for the apoptotic cell identification in this study was consistent with the principle that caspase activation (cleavage of procaspase to active caspase) is a hallmark of almost all apoptotic systems Citation[51]. Also, caspase-3 is a central effector caspase in many cells and mediates the cleavage of itself, other downstream caspases, and other caspase substrates. The immunostaining assay revealed a significant amount of apoptotic cells in the tumour treatment group 4 h after the last treatment, decreasing. In comparison, the index found in control group II was significantly lower. Still, the obtained value (6%) may be explained by the apoptosis processes that occur spontaneously in almost all neoplastic lesions. However, it is important to state that many different cell lines are hyperthermia induced apoptosis competent, including melanoma cells Citation[52] but with varying degrees of sensitivity Citation[53]. Sakaguchi et al. Citation[5] have studied the extent and the kinetics of a hyperthermia treatment method in the apoptosis induction in two tumours and in normal tissues, showing a significant difference between the tumours and, importantly, between tumour and normal tissues. They concluded that the extent and duration of apoptosis seem to be correlated with tumour response to the hyperthermia treatment.
HFT allows the control of the heat generation within the tumour by controlling the magnetic field intensity around the subject. In future experiments we intend to increase the tumour temperature by approximately 8–10°C (to 41–42°C). Some studies have used hyperthermia in this melanoma mouse model and the temperature increase varied between 5° and 10°C Citation[23], Citation[46], Citation[47], Citation[53], Citation[54]. In this work, our choice for the 5°C increase was based on the principle that lower temperatures will induce apoptosis and minimise the damage to the surrounding healthy tissues but, as in other studies Citation[5], this temperature was not high enough to completely destroy the malignant melanoma, at least with the experimental protocol used.
Conclusion
The FC tested in the present study appears to present important features to be used as a magnetic vehicle for HFT. These include the ability to be applied by injection and to remain locally, with minimal adjacent and systemic distribution, in addition to allow for repeated hyperthermia treatments. The HFT protocol using low temperature hyperthermia, with repeated treatments, proved to be a minimally invasive technique that statistically inhibited tumour growth in a melanoma mouse model, due to necrosis and apoptosis. Results suggested that HFT using the tested FC appears to be a promising technique to be applied in low temperature hyperthermia.
Acknowledgements
The authors would like to thank the Laboratory of Pathology from the Abel Salazar Institute of Biomedical Sciences, University of Porto, and the Faculty of Engineering of the University of Porto (FEUP) which provided the thermographic camera.
Declaration of interest: This work was supported by the Portuguese Foundation of Science and Technology (FCT) and FEDER/Compete program: PTDC/SAU-BEB/69497/2006.
References
- Lee CT, Mace T, Repasky EA. Hypoxia-driven immunosuppression: A new reason to use thermal therapy in the treatment of cancer?. Int J Hyperthermia 2010; 26: 232–246
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22: 197–203
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and the molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Pappeti M, Herman I. Mechanism of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 2002; 282: c947–c970
- Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR, Danhauser LL, et al. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res 1995; 55: 5459–5464
- Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia 2010; 26: 211–223
- Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 1999; 201: 413–419
- Sakuray H, Kitamoto Y, Saitoh J, Nonaka T, Ishikawa H, Kiyohara H, et al. Attenuation of chronic thermotolerance by KNK437, a benzylidene lactam compound, enhances thermal radiosensitization in mild temperature hyperthermia combined with low dose-rate irradiation. Int J Radiat Biol 2005; 81: 711–718
- Armour EP, McEachern D, Wang Z, Corry PM, Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res 1993; 53: 2740–2744
- Mackey MA, Anolik SL, Roti Roti JL. Cellular mechanisms associated with the lack of chronic thermotolerance expression in HeLa S3 cells. Cancer Res 1992; 52: 1101–1106
- Ryu S, Brown SL, Kim SH, Khil MS, Kim JH. Preferential radiosensitization of human prostatic carcinoma cells by mild hyperthermia. Int J Radiat Oncol Biol Phys 1996; 34: 133–138
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 1996; 12: 367–373
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, et al. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int J Hyperthermia 2002; 18: 563–575
- O’Neill KL, Fairbairn DW, Smith MJ, Poe BS. Critical parameters influencing hyperthermia-induced apoptosis in human lymphoid cell lines. Apoptosis 1998; 3: 369–375
- Field SB. Physics and technology of hyperthermia. NATO ASI series, E: Applied Sciences, SB Field, C Franconi. Martinus Nijhoff, Dordrecht 1987; 19–27
- Szasz A. Hyperthermia, a modality in the wings. J Cancer Res Ther 2007; 3: 56–66
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hyperthermia 2002; 18: 267–284
- van der Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13: 1173–1184
- Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia 2008; 24: 251–261
- Jordan A, Wust P, Scholz R, Tesch B, Fähling H, Mitrovics T, et al. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int J Hyperthermia 1996; 12: 705–722
- Sato K, Watanabe Y, Horiuchi A, Yukumi S, Doi T, Yoshida M, et al. Feasibility of new heating method of hepatic parenchyma using a sintered MgFe2O4 needle under an alternating magnetic field. J Surg Res 2008; 146: 110–116
- Suzuki M, Shinkai M, Honda H, Kobayashi T. Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res 2003; 13: 129–135
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferromagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 2009; 25: 499–511
- Jones SK, Winter JG. Experimental examination of a targeted hyperthermia system using inductively heated ferromagnetic microspheres in rabbit kidney. Phys Med Biol 2001; 46: 385–398
- Kawashita M, Tanaka M, Kokubo T, Inoue Y, Yao T, Hamada S, et al. Preparation of ferrimagnetic microspheres for in situ hyperthermic treatment of cancer. Biomaterials 2005; 26: 2231–2238
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993; 9: 51–68
- Johannsen M, Thiesen B, Jordan A, Taymoorian K, Gneveckow U, Waldofner N, et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005; 64: 283–292
- Schneider S, Rusconi S. Magnetic selection of transiently transfected cells. Biotechniques 1996; 21: 876–880
- Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: An in vivo study. Jpn J Cancer Res 1998; 89: 463–469
- Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposome: In vitro study. Jpn J Cancer Res 1996; 87: 1179–1183
- Ohno T, Wakabayashi T, Takemura A, Yoshida J, Ito A, Shinkai M, et al. Effective solitary hyperthermia treatment of malignant glioma using stick type CMC-magnetite. In vivo study. J Neurooncol 2002; 56: 233–239
- Saito H, Mitobe K, Ito A, Sugawara Y, Maruyama K, Minamiya Y, et al. Self-regulating hyperthermia induced using thermosensitive ferromagnetic material with low Curie temperature. Cancer Sci 2008; 99: 805–809
- Kokubo T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991; 12: 155–163
- Matsumine A, Takegami K, Asanuma K, Matsubara T, Nakamura T, Uchida A, et al. A novel hyperthermia treatment for bone metastases using magnetic materials. Int J Clin Oncol 2011; 16: 101–108
- Jiang Y, Ou J, Zhang Z, Qin Q. Preparation of magnetic and bioactive calcium zinc iron silicon oxide composite for hyperthermia treatment of bone cancer and repair of bone defects. J Mater Sci Mater Med 2011; 22: 721–729
- Kawashita M, Kawamura K, Li Z. PMMA-based bone cements containing magnetite particles for the hyperthermia of cancer. Acta Biomaterialia 2010; 6: 3187–3192
- Portela A, Vasconcelos M, Branco R, Gartner F, Faria M, Cavalheiro J. An in vitro and in vivo investigation of the biological behaviour of a ferrimagnetic cement for highly focalised thermotherapy. J Mat Sci Mat Med 2010; 1: 2413–2423
- Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: An in vivo study. Jpn J Cancer Res 1998; 89: 463–469
- Norma Portuguesa. Avaliação biológica dos dispositivos médicos, Parte 2: Requisitos para o bem estar dos animais [Biological evaluation of medical devices, Part 2: Animal Welfare Requirements]. 2000, NP EN ISO 10993–2
- Buhtoiarov IN, Lum HE, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cells independent anti-tumor effects. J Immunol 2006; 176: 308–318
- Bancroft JD, Gamble M. Theory and Practice of Histological Techniques6th. Churchill Livingstone, New York 2007
- Tucker RD, Loening SA, Landas S, Paulus JA, Ren ZY, Park JB. The effect of interstitial hyperthermia on the Dunning prostate tumor model. J Urol 1992; 147: 1129–1133
- Dings RPM, Loren ML, Zhang Y, Mikkelson S, Mayo KH, Corry P, et al. Tumour thermotolerance, a physiological phenomenon involving vessels normalisation. Int J Hyperthermia 2011; 27: 42–52
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol 2006; 34: 455–465
- Balivada S, Rachakatla RS, Wang H, Samarakoon TN, Dani RK, Pyle M, et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010; 10: 119
- Ito A, Fujioka M, Yoshida T, Wakamatsu K, Ito S, Yamashita T, et al. 4-S-cysteaminylphenol-loaded magnetite cationic liposomes for combination therapy of hyperthermia with chemotherapy against malignant melanoma. Cancer Sci 2007; 98: 424–430
- Ito A, Saito H, Mitobe K, Minamiya Y, Takahashi N, Maruyama K, et al. Inhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperature. Cancer Sci 2009; 100: 558–564
- Krysko D, Berghe TV, D’Herde K, Vandenabeele P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008; 44: 205–221
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257
- Green DR. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000; 102: 1–4
- Garcia MP, Cavalheiro J, Fernandes MH. Acute and long-term effects of hyperthermia in B16-F10 melanoma cells. PLoS ONE 2012; 7: e35489, doi:10.1371/journal.pone.0035489
- Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother 2004; 53: 26–32
- Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, et al. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003; 94: 308–313