Abstract
Background: Adjuvant intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) is a therapy which combines thermotherapy and intraperitoneal chemotherapy. It is theoretically powerful for patients with advanced gastric cancer (AGC), but is there evident advantage in clinical practice? We need evidence to guide our decision-making.
Objectives: Meta-analysis was performed to assess the effectiveness and safety of adjuvant intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for patients with resectable locally advanced gastric cancer, and to provide the reference for clinical practice and study.
Methods: We searched the Cochrane Library, PubMed, Embase, Web of Science and Chinese databases (Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI) and Wanfang) electronically and also retrieved papers from other sources (tracing related references and communication with other authors). All relevant randomised controlled trials (RCTs) were collected to compare surgery combined with IHIC to surgery without IHIC for AGC. There were no language restrictions. After independent quality assessment and data extraction by two reviewers, meta-analysis was conducted by RevMan 5.1 software.
Results: 16 RCTs involving 1,906 patients were included. Compared with surgery alone, combination therapy (surgery plus IHIC) was associated with a significant improvement in survival rate at 1 year (hazard ratio (HR) = 2.99; 95% confidence interval (CI) = 2.21 to 4.05; p < 0.00001), 2 years (HR = 2.43; 95%CI = 1.81 to 3.26; p < 0.00001), 3 years (HR = 2.63; 95%CI = 2.17 to 3.20; p < 0.00001), 5 years (HR = 2.49; 95%CI = 1.97 to 3.14; p < 0.00001), and 9 years (HR = 2.14; 95%CI = 1.38 to 3.32; p = 0.0007). Compared with surgery alone, combination therapy was associated with a significant reduction in recurrence rate at 2 years (RR = 0.42; 95%CI = 0.29 to 0.61; p < 0.00001), 3 years (RR = 0.35; 95%CI = 0.24 to 0.51; p < 0.00001) and 5 years (RR = 0.47; 95%CI = 0.39 to 0.56; p < 0.00001). IHIC was not found to be associated with higher risks of anastomotic leakage, ileus, bowel perforation, myelosuppression, gastrointestinal reaction and hypohepatia, but it increased the incidence of abdominal pain (RR = 21.46; 95%CI = 5.24 to 87.78; p < 0.00001).
Conclusions: Compared with surgery alone, surgery combined with IHIC can improve survival rate and reduce the recurrence rate, with acceptable safety. However, safety outcomes should be further evaluated by larger samples and high quality studies. Additionally, hyperthermia for the intraperitoneal chemotherapy needs more clinical research.
Introduction
A total of 989 600 new stomach cancer cases and 738 000 deaths are estimated to have occurred in 2008, accounting for 8% of the total cases and 10% of total deaths worldwide. Over 70% of new cases and deaths occur in developing countries. Generally, stomach cancer rates are about twice as high in men as in women Citation[1]. Currently, surgical resection is the mainstay of treatment for gastric cancer, but the therapeutic effect after operation is reduced immensely because of recurrence and metastasis. Despite the use of radiotherapy or adjuvant or neoadjuvant systemic chemotherapy, the long-term survival in patients with locally advanced gastric cancer remains limited. We need to search for more effective adjuvant treatment regimens to change the current situation.
There are many adjuvant treatments being investigated to reduce the recurrence rate and metastasis rate. Intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) is the synthesis of thermotherapy, chemotherapy and peritoneal perfusion, which as a targeted adjuvant treatment after surgery may be considered a rational prophylactic and therapeutic approach. Although the theoretical rationale is very appealing, the real situation in clinical practice is unclear. Is there any significant impact of adjuvant IHIC on the outcomes of patients with AGC? A systematic review Citation[2] has proved that IHIC has a positive effect on gastric cancer with peritoneal metastasis, but what about the effect on patients without peritoneal metastasis who have undergone radical surgery? The current randomised controlled trials (RCTs) have mostly small sample sizes and have shown inconclusive results. So we performed this meta-analysis of RCTs to reliably assess the effectiveness and safety of adjuvant IHIC in the treatment of resectable AGC without peritoneal metastasis, and to provide the reference for further clinical practice and study.
Methods
Study selection criteria
The studies were selected for review if they fulfilled the following inclusion criteria:
Study type: RCTs regardless of use of blinding.
Participants: Patients with histologically diagnosed primary cancer of the stomach having radical resection. Studies of patients having non-radical resection were excluded. Locally advanced gastric cancer was defined as macroscopic serosal invasion, excluding peritoneal or distant metastases.
Intervention and comparison: Trials testing the efficacy and safety of radical surgery (RS) combined with IHIC versus RS without IHIC, no matter whether to implement post-operative chemotherapy.
Outcomes: The primary end point of this meta-analysis was overall survival, defined as the time from random assignment to the last follow-up or death.
There were no language restrictions. When multiple publications from the same institution were identified as duplicates, only the most recent update with the largest number of patients or longer follow-up group was included.
Literature search strategy
We performed literature searches of the Cochrane Library, PubMed, Embase, Web of Science, the Chinese Biomedical Literature Database, Chinese Journal Full Text Database and the Chinese Wanfang Literature Database. The search terms contained the target disease group and intervention group, and all searches used a topic search combined with a non-topic search. The search had no language restrictions and the period of searching was from the inception of databases to October, 2012. All relevant RCTs were collected to evaluate the effectiveness and safety of adjuvant IHIC for patients with resectable locally advanced gastric cancer. The reference lists of articles identified were reviewed for further identification of potentially relevant studies. We also used Google Scholar and Medical Matrix to search for relevant papers. Both published and unpublished trials were sought to limit publication bias. We also communicated with some experts to ask whether they knew about any unpublished trials.
Literature screening
Studies were selected according to the inclusion and exclusion criteria. The title and abstract were assessed and if potentially relevant the publication was retrieved to assess the full text. If necessary, we contacted authors for missing data. All steps were completed by two investigators (Zheng Li and Deng-hai Mi) independently, and all steps were cross-checked. We address discrepancies in the discussion. Problems with discrepancies were judged by the senior investigator (Ke-hu Yang) if they could not be resolved by the two investigators. The final results were reviewed by all three senior investigators.
Data extraction and critical appraisal
Two investigators (Zheng Li and Deng-hai Mi) independently read each article included. Data extracted included the study design, year of publication, number of patients, methodology, quality criteria, completeness of cytoreduction, criteria used to define IHIC, IHIC protocol, treatment outcomes, and prognostic factors associated with outcomes. All data were extracted from texts, tables, and figures of the articles and then tabulated.
The quality of studies was appraised independently using the following criteria: (1) whether the method of allocation was truly random; (2) whether there was proper concealment of allocation; (3) whether the groups were similar at baseline in terms of prognostic features; (4) whether loss to follow-up in each treatment group was specified and (5) whether intention-to-treat (ITT) analysis was conducted. When studies did not report adequate information to determine the above-mentioned assessment criteria, we tried to obtain additional data direct from the investigators.
Discrepancies between the two investigators were resolved by discussion and consensus with a senior investigator (Nong Cao). The final results were reviewed by all three senior investigators to avoid bias.
Statistical analysis
Data were synthesised using RevMan 5.1 provided by the Cochrane organisation. The heterogeneity between the results of the research included was examined by the chi-square test. Chi-square tests were used to study heterogeneity between trials. The I-squared value was used to estimate the percentage of total variation across studies.
When the homogeneity of statistics between studies was considered adequate (P > 0.1, I2 < 50%), we used the fixed effects model; if there was obvious heterogeneity between the studies (P < 0.1, I2 > 50%), we analysed the sources of heterogeneity, and then performed subgroup analysis according to factors accounting for the heterogeneity. When there was enough similarity between the studies within the group or between the groups (P > 0.1, I2 < 50%) we used the fixed effects model to perform meta-analysis. If there was statistical heterogeneity but no clinical and methodological heterogeneity between the subgroups we used the random effects model. If the heterogeneity was substantial between the studies included we used descriptive analysis. We performed sensitivity analysis to examine the stability of the results where necessary.
The primary end point of this meta-analysis was overall survival, defined as the time from random assignment to the last follow-up or death. Secondary end points were the incidence of recurrence and quality of life. Safety was also assessed, although the frequency of important long-term adverse events may not be adequately captured by the information provided in RCTs. Results regarding the overall survival were expressed as hazard ratios (HR) with 95% confidence intervals (CI). Other indicators used relative risk (RR) with 95%CI. All p-values were two-sided. All statistical analysis was conducted by Cochrane Review Manager 5.1.
Results
Results of the search and identified studies
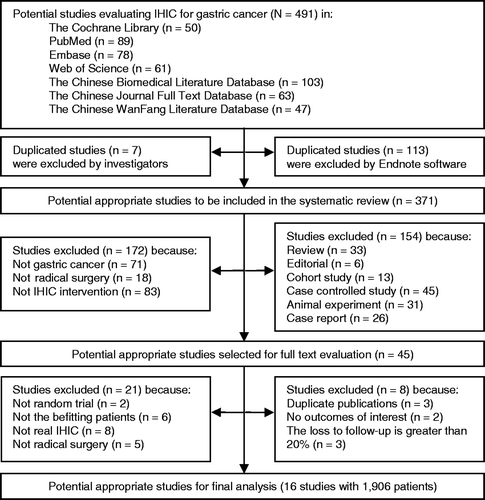
There were 491 references identified through electronic database and other searches. A total of 113 articles were excluded as duplicates by Endnote and seven articles were excluded as duplicates by reviewers. The titles and abstracts of 371 potentially appropriate articles were read by the reviewers, and 326 were excluded because they did not meet the inclusion criteria. A total of 45 articles were appraised by full text to confirm the studies included. Articles were also excluded where patients had peritoneal metastasis or had palliative surgery. Finally, 16 RCTs Citation[3–18] were included for appraisal and data extraction for meta-analysis. The 16 RCTs had a total sample of 1906 patients. The full details are listed in ).
Characteristics of studies included
In these 16 studies Citation[3–18], 1906 patients were randomly assigned, of whom 935 patients were to receive radical resection with adjuvant IHIC and 909 patients were to receive radical resection without IHIC. A total of 62 patients with intraoperative normothermic intraperitoneal chemotherapy (INIC) were randomly assigned in two studies Citation[7], Citation[15]. Two trials were reported in one article Citation[5], and we used the one in which the patients underwent radical resection, with the exclusion of the other trial where patients had palliative surgery. All of the 16 studies Citation[3–18] had RS with IHIC and RS alone, but only some of them implemented post-operative chemotherapy. Although there was a difference, it was irrelevant to our analysis because the post-operative treatment was balanced in the two comparative groups of every study. The full details are listed in .
Table I. Trial design and baseline characteristics of studies included.
Quality of trials
There was good agreement between the reviewers on the eligibility and quality of the studies. demonstrates the quality of all 16 RCTs included in the systematic review Citation[3–18]. An attempt was made to contact the corresponding authors of RCTs, where necessary, to obtain missing details relating to methodological quality.
Eight RCTs Citation[3], Citation[6–8], Citation[14–17] used an adequate approach to sequence generation using computer-generated random numbers or random-number tables. The adequacy of randomisation was unclear in the remaining eight RCTs. In the same eight RCTs Citation[3], Citation[6–8], Citation[14–17], the method of allocation concealment was adequate; randomisation was performed on a central site and transmitted to treatment providers by telephone, fax or sealed opaque envelopes. In the remaining eight RCTs Citation[4], Citation[5], Citation[9–13], Citation[18], the information regarding approaches to allocation concealment could not be determined. The baseline features were similar between treatment groups in all 16 RCTs. Four RCTs Citation[3], Citation[10], Citation[13], Citation[16] had no loss to follow-up, two RCTs Citation[4], Citation[17] specified numbers lost to follow-up in each treatment group, and this was unclear in the remaining 10 RCTs. Six Citation[6–8],Citation[14],Citation[15],Citation[17] RCTs analysed the data on an intention-to-treat (ITT) basis, whereby participants were analysed in the groups to which they were initially randomised; and 10 RCTs Citation[3–5], Citation[9–13], Citation[16], Citation[18] did not perform ITT analysis. Blinding after allocation was impossible because of the nature of the trials. The full details are listed in .
Table II. Quality assessment of RCTs included for meta-analysis.
Assessment of overall survival
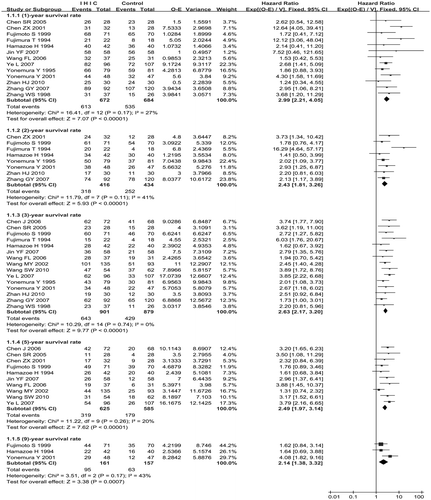
Subgroup analysis was used to evaluate total survival of different follow-up years. A significant survival improvement was found in favour of IHIC: 1 year (HR = 2.99; 95%CI = 2.21 to 4.05; p < 0.00001), 2 years (HR = 2.43; 95%CI = 1.81 to 3.26; p < 0.00001), 3 years (HR = 2.63; 95%CI = 2.17 to 3.20; p < 0.00001), 5 years (HR = 2.49; 95%CI = 1.97 to 3.14; p < 0.00001), 9 years (HR = 2.14; 95%CI = 1.38 to 3.32; p = 0.0007). There was no substantial statistical heterogeneity among the trials, and the meta-analysis was performed using the fixed effects model in all subgroups. We did not synthesise the subgroups of different years because it was inapposite. All the details are shown in .
Assessment of disease recurrence
Two RCTs Citation[6], Citation[17], three Citation[6], Citation[9], Citation[16], five Citation[3], Citation[6], Citation[7], Citation[12], Citation[17], and eight RCTs Citation[3], Citation[6], Citation[8], Citation[10–13], Citation[15] documented the incidence of 1-year, 2-year, 3-year and 5-year recurrence respectively; we performed the subgroup meta-analysis for these different follow-up years. There was no substantial statistical heterogeneity among these RCTs and we performed the meta-analysis using the fixed effects model in all subgroups. It was found that IHIC could reduce recurrence rate significantly: 2 years (RR = 0.42; 95%CI = 0.29 to 0.61; p < 0.00001), 3 years (RR = 0.35; 95%CI = 0.24 to 0.51; p < 0.00001), 5 years (RR = 0.47; 95%CI = 0.39 to 0.56; p < 0.00001). However, there was no significant difference in the 1-year recurrence rate (RR = 0.66; 95%CI = 0.25 to 1.78; p = 0.41) because of the limited sample size. We did not combine the subgroups of different years because it was inapposite. The full details are listed in .
Table III. Meta-analysis of recurrence rate and safety for patients in the two arms.
Assessment of safety
Four studies Citation[3], Citation[4], Citation[9], Citation[12] reported the incidence of abdominal pain, comparing 212 patients in the IHIC groups with 218 patients in the control groups. There was statistical difference in the meta-analysis of the incidence of abdominal pain between the two groups and no substantial heterogeneity was identified (RR = 21.46; 95%CI = 5.24 to 87.78; p < 0.00001). But all the reports explained that pain naturally resolved. There was no statistical heterogeneity in the meta-analysis of anastomotic leakage, ileus, bowel perforation, myelosuppression, gastrointestinal reaction and hypohepatia between the two groups. We used the fixed effects model, and the results of meta-analysis showed no statistical differences in these morbidity rates between the IHIC and without IHIC groups. The full details are listed in .
Analysis of publication bias
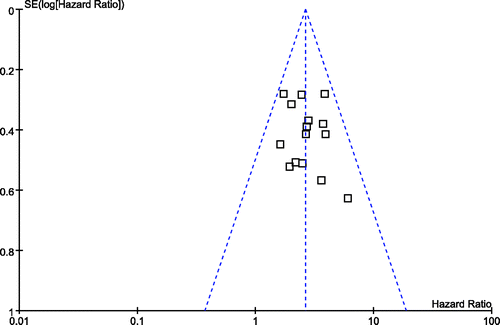
We used the funnel plot to examine the publication bias in the outcome of meta-analysis with the most RCTs contributing data. The horizontal axis of the plot was the HR effect estimate and the vertical axis of the plot was the standard error (SE) of the log (HR). The funnel plot of 3-year survival rate suggests that the quantity distribution of the RCTs is generally balanced, so the influence of publication bias is limited to the meta-analysis of the 3-year survival rate. All the details are shown in .
Discussion
Surgical treatment is still the main treatment for stomach cancer, but 5-year survival rate after surgery alone is very low. The main reason for death is post-operative loco-regional (peritoneal) recurrence and distant metastases. One of the long-term goals of treatment is to reduce the recurrence rate of stomach cancer, which is critical to improve overall survival. IHIC is the organic combination of hyperthermia, chemotherapy and intraperitoneal perfusion, which can improve the sensitisation and antipersonnel force for cancer. So theoretically, IHIC has a very obvious advantage compared with conventional systemic chemotherapy.
Effectiveness evaluation
The existing systematic review Citation[2] has suggested that IHIC has a positive effect on gastric cancer with peritoneal metastasis, but what about the effect of this treatment on patients without peritoneal metastasis and undergoing radical surgery? There are only a few available trials with small sample sizes for the comparison of RS combined with IHIC and RS alone, but the meta-analysis was still able to demonstrate statistically significant and clinically relevant differences. The results of this meta-analysis indicate that compared with RS alone, RS combined with IHIC is more beneficial and superior for primary AGC without peritoneal metastasis, and is associated with improving overall survival and reducing recurrence.
To further clarify the effects of adjuvant IHIC and explore variation among trials, we categorised the trials according to the post-operative treatment with or without chemotherapy. We performed both subgroup analysis and sensitivity analysis. The results of meta-analysis did not change, suggesting that these findings are robust. The data show that the post-operative treatment is irrelevant to compare effectiveness of RS combined with IHIC and RS alone, which is because the post-operative treatments are balanced between the two groups in each RCT.
There is no consistent method in the reports of loco-regional peritoneal recurrence and distant metastases, so we performed the meta-analysis using the disease recurrence rate which contains loco-regional peritoneal recurrence and distant metastases. Although there is some clinical heterogeneity in the recurrence rate of different RCTs, it is appropriate to combine studies and we can get the general trend to evaluate IHIC.
Historical analyses of treatment failure after curative resection for gastric cancer showed that approximately half of patients had a first site of recurrence in their peritoneal cavity and the development of local-regional recurrence had a negative impact on overall survival Citation[19–21]. We searched for the best combination treatment for the eradication of carcinomatosis. IHIC can effectively prevent the recurrence of cancer, especially for patients with serosal invasion but without peritoneal metastasis, who have undergone radical surgery Citation[6], Citation[8], Citation[14]. It is one of the reasons why IHIC can improve overall survival. IHIC can improve survival rates mainly through eradicating residual disease in the peritoneal cavity, but the recurrence also occurred through lymphatic and haematogenous dissemination, so IHIC should be combined with post-operative intravenous chemotherapy.
We should also pay attention to intraoperative normothermic intraperitoneal chemotherapy (INIC) versus IHIC. Hyperthermia is an effective treatment modality to augment chemotherapy-based anti-cancer treatments by various forms, which has been demonstrated by experiments Citation[22–23]. Hyperthermia also has been proven to modulate directly or indirectly the cells of the innate and adaptive immune system, thereby improving effectiveness Citation[24–26]. The data of two meta-analyses Citation[27–28] demonstrated a positive effect of INIC, and the authors also emphasise that IHIC is superior because hyperthermia has a synergistic and additional anti-tumour activity. Two Citation[7], Citation[15] included RCTs of the present meta-analysis and Tan et al.'s RCT Citation[29] performed the comparison of IHIC and INIC. The results indicate that IHIC is superior in terms of significantly improving overall survival and reducing recurrence. The control group underwent post-gastric resection lavage with saline only in every RCT included, but the effectiveness was limited compared to those with INIC or IHIC, as demonstrated by the trials and this review.
Berardi et al.'s systematic review Citation[30] indicates that neoadjuvant chemotherapy (NAC) could improve the global outcome of patients with locally advanced gastric cancer allowing a radical resection. Li et al.'s meta-analysis Citation[31] suggests that NAC could improve tumour stage and survival rate of patients with AGC with reasonable safety. Three meta-analyses Citation[32–34] suggest that adjuvant chemotherapy (AC) may produce a small survival benefit in patients with curatively resected gastric carcinoma. Chen et al.'s meta-analysis Citation[35] recommends that combined NAC and AC should be used to improve the overall survival of AGC patients. Comprehensive tumour treatment to improve the curative effect is very important, so that in the future there might also be the possibility of NAC + surgery + IHIC + AC as a potential therapy, which suggests that gastric cancer would be treated by an interdisciplinary approach in general.
Safety evaluation
There are two kinds of post-operative treatment in the RCTs: with or without chemotherapy. Post-operative factors were balanced in the two treatment groups of every RCT, so the contrasts of overall survival and recurrence rate were independent of the post-operative chemotherapy (PC). And it is appropriate to synthesise the data of all RCTs for meta-analysis of overall survival and recurrence rate. But the PC is relevant to the safety evaluation because many complications and adverse effects were caused by chemotherapy. For example, it is not appropriate to compare myelosuppression between the two groups of the RCTs without PC because only the IHIC group had chemotherapy while the other group had no chemotherapy. So the data from the RCTs without PC could not be pooled together for the meta-analysis of myelosuppression, gastrointestinal reaction, hypohepatia and renal dysfunction, which were the side effects of chemotherapy.
The reports of safety evaluation are not consistent, so we performed the meta-analysis using the data discussed by the investigators. Although there is some clinical heterogeneity in the safety evaluation of different RCTs, it is appropriate to combine studies and we can get the general trend to evaluate the IHIC.
The meta-analysis showed that IHIC does not increase the incidence of anastomotic leakage, ileus, bowel perforation, myelosuppression, gastrointestinal reaction and hypohepatia. Abdominal pain was increased by IHIC but all the reports explained that it disappears naturally. So we suggest that surgery combined with IHIC for advanced gastric cancer is a feasible treatment.
Study limitations
Only acceptable RCTs were included in order to ensure the quality of the meta-analysis, but the data in some types of trials have also investigated this issue. One study Citation[36] of 174 patients was excluded because it was a retrospective study. Three RCTs Citation[37–39] were excluded because the rate of loss to follow-up was more than 20%, which might cause bias. All of these studies indicated that IHIC has superior curative effects and is feasible.
The quality of some of the RCTs included was not adequate in this meta-analysis as they did not report the detailed method of random sequence generation and concealment of allocation. Recurrence and safety were not reported in a unified standard throughout the trials, thus there may be lack of precision in the meta-analysis; however, the sensitivity analysis indicated that findings were not markedly changed. The data were insufficient to evaluate the safety of IHIC adequately. In addition, economic outcomes and quality of life were not reported in any of the RCTs included. Although the surgical methods in some RCTs are not currently acceptable anymore, they are irrelevant to the results of this meta-analysis because the surgical factors were balanced in the two groups of every RCT.
Because of the reasons above, we suggest that prospective trials should be well-designed, well-executed and well-reported, in order to adequately evaluate the role of IHIC. We also need to collect the data of non-randomised studies to adequately assess the safety of IHIC.
The conclusion and the prospect of thermatology
Compared with RS alone, RS combined with IHIC for AGC without peritoneal metastasis can reduce post-operative recurrence rates and improve overall survival without increasing the risk of anastomotic leakage, ileus, bowel perforation, myelosuppression, gastrointestinal reaction and hypohepatia. Although IHIC increased the incidence of abdominal pain, this disappeared naturally. The safety of IHIC can be generally accepted. There are also some problems with IHIC, which should be improved by clinical practice and study. Some of these include the best temperature for perfusion, the dosage and compatibility of medicines for perfusion, and other issues. In addition, we should pay attention to try to prevent and reduce adverse reactions and complications.
Hyperthermia therapy has positive clinical value for the tumour. There have been many evidence-based studies for hyperthermia therapy. Two reviews Citation[40–41], from Lancet Oncology and the International Journal of Hyperthermia, have introduced various clinical applications of hyperthermia, and have summarised the clinical trials comprehensively regarding hyperthermia combined with radiotherapy and chemotherapy for various tumours.
Chua et al.'s study Citation[42] demonstrated a superior effect in patients with advanced ovarian cancer who received cytoreductive surgery and IHIC, when compared with the traditional standard of care. Two Cochrane systematic reviews Citation[43–44] investigated hyperthermia treatment for cervical cancer and rectal cancer respectively, and suggested there were positive effects of hyperthermia therapy. Two trials Citation[45–46] indicated that radiotherapy combined with hyperthermia had significant benefits compared to radiotherapy alone for the lung or nasopharyngeal cancer. Two trials Citation[47–48] suggested that chemotherapy combined with hyperthermia had significant benefits for lung or pancreatic cancer. Bergs et al.'s review Citation[49] suggested that trimodality therapy consisting of hyperthermia, cisplatin and radiation is effective and feasible in patients and seems to be promising. Mi et al.'s meta-analysis Citation[50] indicated that chemoradiotherapy combined with hyperthermia for non-small-cell lung cancer had superior effects. Three trials Citation[51–53] indicated that neo-adjuvant chemoradiation combined with regional hyperthermia followed by oesophageal resection for patients with oesophageal cancer resulted in good loco-regional control and overall survival. Kang et al.'s trial Citation[54] indicated that hyperthermia seemed to increase the response of both primary tumour and lymph nodes to preoperative radiochemotherapy in patients with locally advanced rectal cancer. All these studies have demonstrated the value of hyperthermia therapy for cancer, and provided the reference for clinical practice and research of hyperthermia at the same time.
Non-invasive temperature measurement, targeted therapy and control of thermal dose are the crux of hyperthermia treatment. Two studies Citation[55–56] suggested that targeted hyperthermia by nanoparticles has great value and is promising. Three studies Citation[57–59] researching the method of non-invasive temperature measurement are valuable for the improvement of the efficacy of hyperthermia treatment. We hope that there will be more high quality experiments and clinical trials researching hyperthermia therapy for tumours, and breaking through the technical bottleneck of hyperthermia treatment, which is critical to popularise this treatment. We hope that hyperthermia therapy for tumours can play an increasing role in clinical practice.
Acknowledgements
We wish to thank Qi Zhou, of the Department of Clinical Epidemiology and Biostatistics, McMaster University, for her valuable guidance in data analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90
- Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J Surg Oncol 2011; 104: 692–698
- Chen J, Su Y, Song Z, Lin LM. Clinical application of postoperative intraperitoneal hyperthermic perfusion combined with venoclysis for gastric cancer. Chin J Cancer Prev Treat 2006; 13: 937–938
- Chen SR, Zhao F, Tan ZW, Lv JS. Efficacy of intraoperative hyperthermic intraperitoneal chemotherapy for patients with advanced gastric carcinoma. J Youjiang Med Coll Nation 2005; 27: 357
- Chen ZX, Hu JK, Cheng Z, Chen JP, Peng DS, Tao QP. Positive results of therapy of gastric cancer patients after operation by intraperitoneal hyperthermic chemotherapy. J Chin Pract Med 2001; 3: 39–40 & 44
- Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999; 85: 529–534
- Fujimura T, Yonemura Y, Muraoka K, Takamura H, Hirono Y, Sahara H, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: Randomized controlled study. World J Surg 1994; 18: 150–155
- Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994; 73: 2048–2052
- Jin YF. Efficacy of intraoperative hyperthermic intraperitoneal chemotherapy for patients with advanced gastric carcinoma. Zhejiang J Clin Med 2007; 9: 357–358
- Wang FL, Hou AJ, Peng Z. Clinical study of hypotonic intraoperative intraperitoneal chemohyperthermia for gastric cancer. Clin J Med Offic 2006; 34: 17–19
- Wang MY, Sun G, Zhou JP, He FL. Clinical application of intraperitoneal hyperthermia chemoperfusion in the operation for the advanced gastric cancer. World J Med Today 2002; 3: 293–294
- Wang SW. The analysis for curative effect of intraoperative hyperthermic intraperitoneal chemotherapy combined with postoperative veins chemotherapy. Chron Pathem J (China) 2010; 12: 404–405
- Ye L, Jiang T, Hu D, Xiong GY. Application of intraperitoneal peirtoneal hyperthermia chemoperfusion in advanced gastric cancer. Int Med Health Guid News (China) 2007; 13: 29–31
- Yonemura Y, Ninomiya I, Kaji M, Sugiyama K, Fujimura K, Sawa T, et al. Prophylaxis with intraoperative chemohyperthermia against peritoneal recurrence of serosal invasion-positive gastric cancer. World J Surg 1995; 19: 450–454, discussion: 455
- Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepatogastroenterology 2001; 48: 1776–1782
- Zhang GY, Chen XC, Pan K, Xia LG, Zuo M, Zheng T. Application of hyperthermic intraoperative intraperitoneal chemotherapy in patients with gastric cancer. Chin J Gastrointest Surg 2007; 10: 362–364
- Zhan HJ, Liang H, Wang BG, Deng JY, Hao XS. Efficacy of intraoperative hyperthermic peritoneal perfusion on 60 patients with advanced gastric carcinoma. Chin J Clin Oncol 2010; 37: 229–231
- Zhang WS, Su DM, Wang K, Yi PT, Zhou HT, Guo JJ. Clinical study of intraoperative hyperthermic intraperitoneal chemotherapy for preventing the peritoneal recurrence of advanced gastric carcinoma. Shanxi Med J 1998; 27: 67–69
- Cascinu S, Labianca R, Barone C, Santoro A, Carnaghi C, Cassano A, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst 2007; 99: 601–607
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725–730
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20
- Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 2012; 28: 509–517
- Routt SM, Zhu J, Zaleski JM, Dynlacht JR. Potentiation of metalloenediyne cytotoxicity by hyperthermia. Int J Hyperthermia 2011; 27: 435–444
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012; 28: 528–542
- Knippertz I, Stein MF, Dorrie J, Schaft N, Muller I, Deinzer A, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011; 27: 591–603
- Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-gamma production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012; 28: 9–18
- Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007; 14: 2702–2713
- Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004; 10: 2727–2730
- Tan CQ, Wang XJ, Xu YK, Jiang BN, Zeng FH. Clinical studies on prevention of post-operational intraperitoneal neoplasm seeding of advanced gastric cancer by surgery combined with intraperitoneal perfusion of hyperthermic mitomycin C. J Pract Oncol 2000; 15: 165–168
- Berardi R, Scartozzi M, Romagnoli E, Antognoli S, Cascinu S. Gastric cancer treatment: A systematic review. Oncol Rep 2004; 11: 911–916
- Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: A meta-analysis. World J Gastroenterol 2010; 16: 5621–5628
- Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: Revisiting a meta-analysis of randomised trials. Eur J Cancer 1999; 35: 1059–1064
- Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: A meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol 2000; 11: 837–843
- Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol 2008; 34: 1208–1216
- Chen XZ, Yang K, Liu J, Chen XL, Hu JK. Neoadjuvant plus adjuvant chemotherapy benefits overall survival of locally advanced gastric cancer. World J Gastroenterol 2011; 17: 4542–4544
- Ikeguchi M, Kondou A, Oka A, Tsujitani S, Maeta M, Kaibara N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg 1995; 161: 581–586
- Lu Q, Huang XJ, Luo YF, Yang DS, Chen C. Intraoperative intraperitoneal hypotonic thermoperfusion chemotherapy for advanced gastric cancer. J Clin Experim Med 2007; 6: 29–30
- Luo YF, Lu Q, Huang XJ, Yang DS, Chen C. Countermeasures of nursing in advanced gastric cancer by intraoperative intraperitoneal hypotonic thermoperfusion chemotherapy. Int Med Health Guid News (China) 2008; 14: 125–127
- Zhang B, Jiang ZH, Fang XD, Hong Y. Influence of hypotonic intraoperative intraperitoneal chemohyperthermia (HIPCH) on the long term survival of patients with gastric cancer. Chin J Gastrointest Surg 2004; 7: 289–291
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: Systematic review of current results. J Cancer Res Clin Oncol 2009; 135: 1637–1645
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, van Mastrigt GA, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev 2009; 3: CD006269
- Lutgens L, van der Zee J, Pijls-Johannesma M, De Haas-Kock DF, Buijsen J, Mastrigt GA, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervical carcinoma. Cochrane Database Syst Rev 2010; 1: CD006377
- Sakurai H, Hayakawa K, Mitsuhashi N, Tamaki Y, Nakayama Y, Kurosaki H, et al. Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia 2002; 18: 472–483
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia 2011; 27: 180–186
- Shen H, Li XD, Wu CP, Yin YM, Wang RS, Shu YQ. The regimen of gemcitabine and cisplatin combined with radio frequency hyperthermia for advanced non-small cell lung cancer: A phase II study. Int J Hyperthermia 2011; 27: 27–32
- Ishikawa T, Kokura S, Sakamoto N, Ando T, Imamoto E, Hattori T, et al. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperthermia 2012; 28: 597–604
- Bergs JW, Franken NA, Haveman J, Geijsen ED, Crezee J, van Bree C. Hyperthermia, cisplatin and radiation trimodality treatment: A promising cancer treatment? A review from preclinical studies to clinical application. Int J Hyperthermia 2007; 23: 329–341
- Mi DH, Li Z, Yang KH, Tian JH, Wang DY. HRCT for non-small cell lung cancer: A meta-analysis. Chin J Evidence-Based Med 2011; 11: 1262–1267
- Sugimachi K, Kitamura K, Baba K, Ikebe M, Morita M, Matsuda H, et al. Hyperthermia combined with chemotherapy and irradiation for patients with carcinoma of the oesophagus – A prospective randomized trial. Int J Hyperthermia 1992; 8: 289–295
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 10: 485–493
- Hulshof MC, Van Haaren PM, Van Lanschot JJ, Richel DJ, Fockens P, Oldenborg S, et al. Preoperative chemoradiation combined with regional hyperthermia for patients with resectable esophageal cancer. Int J Hyperthermia 2009; 25: 79–85
- Kang MK, Kim MS, Kim JH. Clinical outcomes of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia 2011; 27: 482–490
- Ghahremani FH, Sazgarnia A, Bahreyni-Toosi MH, Rajabi O, Aledavood A. Efficacy of microwave hyperthermia and chemotherapy in the presence of gold nanoparticles: An in vitro study on osteosarcoma. Int J Hyperthermia 2011; 27: 625–636
- Golneshan AA, Lahonian M. The effect of magnetic nanoparticle dispersion on temperature distribution in a spherical tissue in magnetic fluid hyperthermia using the lattice Boltzmann method. Int J Hyperthermia 2011; 27: 266–274
- Ishihara Y, Ohwada H. Non-invasive temperature measurement by using phase changes in electromagnetic waves in a cavity resonator. Int J Hyperthermia 2011; 27: 726–736
- Raoof M, Zhu C, Kaluarachchi WD, Curley SA. Luciferase-based protein denaturation assay for quantification of radiofrequency field-induced targeted hyperthermia: Developing an intracellular thermometer. Int J Hyperthermia 2012; 28: 202–209
- van Dongen KW, Verweij MD. A feasibility study for non-invasive thermometry using non-linear ultrasound. Int J Hyperthermia 2011; 27: 612–624