Abstract
Purpose: Extracellular Hsp70 has anti-inflammatory potential, demonstrated in different models of inflammatory diseases. We investigated probable mechanisms used by Hsp70 to down-regulate pro-inflammatory cytokines.
Materials and methods: We analysed cytokine mRNA levels in bone marrow-derived murine dendritic cells treated with Hsp70, lipopolysaccharide (LPS) and peptidoglycan (PGN) or OVA (an irrelevant protein control), hypothesising that this was mediated by C/EBPβ and C/EBPδ transcription factors. We also tested the involvement of TLR2, IL-10, ERK and STAT3, using genetically deficient mice and pharmacological inhibitors.
Results: C/EBPβ and C/EBPδ levels were inhibited in bone marrow derived dendritic cells (BMDCs) treated with Hsp70, and that correlated with inhibition of TNF-α, IFN-γ and MCP-1. Such inhibition was not observed in TLR2 or IL-10 knockout mice, and was also abrogated upon pretreatment of cells with ERK and JAK2/STAT3 inhibitors.
Conclusions: C/EBPβ and C/EBPδ transcription factors are inhibited by Hsp70 treatment, and their inhibition occurs via the TLR2-ERK-STAT3-IL-10 pathway in BMDCs, mediating the anti-inflammatory effects of Hsp70.
Introduction
The heat shock protein 70 (Hsp70) is a ubiquitously expressed protein in cells following exposure to heat, UV radiation and other stressors [Citation1]. Hsp70 has been demonstrated to have anti-inflammatory and protective effects in diverse mouse models of inflammation [Citation2]. For example, treatment with Hsp70 whole protein or Hsp70 peptides can prevent arthritis in animal models [Citation3–6] in an IL-10-dependent manner [Citation7]. Hsp70 can also delay acute rejection in tumour and tissue allograft models [Citation8] and protect mice against dextran sulphate sodium (DSS)-induced colitis [Citation9].
We have previously observed that treatment of synovial cells from arthritis patients with mycobacterial Hsp70 not only induced IL-10 production by these cells, but also led to the inhibition of both IFN-γ and TNF-α production in these cells, as well as in healthy control monocytes [Citation10].
Dendritic cells (DCs) are the major antigen-presenting cells (APCs), playing a crucial role in immunity and tolerance [Citation11,Citation12]. These cells uptake antigens in peripheral tissues, migrate to draining lymph nodes (dLNs) through lymphatic vessels, and present antigens to T cell lymphocytes [Citation13,Citation14]. DCs can be found in synovium of arthritis patients, where they are involved in arthritis pathogenesis [Citation15]. These cells are involved in maintenance and progression of arthritis, presenting arthritogenic antigens to T cells and producing pro-inflammatory cytokines, such as TNF-α, IL-1 and IL-6 into the joint [Citation16]. Hsp70 was found to inhibit maturation of murine bone marrow derived dendritic cells (BMDCs) and induce IL-10 production in vitro [Citation10,Citation17]. Nevertheless, the molecular pathways involved in this process were not elucidated.
In DCs, inflammatory cytokines are released following inflammatory stimuli [Citation18]. NF-κB is a transcription factor that plays a key role in the induction of pro-inflammatory cytokines [Citation19]. Under a condition without inflammation, NF-κB is maintained inactive in the cytoplasm as complex with its inhibitor IκBα (NF-κB/IκBα complex). In an inflammatory state, IκBα is degraded and NF-κB is liberated to translocate into the nucleus and direct the transcription of pro-inflammatory genes [Citation20]. Hsp70 can prevent lipopolysaccharide (LPS)-induced production of inflammatory cytokines by interfering with the NF-κB-dependent transcription of cytokines [Citation21]. Overexpression of Hsp70 in human mononuclear cells prevents LPS-induced NF-κB p65 nuclear translocation into the nucleus [Citation22], potentially inhibiting the downstream induction of pro-inflammatory cytokines by LPS. It was suggested that Hsp70 stabilise the NF-κB/IκBα complex by the inhibition of IκBα degradation [Citation23].
Another group of transcription factors that are important for pro-inflammatory cytokines production comprise the CCAAT/enhancer-binding proteins (C/EBPs). These transcription factors compose a family involved in several aspects of cellular functions, such as proliferation, differentiation and cytokine production [Citation24]. C/EBPβ and C/EBPδ were demonstrated to be important for induction of inflammatory cytokines, such as TNF-α and IL-6, in TLR-stimulated macrophages [Citation25], and both C/EBPβ and C/EBPδ mRNA and protein levels have been demonstrated to be induced upon inflammatory stimuli [Citation26]. Upon LPS stimulation, cells from C/EBPβ knockout (KO) mice can express pro-inflammatory cytokines normally, because of the compensatory expression of C/EBPδ, suggesting that these two members of C/EBP family seem to have overlapping roles [Citation27].
In this study we investigated mechanisms by which Hsp70 can down-regulate basal levels of pro-inflammatory cytokines in BMDCs, and asked whether this is mediated by C/EBPβ or C/EBPδ transcription factors. Our results indicate that Hsp70 decreases basal levels of TNF-α, IFN-γ and MCP-1 cytokines in BMDCs concomitantly with down-regulation of C/EBPβ and C/EBPδ. Furthermore, TNF-α, IFN-γ and MCP-1 impairment, as well as C/EBPβ and C/EBPδ inhibition depend on a TLR2-ERK-STAT3-IL-10 cascade.
Materials and methods
Animals
Female C57Bl/6 mice were purchased from FEPPS (Rio Grande do Sul, Brazil). C57Bl/6 TLR2−/− mice were kindly provided by João Santana da Silva (University of São Paulo, Brazil). 129SV wild type (WT) and IL-10−/− mice were kindly provided by Ana M.C. Faria (Federal University of Minas Gerais, Belo Horizonte, Brazil). All mice were used between 6–10 weeks of age and housed in individual and standard mini-isolators (Tecniplast, Buguggiate, Varese, Italy) in a specific pathogen free facility (School of Biosciences, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS)) with free access to water and food. All procedures were previously reviewed and approved by the Ethics Committee for the Use of Animals of the University (CEUA-PUCRS) under protocol ID CEUA 08/00048.
Protein purification and LPS extraction
Recombinant mycobacterial Hsp70 was produced in XL1-blue Escherichia coli as described previously [Citation17]. Briefly, it was purified according to Mehlert and Young [Citation28], and triton X-114 was used to remove LPS, according to the method described in Aida and Pabst [Citation29]. Contaminating Triton X-114 was removed by incubating overnight with Bio-Beads® (Bio-Rad, Hercules, California, United States) at 4°C with agitation. Protein concentration was determined using a Qubit® Protein Assay Kit and the Qubit® Fluorometer (both purchased from Invitrogen, Eugene, OR).
Bone marrow dendritic cells cultures
Dendritic cells were differentiated from bone marrow of C57Bl/6 WT mice or TLR2−/− and 129 WT or IL-10−/− mice with GM-CSF and IL-4 (both purchased from Peprotech, Rocky Hill, NJ), as described by Inaba et al. [Citation30]. Cells were cultured in 24-well plates in medium AIM-V® (Gibco, Grand Island, NY). On the fifth day of culture, BMDCs were incubated with either 30 μg/mL of Hsp70, 30 μg/mL of OVA (Sigma, St. Louis, MO), 500 ng/mL of LPS (Sigma) or 10 μg/mL of peptidoglycan (PGN) (Sigma) for 24 h and total RNA was extracted. The supernatant was collected and used for cytokine analysis. For ERK inhibition, BMDCs were treated with 30 μM of selective inhibitor PD98059 (Cayman, Ann Arbor, MI) for 60 min prior to Hsp70 stimulation. To inhibit the JAK2/STAT3 pathway we used 50 μM of AG490 inhibitor (Sigma) for 60 min prior to stimulation.
Cytokine measurement
Cytokines present in BMDC supernatants were measured using a CBA mouse inflammation kit (BD Biosciences, San Diego, CA), according to manufacturer’s instructions. Samples were analysed by flow cytometry using a FACSCanto II and FACSDiva software (both from BD Biosciences). Cytokine concentrations were obtained using the FCAP software (version 1.01, BD Biosciences).
Real time qPCR
Total RNA was isolated from BMDC cultures using an RNAeasy kit (Qiagen, Germantown, MD) according to manufacturer’s instructions. The concentration of the purified total RNA samples was measured using a Qubit® RNA assay kit and the Qubit® fluorometer (both purchased from Invitrogen). An aliquot of 50 ng of RNA was reverse transcribed with 100 U of Sensiscript (Qiagen). cDNA concentrations were measured using Qubit® dsDNA HS assay kit and the Qubit® fluorometer (purchased from Invitrogen). In a final volume of 10 μL, 8 ng of cDNA was amplified using the following Taqman® gene expression assays (Applied Biosystems, Foster City, CA): Cebpb (Mm00843434_s1), Cepbd (Mm00786711_s1) and β-actin (4352933E). Real-time qPCR was performed with a StepOne™ real-time PCR system (Applied Biosystems). The relative mRNA levels were calculated using the comparative Ct method [Citation31]. The housekeeping gene β-actin was used as a normaliser. Non-treated BMDCs served as a reference for Hsp70-, OVA-, PGN- or LPS-treated BMDCs.
Results
Hsp70 treatment results in decreased basal levels of IFN-γ, TNF-α and MCP-1 in BMDCs with concomitant C/EBPβ and C/EBPδ down-regulation
To determine whether Hsp70 treatment could modulate pro-inflammatory cytokines in BMDCs, DCs differentiated from murine bone marrow were treated with Hsp70, OVA (negative control) or LPS for 24 h. Subsequently, IFN-γ, TNF-α and MCP-1 protein levels were measured in the supernatant by flow cytometry. As expected, stimulation with LPS increased the production of IFN-γ, TNF-α and MCP-1 when compared with OVA (). Interestingly, we found that Hsp70 treatment decreased basal levels (BMDCs treated with OVA) of the analysed cytokines ().
Figure 1. Hsp70 treatment decreases basal levels of TNF-α, IFN-γ, MCP-1 and down-regulates C/EBPβ and C/EBPδ. BMDCs were treated with OVA (30 μg/mL), Hsp70 (30 μg/mL) or LPS (500 ng/mL) for 24 h. Supernatants were analysed for (A) TNF-α, (B) IFN-γ, (C) MCP-1 using a CBA mouse inflammation kit. (D) C/EBPβ and (E) C/EBPδ expression evaluation by qPCR in BMDCs treated as described in A. β-actin was used as a normaliser as described in Materials and methods. *p < 0.05; **p < 0.01; ***p < 0.001. Experiments were performed three times in triplicates.
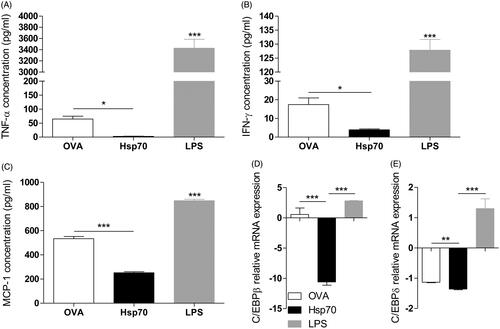
Because C/EBPβ and C/EBPδ are transcription factors that are largely associated with the production of pro-inflammatory cytokines, we tested the hypothesis that C/EBPβ and C/EBPδ modulation could be involved in this effect mediated by Hsp70. mRNA levels of C/EBPβ and C/EBPδ in BMDCs treated as described above were analysed by qPCR, and while LPS treatment induced an increase in C/EBPβ and C/EBPδ expression, Hsp70 treatment led to down-regulation of C/EBPδ () and C/EBPδ (). These data suggested that the decrease in basal levels of IFN-γ, TNF-α and MCP-1 was related to the down-regulation of C/EBPβ and C/EBPδ transcription factors.
Down-regulation of C/EBPβ, C/EBPδ and IFN-γ, TNF-α and MCP-1 inhibition by Hsp70 is dependent on TLR2 expression
TLR2 has been associated with Hsp70-induced suppressive effects in MDSCs, when Hsp70 release in tumour derived-exossomes activated STAT3 in a toll-like receptor (TLR)2-dependent manner in myeloid-derived suppressor cells (MDSCs) [Citation32]. Zymosan, Pam3Cys and Vitamin D3, which are TLR2 ligands, have been described to have anti-inflammatory effects in DCs [Citation33,Citation34] and tolerance in a type 1 diabetes model [Citation35]. In order to further investigate the molecular mechanisms involved in the observations described above, we analysed whether TLR2 was required for the anti-inflammatory Hsp70 effects.
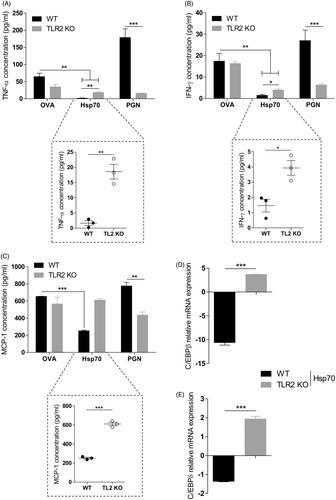
BMDCs from WT or TLR2 KO mice were treated for 24 h with OVA, Hsp70, PGN (a TLR2 agonist), or left unstimulated. After this period, IFN-γ, TNF-α and MCP-1 protein levels were measured in the supernatant by flow cytometry. C/EBPβ and C/EBPδ mRNA levels were analysed by qPCR.
WT PGN-treated cells exhibited a higher production of IFN-γ, TNF-α and MCP-1 when compared with cells lacking TLR2 (). WT BMDCs treated with Hsp70, however, presented an inhibition of IFN-γ, TNF-α and MCP-1 production when compared with OVA. However, the production of TNF and IFN was only partially recovered in TLR2 KO BMDCs treated with Hsp70 (), while MCP-1 production in TLR2 KO BMDCs treated with Hsp70 was completely recovered and comparable to the basal levels (). Concomitantly, down-regulation of both C/EBPβ () and C/EBPδ () induced by Hsp70 was abolished by the absence of TLR2 in BMDCs.
Figure 2. Pro-inflammatory cytokine inhibition and C/EBPβ and C/EBPδ down-regulation induced by Hsp70 is dependent on TLR2. WT or TLR2 KO BMDCs were treated with OVA, TBHsp70 or PGN for 24 h. Cytokines in the supernatants were analysed using a CBA mouse inflammation kit: (A) TNF-α, (B) IFN-γ and (C) MCP-1; (D) C/EBPβ and (E) C/EBPδ expression evaluation by qPCR in WT or TLR2 KO BMDCs treated with Hsp70 or left unstimulated for 24 h. β-actin was used as a normaliser as described in Materials and methods. *p < 0.05; **p < 0.01; ***p < 0.001. Experiments were performed three times in triplicates.
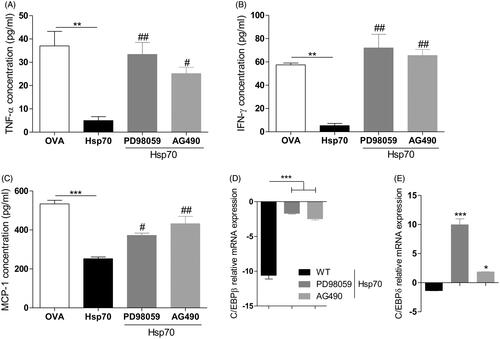
ERK and STAT3 are required for Hsp70-driven impaired IFN-γ, TNF-α and MCP-1 production and C/EBPβ/δ down-regulation
Recently, Hsp70 has been demonstrated to activate ERK and STAT3 in MDSCs [Citation32]. IL-10 production has been linked to ERK and STAT3 activation [Citation36] and STAT3 has also been shown to mediate anti-inflammatory responses [Citation37–39]. Thus we analysed the role of these two molecules on the inhibition of pro-inflammatory cytokines basal levels and down-regulation of both C/EBPβ and C/EBPδ by Hsp70. To inhibit ERK, we used the specific inhibitor PD98059. We inhibited the JAK2/STAT3 pathway using the AG490 inhibitor. Once again, Hsp70 treatment impaired IFN-γ, TNF-α and MCP-1 production when compared with OVA. However, when BMDCs were pre-treated with ERK or STAT3 inhibitors and then stimulated with Hsp70, the production of IFN-γ, TNF-α and MCP-1 observed were very similar to the basal levels (BMDCs treated with OVA) (). Both ERK and STAT3 were required for Hsp70-driven down-regulation of C/EBPβ () and C/EBPδ mRNA levels ().
Figure 3. ERK and STAT3 are required for Hsp70-induced pro-inflammatory cytokines inhibition and C/EBPβ and C/EBPδ down-regulation. BMDCs from WT mice were treated with inhibitors of ERK PD98059 or JAK2/STAT3 AG490 for 1 h prior to stimulation. After that, cells were treated with OVA, Hsp70, LPS or left unstimulated for 24 h. Culture supernatants were analysed for the presence of (A) TNF-α, (B) IFN-γ, and (C) MCP-1 using a CBA inflammation kit, (D) C/EBPβ and (E) C/EBPδ expression evaluation by qPCR. β-actin was used as a normaliser as described in Materials and methods. *p < 0.05; **p < 0.01; ***p < 0.001. #p < 0.05, ##p < 0.01 when compared with Hsp70. Experiments were performed three times in triplicates.
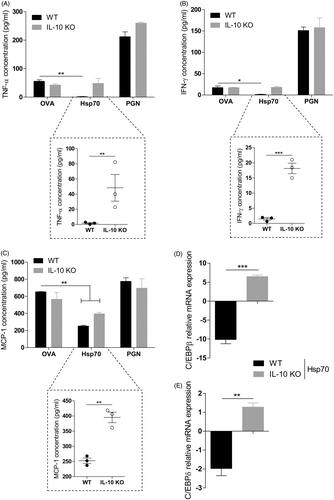
Hsp70 down-regulation of basal levels of IFN-γ, TNF-α and MCP-1 is dependent on IL-10 production by BMDC
IL-10 is the most powerful anti-inflammatory cytokine [Citation40] and its production can be triggered by Hsp70 [Citation10,Citation17]. We asked whether IL-10 is involved in Hsp70-driven down-regulation of pro-inflammatory cytokines. In order to do that, we treated WT or IL-10 KO BMDCs with either OVA, Hsp70 or PGN for 24 h. Indeed, IFN-γ and TNF-α inhibition was dependent on IL-10 expression in BMDCs treated with Hsp70 (). MCP-1 production was down-regulated in WT BMDCs treated with Hsp70 when compared with OVA. This production was re-established in IL-10 KO BMDCs (). In addition, Hsp70-driven reduced expression of C/EBPβ and C/EBPδ were both dependent on IL-10 as shown in and , respectively.
Figure 4. IL-10 is necessary for Hsp70 anti-inflammatory effects. BMDCs from WT or IL-10 KO mice were treated with OVA, Hsp70, PGN or left unstimulated for 24 h. Cytokines in the supernatants were analysed using a CBA mouse inflammation kit: (A) TNF-α, (B) IFN-γ and (C) MCP-1; (D) C/EBPβ and (E) C/EBPδ expression evaluation by qPCR. β-actin was used as a normaliser as described in Materials and methods. *p < 0.05; **p < 0.01; ***p < 0.001. #p < 0.05 and ##p < 0.01 when compared with Hsp70. Experiments were performed two or three times in triplicates.
Discussion
During acute or chronic inflammatory conditions, DCs acquire a mature phenotype in which they can produce high amounts of pro-inflammatory cytokines [Citation41]. This phenotype can be induced upon a microbial inflammatory stimulus [Citation42]. Moreover, DCs play a crucial role in the pathogenesis of autoimmunity conditions [Citation43]. Therewith, the modulation of DC activation has been suggested as an interesting strategy in the attempt to abrogate chronic inflammatory diseases, such as arthritis [Citation16,Citation44]. Indeed, one of the contributions to the powerful effect of TNF-α blockade in arthritis patients is that this treatment leads to DC impaired functions such as down-regulation of co-stimulatory signals [Citation45]. In the present work we demonstrated that immature BMDC stimulation with Hsp70 decreased the basal expression of C/EBPβ or C/EBPδ, leading to impairment in TNF-α, IFN-γ and MCP-1 production. However, we did not analyse whether Hsp70 can exert its anti-inflammatory proprieties in mature BMDCs which had been stimulated with LPS before Hsp70 treatment, for example. Moreover, we also did not analyse whether BMDCs treated with Hsp70 can prevent the up-regulation of both C/EBPβ and C/EBPδ and pro-inflammatory cytokines induced by LPS. These questions need to be further elucidated in our system. In a different study, Spiering et al. [Citation46] recently demonstrated that the administration of Hsp70-pulsed BMDCs can prevent proteoglycan-induced arthritis (PGIA) in mice. In the same work, the treatment of BMDCs with Hsp70 generates a semi-mature phenotype that was stable after the addition of LPS in culture.
In accordance with our findings, the treatment of synovial cells from arthritis patients with Hsp70 diminished TNF-α and IFN-γ production by these cells [Citation10]. MCP-1 was not measured in that previous work, but this chemokine is important for cell migration in inflammatory responses [Citation47]. We believe that MCP-1 production should be analysed in future works with human cells treated with Hsp70.
TLR2 has an interesting feature that, depending on the nature of TLR2 ligand, it can induce pro-inflammatory or anti-inflammatory responses [Citation33,Citation48,Citation49]. We observed that TLR2 plays a critical role in mediating down-regulation of C/EBPβ and C/EBPδ and inhibition of pro-inflammatory cytokines (). In contrast with our findings, macrophages treated with a Mycobacterium tuberculosis 19-kDa lipoprotein leads to up-regulation of both via TLR2 [Citation50]. This is the first time that TLR2 has been associated with a down-regulation of C/EBPβ and C/EBPδ expression.
We next tried to elucidate other molecules that could be involved in Hsp70-mediated effects found in BMDCs. Tumour-associated Hsp70 can activate ERK and STAT3 in a TLR2-dependent manner [Citation32], and both molecules can be activated downstream of TLR activation [Citation51]. We observed that after ERK inhibition, Hsp70 tolerogenic effects on BMDCs could not be observed anymore. C/EBPβ or C/EBPδ activation via the ERK pathway has been reported in chondrocytes [Citation52], monocytes [Citation53] and macrophages [Citation54]. ERK is also required for the stability of tolerogenic phenotype in DCs [Citation55,Citation56]. We believe that ERK interaction with C/EBPβ and C/EBPδ could be a possible explanation for our observations.
In addition, we observed that STAT3 was required for decreasing the basal expression of C/EBPβ and C/EBPδ, leading to an impairment in TNF-α, IFN-γ and MCP-1 production in Hsp70-treated BMDCs. Previous studies suggested that STAT3 could control transcription of C/EBPβ [Citation54] and C/EBPδ [Citation57] genes. Macrophages from STAT3 KO mice failed to induce C/EBPβ upon LPS or IL-10 stimulation [Citation58]. This regulation was suggested to be due to STAT3 binding in the distal region of C/EBPβ promoter [Citation59]. These findings strongly corroborate our observations that STAT3 can mediate C/EBP family members’ expression.
In our system, IL-10 production by BMDCs induced by Hsp70 was necessary for C/EBPβ and C/EBPδ down-regulation and concomitant inhibition of pro-inflammatory cytokines. ERK and STAT3 have been described to be involved in IL-10 signalling [Citation36] and Hsp70 effects were also dependent on this cytokine [Citation60]. C/EBPβ and C/EBPδ have been associated with the production of IL-10 in macrophages [Citation61,Citation62]; however, the effects of IL-10 over the expression of these transcription factors in different immune cells have not been investigated in detail. One study suggested that IL-10 treatment could either up-regulate C/EBPβ in THP-1 macrophages, or yet that it has no effect over undifferentiated monocytes, [Citation58]. It is possible that the regulation of C/EBP transcription factors expression depends not only on a single cytokine stimulus, but rather requires a combination of signals triggered in Hsp70-treated cells.
Aside from the anti-inflammatory effects of extracellular Hsp70, elevation of intracellular Hsp70 levels by chemical agents or thermal stress also demonstrated tolerogenic properties on DCs [Citation63], perhaps due to secretion of this protein when it is produced in elevated levels. Consequently, induction of intracellular HSPs using non-toxic chemical compounds isolated from medicinal plants [Citation64] might constitute an alternative way to induce tolerance in inflammatory conditions.
Conclusion
Our results indicate a probable mechanism employed by Hsp70 to down-regulate levels of IFN-γ, TNF-α and MCP-1, via inhibition of the expression of C/EBPβ and C/EBPδ transcription factors. Hsp70 was not capable to impair pro-inflammatory cytokines production and decrease C/EBPβ and C/EBPδ levels in TLR2 or IL-10 KO cells, or in BMDCs that had ERK or STAT3 signalling pathways inhibited. We suggest that extracellular Hsp70 signals via the TLR2-ERK-STAT3-IL-10 pathway in BMDCs to exert its strong anti-inflammatory effects.
Declaration of interest
We acknowledge financial support from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) Grant 11/0903-1 and PUCRS. T.J.B. is a recipient of a FAPERGS/CAPES fellowship. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank Taiane Garcia for technical support, Dr. Bárbara Porto (PUCRS, Brazil) for the gift of the PD98059 inhibitor, Dr. Alfeu Zanotto Filho (Federal University of Rio Grande do Sul, Brazil) for the gift of the AG490 inhibitor, and Dr. João Santana da Silva (University of São Paulo, Brazil) and Dr. Ana M.C. Faria (Federal University of Minas Gerais, Brazil) for providing mice.
References
- Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: Releasing signal(s) and function. Int J Hyperthermia 2005;21:457–71
- Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, et al. The anti-inflammatory mechanisms of Hsp70. Front Immunol 2012;3:95
- Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, et al. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol 1999;163:5560–5
- Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (HSP) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-Hsp70 homologue. J Immunol 2000;164:2711–17
- van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 2005;5:318–30
- Broere F, Wieten L, Klein Koerkamp EI, van Roon JA, Guichelaar T, Lafeber FP, et al. Oral or nasal antigen induces regulatory T cells that suppress arthritis and proliferation of arthritogenic T cells in joint draining lymph nodes. J Immunol 2008;181:899–906
- Wieten L, Berlo SE, Ten Brink CB, van Kooten PJ, Singh M, van der Zee R, et al. IL-10 is critically involved in mycobacterial Hsp70 induced suppression of proteoglycan-induced arthritis. PLoS One 2009;4:e4186
- Borges TJ, Porto BN, Teixeira CA, Rodrigues M, Machado FD, Ornaghi AP, et al. Prolonged survival of allografts induced by mycobacterial Hsp70 is dependent on CD4+CD25+ regulatory T cells. PLoS One 2010;5:e14264
- Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, et al. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem 2007;282:23240–52
- Detanico T, Rodrigues L, Sabritto AC, Keisermann M, Bauer ME, Zwickey H, et al. Mycobacterial heat shock protein 70 induces interleukin-10 production: Immunomodulation of synovial cell cytokine profile and dendritic cell maturation. Clin Exp Immunol 2004;135:336–42
- Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev 2010;238:76–92
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 2009;10:1237–44
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002;2:151–61
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010;234:45–54
- Sarkar S, Fox DA. Dendritic cells in rheumatoid arthritis. Front Biosci 2005;10:656–65
- Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:566–71
- Motta A, Schmitz C, Rodrigues L, Ribeiro F, Teixeira C, Detanico T, et al. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology 2007;121:462–72
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011;34:637–50
- Baldwin AS Jr.. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol 1996;14:649–83
- Ruland J. Return to homeostasis: Downregulation of NF-kappaB responses. Nat Immunol 2011;12:709–14
- Shi Y, Tu Z, Tang D, Zhang H, Liu M, Wang K, et al. The inhibition of LPS-induced production of inflammatory cytokines by Hsp70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock 2006;26:277–84
- Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: Possible role of NF-kappaB. Cell Stress Chaperones 2010;15:153–63
- Malhotra V, Wong HR. Interactions between the heat shock response and the nuclear factor-kappaB signaling pathway. Crit Care Med 2002;30:S89–95
- Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 1998;273:28545–8
- Lu YC, Kim I, Lye E, Shen F, Suzuki N, Suzuki S, et al. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J Immunol 2009;182:7212–21
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 1998;273:29279–82
- Hu HM, Baer M, Williams SC, Johnson PF, Schwartz RC. Redundancy of C/EBP-alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J Immunol 1998;160:2334–42
- Mehlert A, Young DB. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71-kD antigen, a member of the 70-kD heat-shock protein family. Mol Microbiol 1989;3:125–30
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase-separation using triton X-114. J Immunol Methods 1990;132:191–5
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–8
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010;120:457–71
- Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 2006;116:916–28
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 2009;15:401–9
- Filippi CM, Ehrhardt K, Estes EA, Larsson P, Oldham JE, von Herrath MG. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur J Immunol 2011;41:1399–409
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010;10:170–81
- Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, et al. A critical role for STAT3 signaling in immune tolerance. Immunity 2003;19:425–36
- Barton BE. STAT3: A potential therapeutic target in dendritic cells for the induction of transplant tolerance. Expert Opin Ther Targets 2006;10:459–70
- Dhingra S, Bagchi AK, Ludke AL, Sharma AK, Singal PK. Akt regulates IL-10 mediated suppression of TNFalpha-induced cardiomyocyte apoptosis by upregulating STAT3 phosphorylation. PLoS One 2011;6:e25009
- Kubo M, Motomura Y. Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells. Front Immunol 2012;3:275
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52
- Granucci F, Girolomoni G, Lutz MB, Foti M, Marconi G, Gnocchi P, et al. Modulation of cytokine expression in mouse dendritic cell clones. Eur J Immunol 1994;24:2522–6
- Amodio G, Gregori S. Dendritic cells a double-edge sword in autoimmune responses. Front Immunol 2012;3:233
- Hackstein H, Thomson AW. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004;4:24–34
- Baldwin HM, Ito-Ihara T, Isaacs JD, Hilkens CM. Tumour necrosis factor alpha blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann Rheum Dis 2010;69:1200–7
- Spiering R, van der Zee R, Wagenaar J, van Eden W, Broere F. Mycobacterial and mouse Hsp70 have immuno-modulatory effects on dendritic cells. Cell Stress Chaperones 2012
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol 2000;18:217–42
- Frodermann V, Chau TA, Sayedyahossein S, Toth JM, Heinrichs DE, Madrenas J. A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J Infect Dis 2011;204:253–62
- Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 2009;126:475–84
- Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: A role for toll-like receptors. Nat Rev Microbiol 2010;8:296–307
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–7
- Raymond L, Eck S, Mollmark J, Hays E, Tomek I, Kantor S, et al. Interleukin-1 beta induction of matrix metalloproteinase-1 transcription in chondrocytes requires ERK-dependent activation of CCAAT enhancer-binding protein-beta. J Cell Physiol 2006;207:683–8
- Zhang T, He YM, Wang JS, Shen J, Xing YY, Xi T. Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPbeta expression by ERK pathway activation. Anticancer Drugs 2011;22:158–65
- Niehof M, Streetz K, Rakemann T, Bischoff SC, Manns MP, Horn F, et al. Interleukin-6-induced tethering of STAT3 to the LAP/C/EBPbeta promoter suggests a new mechanism of transcriptional regulation by STAT3. J Biol Chem 2001;276:9016–27
- Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci 2006;42:1–11
- Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, et al. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood 2001;98:2175–82
- Yamada T, Tobita K, Osada S, Nishihara T, Imagawa M. CCAAT/enhancer-binding protein delta gene expression is mediated by APRF/STAT3. J Biochem 1997;121:731–8
- Tanaka N, Hoshino Y, Gold J, Hoshino S, Martiniuk F, Kurata T, et al. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol 2005;33:406–11
- Zhang K, Guo W, Yang Y, Wu J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPbeta transcription. J Cell Biochem 2011;112:488–97
- Stocki P, Dickinson AM. The immunosuppressive activity of heat shock protein 70. Autoimmune dis 2012;2012:617213
- Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 2007;110:2685–95
- Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA 2009;106:17475–80
- Spiering R, van der Zee R, Wagenaar J, Kapetis D, Zolezzi F, van Eden W, et al. Tolerogenic dendritic cells that inhibit autoimmune arthritis can be induced by a combination of carvacrol and thermal stress. PLoS One 2012;7:e46336
- Ahmed K, Furusawa Y, Tabuchi Y, Emam HF, Piao JL, Hassan MA, et al. Chemical inducers of heat shock proteins derived from medicinal plants and cytoprotective genes response. Int J Hyperthermia 2012;28:1–8
