Abstract
Heat shock proteins (HSP) are molecular chaperones and have been implicated in longevity and aging in many species. Their major functions include chaperoning misfolded or newly synthesised polypeptides, protecting cells from proteotoxic stress, and processing of immunogenic agents. These proteins are expressed constitutively and can be induced by stresses such as heat, oxidative stress and many more. The induction of HSP in aging could potentially maintain protein homeostasis and longevity by refolding the damaged proteins which accumulate during aging and are toxic to cells. HSP are shown to increase life span in model organisms such as Caenorhabditis elegans and decrease aging-related proteotoxicity. Thus, decrease in HSP in aging is associated with disruption of cellular homeostasis which causes diseases such as cancer, cell senescence and neurodegeneration. HSP levels are decreased with aging in most organs including neurons. Aging also causes attenuation or alteration of many signalling pathways as well as the expression of transcription factors such as heat shock factor (HSF). The alteration in regulation and synthesis of Forkhead box O3a (FoxO3a) family of transcription factors as well as major antioxidant enzymes (manganese superoxide dismutase, catalase) are also seen in aging. Among many signalling mechanisms involved in altering longevity and aging, the insulin/IGF-1 pathway and the Sir2 deacetylase are highly significant. This review enquires into the role of some of these pathways in longevity/aging along with HSP.
Introduction: aging and life span
In biological systems aging is known to be a complex and multifactorial process in which vital organs undergo major cell death degeneration and loss of function [Citation1]. This process is also associated with appearance of multiple diseases due to patho-physiological changes. Aging is a cumulative and irreversible process triggered by accumulation of damaged cellular macromolecules in non-dividing adult cells [Citation1,Citation2]. However, the exact intracellular lesions occurring in this process and responsible for aging are yet to be defined [Citation2]. It has been shown that protein aggregation and damage occur due to stress as well as protein misfolding and is a fundamental component of the aging process in cells and tissues which decreases life span in many organisms such as Caenorhabditis elegans [Citation3,Citation4]. The aging process in cells can influence the longevity and life span of an organism [Citation5,Citation6]. On the other hand, longevity is a quantitative trait and can be defined in many ways. One such definition includes the increase in the life span or exponential increase in prolonged life [Citation1]. Aging can also be defined as a gradual biological impairment with age during life span [Citation7]. It is a series of events resulting in a functional decline of a species or organism.
It is known that aging is associated with increased proteotoxicity, a fundamental component in the biology of human degenerative diseases [Citation8,Citation9].Therefore, the ability of HSP to counteract the proteotoxic effect in tissues is one of the major mechanisms of these chaperones by which they can promote longevity and long life. This has been demonstrated with laboratory stresses such as reactive oxygen species (ROS) or heat stress, in which native proteins become denatured. HSPs thus become engaged in chaperoning the damaged proteins by triggering the activation of heat shock response by HSF1 [Citation10].
Altered activity of signalling pathways and transcription factors in aging
Two of the major conserved signalling pathways that are responsible for aging were first characterised in C. elegans [Citation5,Citation6]. These mechanisms have been shown to influence longevity in other invertebrates. These include: 1) reduced activity of the insulin/IGF-1/mTOR pathway and 2) enhanced silent mating type information regulation (Sir2) activation (in yeast) [Citation3,Citation4]. Sir2 (in yeast, SIRT in mammals) is an NAD+-dependent histone deacetylase which catalyses a key signalling pathway involving deacetylation of lysine residues in many substrates that can influence longevity pathways [Citation4]. It has been shown in C. elegans and in the fruitfly, Drosophila melanogaster, that starvation or forced expression of Sir2, or its mammalian homologue sirtuin can enhance life span [Citation11]. In addition, it was shown in nematode worms that the loss of IGF-1 receptor/daf-2, (daf-2 in C. elegans) can increase lifespan by activating a major transcription factor, FoxO (daf-16) which in turn is responsible for Hsp16 transcription [Citation11,Citation12]. This transcription factor, daf-16/FoxO can be activated by starvation or caloric restriction, a known factor involved in increased longevity [Citation11]. Daf-16/FoxO activation requires reduced IGF-1 activity during growth factor starvation or a decrease in the PI3K/AKT/mTOR signalling pathway [Citation12]. Also, depletion of growth factors can inhibit synthetic pathways by decreasing mTOR (mammalian target of rapamycin) activity and increasing autophagic degradation (a cell survival pathway) in cells [Citation13]. Recently it has been shown that cyclic AMP responsive element binding protein (CREB) and nuclear factor erythroid-2 related factor 2 (Nrf2) are involved in the aging process and are able to increase the life span of invertebrates by activating multiple cell survival signalling pathways [Citation14,Citation15]. These pathways are also regulated by AMP activated kinase (AMPK). Greer et al. (2007) showed that AMPK can directly phophorylate FoxO3 and activate it without affecting its subcellular localisation [Citation16]. FoxO3 can also be phosphorylated by protein kinase Akt in response to growth factor or insulin and becomes inactive and sequestered in the cytosol, accounting for its inhibition by the IGF-1 pathway. Greer and colleagues [16] also showed that AMPK phosphorylation of FoxO/daf-16 is involved in life span extension during starvation in C. elegans. Thus FoxO, a target of many kinases can promote longevity and reduce the occurrence of age-dependent diseases in invertebrates in dietary restriction [Citation16]. The target genes for the AMPK-FoxO3 pathway include several antioxidants such as Gadd45 which is known to reduce DNA damage. Gadd45 can inhibit age-related inflammatory response by inhibiting NF-κB activity [Citation17]. Longevity is affected only in starvation in nematode worms and Drosophila, i.e. in the absence of growth factor or insulin. In C. elegans, FoxO, can function along with HSF to regulate small HSP synthesis in response to stress and starvation (reduced IGF1-like signalling pathway, IIS) [Citation18,Citation19]. It should be noted that deletion of the FoxO gene in nematode worms and flies has very little effect on life span if the insulin/IIS pathway is active [Citation18].
Although IIS is a key pathway for regulating life span in nematode worms and flies, there are other pathways that protect cells from oxidative stress and increase life span in these organisms. In addition to the HSF1-mediated heat shock response, other transcriptional programmes are activated by age-induced proteotoxicity. For instance, HSP genes in C. elegans are regulated by both HSF1 and FoxO [Citation5]. It was shown in C. elegans that overexpression of both the transcription factors HSF1 and FoxO can increase the life span of this organism [Citation19,Citation20]. It is significant that FoxO and HSF can activate HSP and other genes which can increase resistance of cells against stress, promoting longevity [Citation19,Citation20]. Consensus FoxO binding sites are present on HSP promoters in C. elegans [Citation3,Citation4]. Pro-longevity factors, FoxO and others in turn can be activated by ROS activated c-jun kinase (JNK) [Citation19]. This only happens during reduced IIS pathway which is the negative regulator of starvation-induced FoxO activity [Citation19]. Studies in invertebrates and animal species therefore indicate key roles for these above mentioned factors, HSF1, daf-16/FoxO, in fostering longevity [Citation19,Citation20] (). However, the many processes through which these factors interact to prolong life span in mammalian species is still not clear.
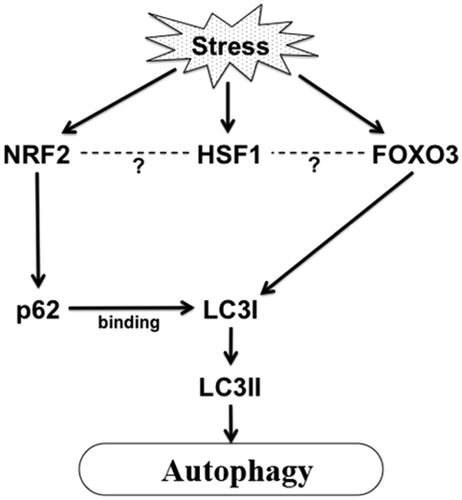
Figure 1. Stress induced activation of autophagy in aging. Proteotoxic stress or ROS or other stress in aging can activate FoxO and Nrf2 which in turn can activate autophagy by activating LC3I and sequestosome 1/p62 respectively. HSF1 is also activated in stress which may regulate transcription of Nrf2 and FoxO3 maintaining protein homeostasis in cells.
FoxO or daf-16 in C. elegans can also be activated by SIRT1 (the mammalian deacetylase homologue of yeast Sir2) dependent deacetylation during oxidative stress [Citation21]. Several studies showed that the SIRT1 signal is associated with extension of life span in caloric restriction conditions [Citation22]. This increase in SIRT1 activity during starvation or caloric restriction can increase cellular stress resistance ability, an important phenomenon in aging. It has also been shown that SIRT1 can directly deacetylate many autophagy genes (discussed below) during starvation, thereby activating the autophagy pathway. Some other findings showed that mammalian SIRT1 can alter the conditions of many age-related diseases, for instance neurodegenerative diseases, and enhance life span [Citation21]. A transcription factor for antioxidant gene products Nrf2, and its C. elegans orthologue skinhead family member 1, (SKN-1) also has the ability to extend the life span by activating many autophagic genes as well as antioxidant gene products during aging [Citation23].
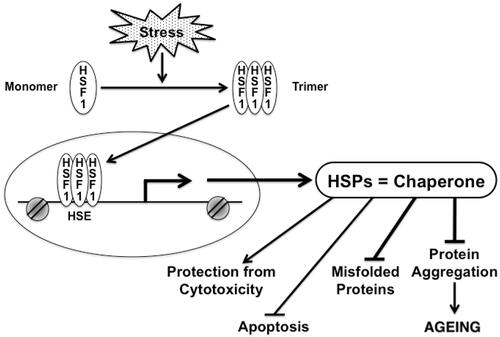
Figure 2. Chaperones and their transcription factor, HSF1 play significant role in multiple processes in cells under stress. HSF1 is activated under stress, which causes HSF1 to form a trimer. Active HSF1 then translocates to the nucleus and directly bind to the promoter of the HSP gene, thereby activating transcription. HSP can fold unfolded protein that accumulates in aging. Chaperones can protect cells from proteotoxicity, protein aggregation, apoptosis and accumulation of misfolded proteins.
HSPs and their induction
The HSP family of molecular chaperones is able to facilitate the folding of newly synthesised polypeptides and refold denatured proteins. This family consists of different molecular weight proteins (from smaller to higher), including Hsp10, Hsp27, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110, all of which are well characterised functionally [Citation24]. However, the major HSP that can bind to unfolded polypeptide or protein in the cytosol and help them to fold properly include Hsp70 and Hsp90 [Citation24,Citation25]. These chaperones are capable of forming large complexes containing accessory proteins. The client polypeptide can be associated or dissociated from these chaperone complexes with the help of energy from ATP [Citation25]. Smaller chaperones such as Hsp27 can work in association with the large ones and they mediate client holding and folding in an energy independent manner [Citation24,Citation25]. Protein folding of proteome requires coordinated activities of all these HSP. HSF1, the principal member of the HSF family of transcription factors is highly conserved and its activity is somewhat regulated through feedback by HSP [Citation26]. HSF1 is chaperoned by HSP in the cytosol and released from the complex (complexed with Hsp90 and Hsp40) under stress to undergo trimerisation followed by several modifications (such as acetylation, phosphorylation and sumoylation) [Citation26–28]. The activated HSF1 then translocates to the nucleus to bind to a consensus heat shock element (HSE) in the HSP genes as shown in . These HSPs can remove protein aggregates, inhibit apoptosis, and protect cells from the cytotoxic effect of toxic proteins as shown in . Mammalian HSF families are found in all eukaryotes [Citation29]. Decrease in HSP transcription as well as HSF1 expression during aging is seen in many tissues such as neurons and muscle which affect the protein folding and refolding ability of cells with aging [Citation30,Citation31]. For instance in the case of neurodegenerative diseases, such as Huntington’s, extended polyglutamine or polyQ (CAG) repeats are shown to be formed in neurons, and are found in toxic insoluble aggregates. Poly Q is a polymorphic CAG tract which codes for polyglutamine and is shown in many neurodegenerative diseases [Citation31,Citation49]. However, such aggregates can be suppressed by the activation of HSF1 and elevated expression of HSP as well as other components of proteotoxic machineries [Citation30,Citation31].
Role of HSP in aging
HSP protects cells from multiple cellular phenotypes during aging such as proteotoxicity, apoptosis and protein aggregation. However, aging cells undergo a decline in transcriptional pathways, including HSF1 potency and thus lose capacity to synthesise HSP [Citation32]. Decreased HSP concentrations leads, by definition to a decline in protein quality control, a change which contributes to protein aggregate formation in neuronal cells, a commonly observed phenotype in neurodegenerative diseases [Citation33]. Many neurodegenerative disorders share a similar pathological symptom, the accumulation of inclusion bodies or insoluble aggregates due to protein aggregation [Citation34]. These changes involve time-dependent loss of protein quality control. Although HSP are expressed at reduced rates in healthy neuronal cells, they appear to be able to maintain their protein quality control throughout life. The exact mechanism of aggregate formation and neuronal cell death is not clearly known, but they can be formed due to loss of protein quality control due to aging [Citation35].
Age-dependent decline of HSP is not restricted to neuronal cells and is also seen in muscle cells. In addition, decreased HSP expression in the liver causes cells to be affected by toxins. Even though the ability of HSF1 to bind to HSP genes with stress such as heat decreases in certain mammalian cells in aging, there is evidence of increased expression of HSP in a variety of tissues in disease conditions in some aging cells. Drosophila aging is associated with up-regulation of Hsp22 and Hsp70 which requires HSF1 binding in the promoter region of both genes [Citation36]. However decrease in HSF1 levels in cells might be due to inhibition by HSP or their chaperone activity in cytosol.
Role of HSP on autophagy in aging
Autophagy is one of the most important survival pathways that is somewhat controlled by HSP. Autophagy was first seen as an emergency response to starvation, and permits a proportion of the cytosol to be autolysed to provide nutrients for survival. However, autophagy is now known also to be a component of protein quality control due to capacity to degrade bulky cellular components such as protein aggregates. Starvation-induced autophagy may thus confer enhanced capacity for protein aggregate resolution and potentially enhanced longevity. The major autophagy pathways in cells are macro-autophagy and chaperone mediated autophagy (CMA) [Citation37]. During CMA, constitutively active HSP, Hsc70 recruits cytosolic proteins to the lysosomes for subsequent degradation. CMA is activated both in starvation and oxidative stress and is involved in degradation of 30% of cellular proteins containing KFERQ (CMA recognition motif) [Citation37]. CMA substrates are delivered to lysosomal membrane receptor LAMP2A, which helps substrate to enter into the lumen, accepted by lumenal Hsc70 and later degraded. Both Hsc70 and LAMP2A expressions are modulated during starvation and oxidative stress and the level of LAMP2A declines during senescence [Citation37]. In neurodegenerative diseases such as in Parkinson’s the CMA pathway declines [Citation38]. Autophagy is also decreased in aging which in turn affects life span. In Drosophila it was shown that when autophagy genes are inactivated, flies become short lived and also protein aggregates are formed in their nervous system, suggesting the importance of this pathway in maintaining protein quality control [Citation39]. It was shown in nematode worms, that autophagy is responsible for longevity and both FoxO and Nrf2 factors can regulate protein synthesis required for the autophagy pathway in mammalian cells [Citation40,Citation15] (). shows how autophagy, a cell survival pathway can be activated under stress. For instance Nrf2 can directly activate sequestosome 1/p62 synthesis, a component of autophagosome formation [Citation40,Citation41] (). Sequestosome 1/p62 protein is localised at the autophagosome formation site and interacts with LC3 (microtubule light chain), the primary component of autophagosome formation. Later it is incorporated into the autophagosome and finally degraded. On the other hand, FoxO3 is able to regulate autophagy through activating LC3I (unpublished data, Murshid and Calderwood) in mammalian cells (). LC3I becomes lipidated by conjugating with PE (phosphatidylethanolamine) to form LC3II, which associates with autophagosomes (). Consensus FoxO binding sites are found in the promoter regions of small HSP in C. elegans. All these findings suggested that these factors; HSF1, FoxO and Nrf2 play vital roles in aging-related complications such as removal of protein aggregates, increasing stress induced autophagy, thereby enhancing the life span of an organism [Citation15,Citation40]. One of the future studies should include exploring the mechanisms in mammalian cells by which life span can be increased by altering the expression of HSF1, Nrf2 and FoxO in vivo ().
Effect of HSP on proteotoxicity in aging
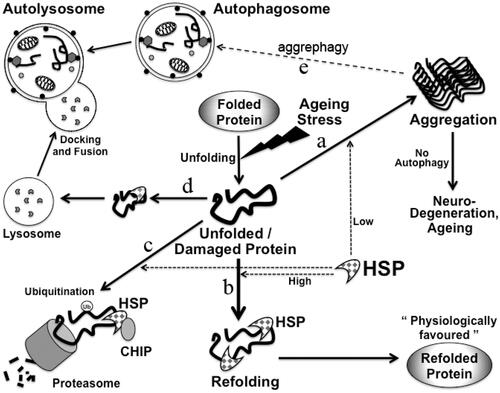
Proteotoxicity is commonly seen in aging. The toxic substances of cells include ROS, damaged or toxic proteins which may arise from apoptosis, cell membrane disruption or misfolded protein aggregation [Citation42]. HSP prevent cells from generation of these toxic products and genes, facilitating survival during stress. Consistent with the role of FoxO in autophagy in C. elegans, HSF1 and FoxO can act simultaneously to remove protein aggregates formed from misfolded proteins [Citation5,Citation21,Citation23]. Thus polyQ aggregates can be removed either by proteasomal or lysosomal degradation with the help of activity of these two transcription factors. Misfolded proteins which arise in aging cells may have different potential fates as summarised in . These proteins can either go on to form aggregates, or can be folded correctly by the help of chaperones such as Hsp70 and Hsp90 [Citation9]. These proteins can also become polyubiquitinylated with the help of ubiquitin E3 ligases such as carboxyl terminus of Hsc70-interacting protein (CHIP) and later degraded. Other fates of misfolded or damaged proteins include lysosomal degradation by CMA as in . Protein aggregates can be degraded by alternate autophagy pathway, also termed as aggrephagy () [Citation43].
Figure 3. Maintenance of protein homeostasis by HSP in cells. Damaged or misfolded proteins accumulate in cells in aging. Protein homeostasis needs to be maintained in cells in aging to avoid aging-related diseases. Possible fates of misfolded proteins are shown: misfolded/unfolded proteins if not refolded can form aggregates (a), unfolded proteins can be refolded by HSP (b). These misfolded proteins can be degraded in proteasome through binding to Hsp70 and CHIP. CHIP leads to ubiquitinylation of these proteins (c). These misfolded proteins can also be transported to the lysosomes for degradation by CMA (d). Protein aggregates can also be degraded by aggrephagy (e).
Effect of HSP on immune response in aging
The immune response decreases with aging but it is not known whether HSP is involved in this phenomenon. HSP level decreases in cells with aging, and it has been shown that the serum concentration of Hsp70 in a healthy individual decreases with age as well [Citation44]. However, in diseased conditions increase in serum Hsp70 level is associated with inflammatory pathology [Citation44]. FoxO3 was also shown to promote immune function especially innate immunity [Citation45]. However, FoxO3 decreases inflammation in the mammalian system in a healthy individual. FoxO3 might thus play a role in controlling inflammation by reducing the activation of some of the inflammatory genes [Citation45].
HSP and apoptosis in aging
Apoptosis plays a key role in aging due to depletion of essential cell populations such as motor neurons that occurs in degenerative processes [Citation46]. Molecular chaperones such as (Hsp27 and 70) are anti-apoptotic and induced in response to anticancer drugs [Citation47]. FoxO is involved in promoting apoptosis. PI3K/Akt phosphorylation of FoxO can increase cell survival by sequestering it in cytosol [Citation16,Citation18]. On the other hand FoxO can indirectly down-regulate BCL-xL a pro-survival Bcl2 family member, thereby increasing apoptosis [Citation48]. FoxO can activate both the intrinsic and extrinsic apoptotic pathways. The intrinsic pathway requires mitochondria and activation of caspase-9 whereas the extrinsic pathway is mediated by the activation of death receptors and caspase-8. FoxO can directly activate pro-apoptotic factors such as Fas ligand (FasL) or CD95L, and TNF-related apoptosis-inducing ligand, extrinsic pathway (TRAIL) [Citation49]. Thus activation of FoxO can be both pro-survival and pro-apoptotic factor and it is therefore necessary to regulate FoxO activity to maintain the balance between survival and death in aging cells.
Conclusion and future prospects
Several aging-related physiological complications such as neurodegenerative diseases are often associated with accumulation of misfolded proteins or toxic protein aggregates. With such conditions it is necessary for chaperones to refold the protein and remove aggregates from cells by CMA or other processes. Altered synthesis of HSP and cochaperones can bring back the normal physiology of cells in aging. It is necessary to understand how the levels of chaperones are maintained in aging and how we can alter their level in order to decrease aging-related complications and hence increase life span.
Our understanding and knowledge about the signalling pathways and role of transcription factors such as HSF1, FoxO and Nrf2 that affect aging and life span is increasing. These factors regulate a highly divergent range of genes and likely involve interaction between the transcriptional networks. However, future studies should involve deciphering the roles of these transcription factors as well as their possible interaction in aging cells of the mammalian system. The protective roles of HSP in cellular processes such as proteotoxicity, apoptosis, neurodegenerative diseases and cancer make it unique as an important component in regulation of aging and longevity. HSP can also inhibit programmed cell death in aging and thereby play vital role in cell survival. It can be anticipated that increase in HSP can enhance life span and longevity, whereas decreased HSP is associated with reduction in life span due to increase in age-related diseases and cell death. The role of this HSF1-HSP system in aging is still needs to be elucidated. Unravelling mechanisms of aging through this factor and others as well as HSP and their interacting partners and finding targets for modification of these in the aging process in mammalian models and human subjects could help to decrease aging and increase the life span of organisms.
Declaration of interest
This work was supported by US National Institutes of Health research grants RO-1CA047407, R01CA119045 and RO-1CA094397. The authors alone are responsible for the content and writing of the paper.
References
- Lithgow GJ. Why aging isn’t regulated: A lamentation on the use of language in aging literature. Exp Gerontol 2006;41:890–3
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative diseases and aging. Genes Dev 2008;22:1427–38
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age onset proteotoxicity. Science 2006;313:1604–10
- Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell 2005;120:449–60
- Tissenbaum HA. Genetics, life span, health span, and the aging process in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 2012;67:503–10
- Leavy SA. The last of life: Psychological reflections on old age and death. Psychoanal Q 2011;80:699–715
- Fontana L, Partridge L, Longo VD. Extending healthy life span – From yeast to humans. Science 2010;328:321–6
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 2008;319:916–19
- Calderwood SK, Murshid A, Prince T. The shock of aging: Molecular chaperones and the heat shock response in longevity and aging – A mini-review. Gerontology 2009;55:550–8
- Kim YH, Park EJ, Han ST, Park JW, Kwon TK. Arsenic trioxide induces Hsp70 expression via reactive oxygen species and JNK pathway in MDA231 cells. Life Sci 2005;77:2783–93
- Weinkove D, Halstead JR, Gems D, Divecha N. Long-term starvation and aging induce AGE-1/PI 3-kinase-dependent translocation of DAF-16/FOXO to the cytoplasm. BMC Biol 2006;4:1
- Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol 2009;7:e60
- Uddin MN, Ito S, Nishio N, Suganya T, Isobe K. Gadd34 induces autophagy through the suppression of the mTOR pathway during starvation. Biochem Biophys Res Commun 2011;407:692–8
- Shenvi SV, Smith E, Hagen TM. Identification of age-specific Nrf2 binding to a novel antioxidant response element locus in the Gclc promoter: A compensatory means for the loss of glutathione synthetic capacity in the aging rat liver? Ageing Cell 2012;11:297–304
- Lewis KN, Mele J, Hornsby PJ, Buffenstein R. Stress resistance in the naked mole-rat: The bare essentials – A mini-review. Gerontology 2012;58:453–62
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007;282:30107–19
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr., DiStefano PS, et al. DNA repair pathway stimulated by the Forkhead transcription factor FOXO3a through the Gadd45 protein. Science 2002;296:530–4
- Ruaud AF, Katic I, Bessereau JL. Insulin/insulin-like growth factor signaling controls non-Dauer developmental speed in the nematode Caenorhabditis elegans. Genetics 2011;187:337–43
- Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem 2009;284:28319–31
- Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, et al. Lifespan extension and elevated HSP gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol 2005;208:697–705
- Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 2005;16:237–43
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: The ‘magnificent seven’, function, metabolism and longevity. Ann Med 2007;39:335–45
- Vanduyn N, Settivari R, Wong G, Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci 2010;118:613–24
- Calderwood SK. Molecular Chaperone and the Ubiquitin Proteasome System in Ageing. New York: Nova, 2007
- Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci 2005;62:670–84
- Wu C. Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol 1995;11:441–69
- Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, et al. Induction of heat shock proteins by heregulin β1 leads to protection from apoptosis and anchorage-independent growth. Oncogene 2005;24:6564–73
- Murshid A, Chou SD, Prince T, Zhang Y, Bharti A, Calderwood SK. Protein kinase A binds and activates heat shock factor 1. PLoS One 2010;5:e13830
- Gidalevitz T, Prahlad V, Morimoto RI. The stress of protein misfolding: From single cells to multicellular organisms. Cold Spring Harb Perspect Biol 2011;3:1--18
- Nagai Y, Fujikake N, Popiel HA, Wada K. Induction of molecular chaperones as a therapeutic strategy for the polyglutamine diseases. Curr Pharm Biotechnol 2010;11:188–97
- Zhang Y, Murshid A, Prince T, Calderwood SK. Protein kinase A regulates molecular chaperone transcription and protein aggregation. PLoS One 2011;6:e28950
- Hou Y, Wei H, Luo Y, Liu G. Modulating expression of brain heat shock proteins by estrogen in ovariectomized mice model of aging. Exp Gerontol 2010;45:323–30
- Gagliano N, Grizzin F, Annoni G. Mechanisms of aging and liver function. Dig Dis 2007;25:118–23
- Gestwicki JE, Garza D. Protein quality control in neurodegenerative disease. Prog Mol Biol Transl Sci 2012;107:327–53
- Wagstaff MJ, Collaço-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem 1999;274:5061–9
- Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of Drosophila survival under normal and stress conditions. J Gerontol A Biol Sci Med Sci 2009;64:828–38
- Massey AC, Zhang C, Cuervo AM. Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol 2006;73:205–35
- Xilouri M, Stefanis L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med 201;13
- Jaiswal M, Sandoval H, Zhang K, Bayat V, Bellen HJ. Probing mechanisms that underlie human neurodegenerative diseases in Drosophila. Annu Rev Genet 2012;46:371–96
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 2012;11:230–41
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010;12:213–23
- Friguet B, Bulteau AL, Petropoulos I. Mitochondrial protein quality control: Implications in aging. Biotechnol J 2008;3:757–64
- Øverbye A, Fengsrud M, Seglen, PO. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy 2007;3:300–22
- Njemini R, Bautmans I, Onyema OO, Van Puyvelde K, Demanet C, Mets T. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol 2011;12:24
- Wątroba M, Maślińska D, Maśliński S. Current overview of functions of FoxO proteins, with special regards to cellular homeostasis, cell response to stress, as well as inflammation and aging. Adv Med Sci 2012;57:183–95
- Chopek JW, Gardiner PF. Life-long caloric restriction: Effect on age-related changes in motoneuron numbers, sizes and apoptotic markers. Mech Ageing Dev 2010;131:650–9
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005;10:86–103
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, et al. The Forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem 2002;277:14255–65
- Marfè G, Tafani M, Fiorito F, Pagnini U, Iovane G, De Martino L. Involvement of FOXO transcription factors, TRAIL-FasL/Fas, and sirtuin proteins family in canine coronavirus type II-induced apoptosis. PLoS One 2011;6:e27313
