Abstract
Purpose: This paper presents the treatment of a 12-year-old female spayed Great Dane who presented with vestibular signs (ataxia, nystagmus, hind end collapse). Thoracic radiographs revealed a discrete pulmonary nodule in the right cranial lung lobe. Ultrasound-guided fine needle aspirate detected primary bronchoalveolar adenocarcinoma, verified via computed tomography, with a second smaller nodule discovered in the right cranial lung lobe.
Materials and methods: A lateral thoracotomy with right cranial lung lobectomy was performed. Histopathological analysis of the nodules and an excised lymph node identified grade III bronchoalveolar adenocarcinoma with vascular infiltration and lymph node metastasis – a grim diagnosis with a reported median survival time of 6–27 days. A 10-g sample of the tumour was processed into a chaperone-rich cell lysate (CRCL) vaccine, which was administered weekly to the patient. Imiquimod – a Toll-like receptor 7 (TLR7) agonist – was applied topically for the first 12 treatments to stimulate local Langerhans cells. A single injection of bacillus Calmette-Guerin (BCG) was administered for additional immune stimulation at week 30 of treatment.
Results: The dog remained stable and in otherwise good health until diffuse relapse occurred 44 weeks after the initial treatment; following gastrointestinal bleeding, the dog was euthanised 50+ weeks post diagnosis.
Conclusion: To the authors’ knowledge, this is the first report of significantly prolonged survival following a diagnosis of grade III/stage III bronchoalveolar adenocarcinoma in a canine patient. This case report suggests that CRCL vaccine combined with topical imiquimod is a safe, effective treatment for canine tumours.
Introduction
Autologously derived cancer vaccines offer a promising avenue for the treatment of many malignancies. Based on a free solution-isoelectric focusing (FS-IEF) technique, we have developed chaperone-rich cell lysate (CRCL) vaccines – capable of eliciting potent anti-tumour immune responses against a wide variety of murine model tumour types [Citation1–8]. This has been demonstrated in vivo in murine models, and in in vitro settings with both murine and human cells [Citation3,Citation6]. To date, however, this is the first study reported of a canine response to actual clinical CRCL treatment.
CRCL vaccines are enriched for a diverse array of heat shock proteins (HSPs) and associated/chaperoned peptides; during FS-IEF, the bulk of the HSPs fractionate out in approximately a pH 5–6 range and serve as both adjuvant-type stimulants (danger signals) while providing antigens in the form of peptides for the adaptive/effector response [Citation2,Citation4,Citation5,Citation8–10].
GRP94/gp96, Hsp90, Hsp70 and calreticulin are examples of the chaperone proteins found in CRCL which have demonstrated immunogenicity individually and we have identified some 60 peptides through proteomic/mass spectrometry analysis [11]. We surmise that the strength of CRCL lies in the collective enrichment of a conglomerate of chaperones/HSPs acting as innate immune danger signals, and other entities (proteins, peptides), contributing to a specific (targeting) stimulus resulting in activation of a more robust antigenic response from T cells, B cells, and NK cells [Citation2,Citation3,Citation6–10,Citation12–16].
While CRCL and other HSP-based vaccines provide adjuvant capacity in the danger signal format, in a clinical setting increased stimulation is often sought. Forms of adjuvant stimuli to increase local inflammation and non-specific stimulation of dermal antigen presenting cells (APCs) include topical applicants such as imiquimod (IMQ) and injectables such as QS21, or BCG [Citation17–19,Citation20–25]. These adjuvants are making their ways into clinical trials.
Primary pulmonary tumours are extremely rare in dogs, accounting for ∼1% of all canine malignancies. High grade tumours of this histology carry a remarkably grim prognosis, and treatment is aimed mainly at palliation [Citation26–29]. The canine patient in this case report presented with stage III, grade III bronchoalveolar adenocarcinoma – a diagnosis indicative of an invasive, aggressive phenotype. This malignancy generally does not metastasise to distant parts of the body, but had already moved into the nearby tracheobronchial lymph nodes in this patient before surgical intervention occurred. Little research has been done into this type of tumour in dogs. Only two studies with an n > 50 dogs were found to reference median survival times with this advanced stage and grade of bronchoalveolar adenocarcinoma following surgical excision. These described a range of 6 to 60 days, leading to median survival times of 6–27 days, with a disease free interval of 6 days [Citation27–30] This patient was enlisted as the first canine CRCL trial participant as the chemotherapeutics employed for this type of canine cancer often display low response rates (e.g., 10–12%, usually <5 months survival) [Citation31]. She received 44 rounds of CRCL, the first 12 of which were supplemented with IMQ. Periodic radiographs, multiple CTs and weekly physical examinations revealed no signs of recurrence or decline in health up until the last couple of weeks of treatment. She displayed few, if any, ill effects of treatment, and outlived her prognosis by >10 months. Serum antibodies over the course of treatment displayed reactivity against the tumour. Preliminary analysis of the primary and metastatic tumours revealed none of the common mutations in BRAF, K-RAS, or EGFR; however, there was a mutation in the DNA binding domain of p53. This case study provides preliminary evidence that the CRCL vaccine is a safe and effective treatment in dogs with strong clinical therapeutic potential, though a larger trial is needed to fortify these results.
Materials and methods
Surgical excision
A right lateral thoracotomy was completed to access the neoplastic tissue in this case. Briefly, an incision was made through the skin and cutaneous trunci musculature parallel to the fifth rib along the intercostal space on the right side – extending from the costovertebral junction to the sternum. Blunt dissection was employed to enter the pleural cavity thereafter. The ribs were retracted with a Finochietto rib spreader to allow visibility of the malignant nodules within the lung. The two malignant nodules were contained within the right cranial lung lobe – necessitating a lung lobectomy to achieve good margins. The right cranial lung lobe was visualised and the affected lobe, including pulmonary vasculature and mainstem bronchus were ligated and excised at the level of the hilus utilising a TA-30 stapling device (V3 cartridge). Additionally, a tracheobronchial lymph node was excised for histopathological analysis to determine the stage of the tumour. A thoracostomy tube was then placed, and the incision was routinely closed. Recovery was successful and uneventful, and the patient was discharged two days after the procedure.
CRCL preparation
Preparation of the CRCL vaccine was kindly completed by Immunovative Therapies, located in Jerusalem, Israel. Ten grams of raw tumour from the patient were collected from surgery, frozen on dry ice, and shipped overnight to Immunovative Therapies. The tumour sample was prepared as CRCL by procedures previously described [Citation2,Citation4,Citation5,Citation7,Citation8,Citation32], under Good Manufacturing Practices (GMP) conditions as established by the World Health Organization (WHO). The purified CRCL vaccine was then shipped to the client and stored at −80 °C until it was thawed to ambient temperature just prior to administration.
CRCL administration
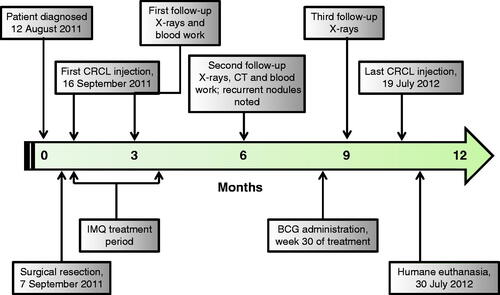
Vaccination with CRCL was performed on a weekly basis for 44 weeks commencing on day 7 after the patient returned home following her lung lobectomy. Prior to each injection, a temperature, heart rate, and respiratory rate were obtained. Additionally, thoracic auscultation was completed, and a thorough veterinary examination was done to ensure that the patient was in good health. Two 8 cm × 6 cm rectangular injection sites were clipped, scrubbed with chlorhexidine and allowed to dry prior to administration of the CRCL vaccine, each on the right side of the patient. One site was located just caudal to the prominence of the scapula at the approximate level of the fourth rib, while the other was positioned just cranial to the flank. Imiquimod topical cream was applied to the scapular site 1 h prior to injection with CRCL for the first 12 treatments, and allowed to absorb. Imiquimod was then applied to this same site immediately following these treatments [Citation17–19]. At each of the two sites, 25 μg of CRCL in 250 μL phosphate-buffered saline was instilled intradermally, for a total dose each week of 50 μg in 500 μL. Temperature, heart rate, and respiratory rates were monitored 10, 20 and 30 min following each injection, with no significant variability from the patient’s normal resting values. BCG was given at week 30 in the flank site as an adjuvant in an attempt to stimulate further immune reaction. This 0.1 mL injection containing 8.1 mg BCG (Sanofi Pasteur, Toronto, Canada) stimulated formation of a large 11 cm × 6 cm welt at the flank site 12 days following its administration. This timeline has been observed in human subjects as well. The site was mildly tender upon manipulation and appeared erythematous, but the overt inflammation subsided approximately 1 month after its formation. The treatment scheme is shown in .
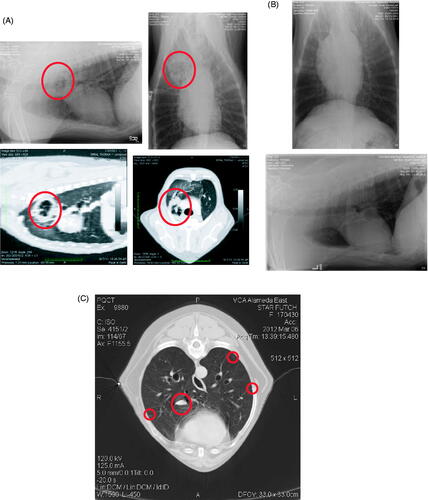
Figure 1. Timeline delineating critical events during the case study. The vaccine was administered once a week for 44 weeks, from the first administration on 16 September 2011. Imiquimod was applied topically before and after the vaccine was given for the first 12 weeks of treatment. BCG was administered prior to the vaccine at week 30. Recurrent nodules were seen on radiographs/CT on 6 March 2012 – 6 months into treatment. The patient was euthanised on 30 July 2012, following development of plural effusion and a significant gastrointestinal haemorrhage.
Monitoring
This case study employed multiple repeated imaging modalities, repeated blood work analysis, and weekly physical examinations for monitoring purposes and to ensure safety of treatment. At the initial veterinary visit, thoracic radiographs were used to initially diagnose the disease, followed by CT and ultrasound-guided biopsy for histopathological evaluation for grading and staging of the tumour. Thoracic radiographs were repeated at 3-month intervals from the surgical resection to monitor for recurrence of the primary tumour and to evaluate for the development of pulmonary metastatic disease. An additional CT was completed at month 6 following surgery for follow-up evaluation. Blood was drawn at initial presentation and a complete blood count and blood biochemistry were evaluated. This was repeated at the month 6 visit, and again 1 week prior to euthanasia. Weekly physical examination took place by a veterinarian, which included assessment of vital signs, thorough auscultation of the thorax, palpation of lymph nodes, abdominal palpation, evaluation of mentation, assessment of appetite and energy level, and gait analysis.
Cell culture techniques
Immediately after humane euthanasia, multiple metastatic pulmonary nodules were excised and placed in Neurobasal-A (NBA) media supplemented with 200 mM L-glutamine, N-2, and B-27 (all purchased from Invitrogen/Life Technologies, Carlsbad, CA, USA). All initial cultures were additionally supplemented with penicillin-streptomycin at a concentration of 20 mL/L and 2.5 μg/mL amphotericin-B to prevent contamination due to non-sterile collection methods (also purchased from Invitrogen Life Technologies). Upon arrival at the laboratory, these tumours were dissociated into single cell suspensions and placed in fresh media supplemented with the additives listed above, and incubated at 37 °C and 5% CO2. Cells were monitored daily and split when at 70% confluence. Cells were fed approximately every 3–10 days, depending on cell growth requirements and nutrient utilisation. Cell stocks were frozen down in NBA media supplemented with ProFreeze freezing medium (Lonza, Allendale, NJ, USA) and 10% DMSO when stability of the individual cultures was established. Thaw and re-freezing experiments were conducted to ensure stability of the lines.
Exosome harvest
Exosomes were harvested from spent cell culture medium by filtration, concentration, and ultracentrifugation as described [Citation33]. Approximately 7 mg of exosomes (by protein) were obtained from 500 mL of spent medium.
Tumour, cell and exosome lysis
Tumour pieces from both primary and metastatic tumours were homogenised in glass/glass homogenisers in radio-immune precipitation assay (RIPA) buffer (Sigma, St Louis, MO, USA) supplemented with Complete Protease Inhibitor Cocktail Tablets (Roche, Indianapolis, IN, USA). Homogenates were clarified by centrifugation at 10 000 × g, and supernatants were used in subsequent experiments. Cells and exosomes from tissue culture were lysed as described [Citation33].
Western blots
Tumour, cell, and exosome lysate proteins were prepared for SDS-PAGE and western blotting as described [Citation33]. Patient anti-sera (from prior to vaccine treatment, mid-stage of treatment, and at euthanasia) were diluted 1:100 in 5% non-fat dry milk (NFDM) (Carnation/Nestle, Wilkes-Barre, PA, USA) Tris-buffered saline with 0.5% Tween 20 (TBST) (Sigma) and probed on blots of tumour/cell/exosome lysates (25 µg per lane) overnight at 4 °C. After washing with TBST, blots were probed with anti-canine IgG (Innovative Research, Novi, MI) at a 1:10 000 dilution in NFDM/TBST for 1 h. Blots were developed with chemiluminescent substrate and imaged as described by Epple et al. [Citation33].
Results
Initial presentation
The 12-year-old female spayed Great Dane presented with likely geriatric vestibular disease with typical signs (ataxia, nystagmus, hind end collapse) on 12 August 2011. An MRI was performed at this time, which displayed no CNS abnormalities. While the presenting complaints resolved without treatment after a few days, other diagnostic studies completed at this initial veterinary visit revealed the presence of a lung mass that was determined to be a carcinoma upon cytological and histopathological analyses of the fine needle aspirate. Other neurological studies did not indicate stroke or nerve damage. The only prior pulmonary indication was an occasional dry cough.
Weekly physical examination
The treatment scheme/vaccination schedule for the course of therapy is shown in . Temperature, heart rate, respiratory rate, mucous membrane colour and character, and overall health status were monitored prior to administration of the vaccine each week. None of these vital signs ever deviated out of the normal range for canines when the vaccine was given, for the duration of the study. Just prior to the third vaccination, the patient contracted a dry, non-productive cough which resolved 2 days after being noticed and did not return. Also of note, the patient developed an 11 cm × 9 cm × 3 cm inflamed welt on day 12 after the BCG injection was given at week 30 of treatment. This inflammation caused some soreness but resolved without incident 3 weeks after the injection was administered. Otherwise, the patient was in good health for the duration of the study, until 1 week prior to euthanasia, when she developed exercise intolerance, lethargy, inappetence, and dyspnoea. Euthanasia was performed after approximately 2 L of pleural fluid was removed and she suffered from an episode of gastrointestinal haemorrhage.
Pathology report
As mentioned above, initial fine needle biopsy performed on the site of the identified lung mass indicated carcinoma; further histopathological analysis of the initial tumour nodules removed by lung lobectomy identified pleomorphic, rounded nuclei with prominent nucleoli, significant numbers of mitotic figures, poorly demarcated margins, marked invasion into surrounding tissues, and diffuse areas of necrosis and inflammation. This biopsy also displayed vascular and lymphatic infiltration into surrounding areas, and tracheobronchial lymph nodes excised at the same time displayed clumps of tumour cells within the lymph node parenchyma. The summation of these findings concluded in a grade III, stage III delineation for this case of bronchoalveolar adenocarcinoma (verified by multiple pathologists at another institution).
After humane euthanasia, a necropsy was completed to determine the extent of disease recurrence, determine any metastatic involvement, and to evaluate for tissue changes at injection sites. At necropsy, both lungs were diffusely riddled with multifocal to coalescing, poorly demarcated, locally invasive, unencapsulated adenocarcinomas. Areas of necrosis admixed with macrophages and fewer neutrophils and lymphocytes were present within a number of the masses. Multiple metastatic nodules were also present within the splenic parenchyma. The vasculature and lymphatics within the mediastinal connective tissue also displayed invasion of neoplastic epithelial cells, similar to those within the lung parenchyma. The injection site tissue was grossly unaffected in the shoulder region. There were no signs of inflammation or tissue remodelling despite >30 repeated injections in the region. However, the flank site tissue where the BCG injection was given was grossly inflamed, and histopathology showed necrotic lesions through multiple tissue layers. At this site, a subcutaneous haemangiosarcoma had formed, with neoplastic spindle cells dissecting through the underlying skeletal muscle layers. These findings support a negative reaction to the BCG administration. However, imiquimod topical treatment (applied to the shoulder site) and autologous vaccine intradermal injection (given at both sites) seemed to be well tolerated. Nervous system tissues including brain and spinal cord were evaluated by a pathologist as well, with no abnormalities found – indicating that the patient’s initial presenting clinical signs were likely unrelated to her malignancy.
Blood work
A blood draw completed on 4 April 2010 at a senior wellness check-up was utilised as a healthy baseline value for this patient. It was determined from prior wellness visits that the patient had a historical elevation in alanine aminotransferase (ALT) – a liver enzyme – that was determined to be inconsequential in monitoring the patient’s overall health status throughout the duration of the case study. At diagnosis, a complete blood count (CBC), blood biochemistry, packed cell volume (PCV) with total protein (TP), urinalysis (UA), and prothrombin time (PT) were completed. The results of repeated CBC and biochemical analyses displayed only clinically insignificant abnormalities throughout treatment, with the exception of a progressive leucopenia. For instance, this patient displayed a high ALT value throughout numerous years of monitoring prior to development and discovery of her malignancy; this finding was deemed clinically irrelevant, given chronicity. The WBC value dropped throughout the study, starting at 9.4 × 103/mm3 at initial diagnosis, dropping below normal to 5.5 × 103/mm3 at the date of tumour resection, and at a 6-month post-op blood work analysis on 6 March 2012, fell to 5.1 × 103/mm3 (Normal range of 6–17 × 103/mm3). The final WBC count taken one week prior to euthanasia displayed an increasingly deficient value of 3.2 × 103/mm3. Differential analysis of the WBC populations was completed at the time of tumour resection, revealing 86.4% neutrophils, 10.2% lymphocytes, 8.4% eosinophils and 3.4% monocytes. A differential count was also completed at the 6 March 2012 visit, finding 84.2% neutrophils, 12.3% lymphocytes, 5.6% eosinophils, and 3.5% monocytes.
Imaging studies
The initial thoracic radiographic studies completed at diagnosis displayed one radio-opaque nodule within the right cranial lung lobe. This instigated more thorough review with CT imaging, at which time two nodules were found and fine needle aspirates were collected. After surgical resection of the affected lung lobe, repeated thoracic radiographs were conducted 3 months post-op to monitor for metastatic disease, as this tumour type, stage and grade almost always displays rapid ipsolateral or contralateral pulmonary metastasis. This initial post-surgical resection study was clean. However, at the patient’s 6-month follow-up study, further nodules were seen on the thoracic radiographs. This 6-month study was then followed up with a repeat CT, which displayed a milliary nodular metastatic pattern despite complete lack of clinical signs. Radiographs and CT images are shown in .
Gene mutation analysis
Typical regions of mutations for EGFR, KRAS, and BRAF in human lung tumours were sequenced from the canine primary and metastatic tumours, as well as from a cell line grown from a metastatic tumour. No mutations were found (data not shown). However, in exon 6 of the TP53 (p53) gene, there was a G-to-T transition that resulted in a missense mutation at residue 192 (Q192H). This is in the DNA binding domain of p53, where such mutations are associated with significantly reduced disease-free and overall survival in patients with breast cancer [Citation34].
Immune response – western blots with patient sera
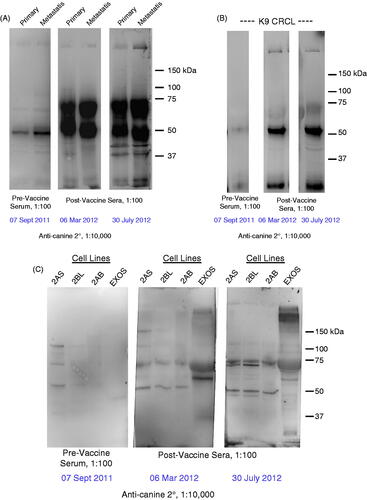
After surgical harvest of the primary tumour, and following euthanasia, solid tumour pieces from the primary and metastatic tumours were obtained for lysate preparation as a source of proteins for western blotting. Additionally, a number of independent cell lines were generated from two of the metastatic nodules; all of these cells were grown in Neurobasal A medium with appropriate supplements. Despite the serum-free conditions, the cells grew as tight, adherent monolayers. Cells and exosomes were collected from the tissue culture cells and were also used as protein sources for western blotting. Probing samples with patient anti-sera from pre-vaccination, approximately midway through treatment, and at euthanasia revealed increased anti-tumour titres over time, as well as increased antibody reactivity against the metastatic tumour versus the primary tumour. The range of antigens also increased upon vaccination, but the immunoreactive species did not appear to change considerably over the course of vaccination. Results are displayed in .
Figure 3. Western blot results using patient anti-sera to probe lysates prepared from (A) primary and metastatic tumours, (B) the CRCL preparation that was used as a vaccine, (C) cell lines generated from metastatic tumours and exosomes harvested from those lines. In each case, the sera are diluted 1:100, and consist of serum taken prior to receiving any vaccines (pre-vaccine serum), and sera taken ∼6 months post-vaccine initiation and ∼10 months post-vaccine initiation. Anti-canine IgG-HRP (1:10 000 dilution) was used as a secondary antibody, and blots were developed with ECL reagents. Molecular weight markers are shown on the right.
Discussion
Given the extremely poor prognosis of this patient’s disease, there were very few options for treatment beyond palliative care. Thus, a novel cancer vaccine strategy was employed. While undergoing treatment with CRCL and other immune-promoting adjuvants, the patient experienced prolonged survival of 10 months beyond the expectation [Citation27,Citation28]. Despite extensive resection, surgical excision of the initial nodules did not successfully contain this patient’s disease, as metastatic cells had already disseminated into the surrounding lung parenchyma, lymph nodes, and blood vessels as seen upon initial histopathological analysis. Though this specific tumour did not display the more common mutations seen in human pulmonary tumours in BRAF, KRAS, and EGFR, there was a mutation in the DNA binding domain of p53. Those mutations are associated with shorter disease-free and overall survival in patients with breast cancer [Citation34]. While in various human tumour types, as many as 50% of the tumours have mutated p53 expression [Citation35], the prevalence of p53 mutations in canine tumours seems much lower [Citation36,Citation37] but do appear to be associated with worse prognosis [Citation38,Citation39]. The particular mutation we identified (Q192H) does not appear to have been previously identified in canine tumours. The mutational status of this particular tumour notwithstanding, the invasive, aggressive nature was clearly demonstrated. Repeated analyses of radiographs and blood work showed that in the context of this treatment visual and systemic signs of disease were staved off until month 6. The patient maintained good lung function and quality of life for an additional 4 months, despite known recurrence.
IMQ is a topical TLR7 agonist licensed to treat basal cell carcinoma, superficial squamous cell carcinoma and superficial malignant melanoma in humans [Citation17–19]. Dermal Langerhans cells, APCs distributed through the skin, become activated by imiquimod and migrate to local lymph nodes to secrete proinflammatory cytokines such as interleukin-6, tumour necrosis factor-α, and interferon-α. These immunostimulatory cytokines act downstream to activate natural killer cells, B-cells and macrophages. Hence, imiquimod was topically applied in this study prior to vaccination with CRCL to ramp up the patient’s APC migration and immune response. The inflammatory side effects seen with imiquimod treatment in humans were not demonstrated to be detrimental in this case study. The treatment was well-tolerated, with only mild, transient erythema presenting at the site of application. On pathologic evaluation, treatment with imiquimod left no visible or histologic evidence of harmful effect. Though efficacy was not directly evaluated, imiquimod was found to be a safe treatment in this case. Thus, pretreatment with topical imiquimod appeared to be safe, though a skin biopsy would have been necessary to prove efficacy of this adjunctive treatment.
The patient received an extensive number of vaccinations (44), due in part to the high yields obtainable with the CRCL preparation protocol resulting in many applicable doses from a given amount of material (∼1 mg of vaccine material per gram of tumour starting material). The rationale for high numbers of treatments is based on the exploratory landmark analyses from a phase III clinical trial using autologous, tumour-derived gp96 (GRP94 under the trade name vitespen) to treat patients with metastatic melanoma [Citation40]. In that trial it was shown that patients with less advanced disease who received 10 or more immunisations had clinically significant survival benefit over the same sub-set of patients receiving physicians’ choice for treatment. Thus, given the lack of obvious side-effects from CRCL immunisations, we chose to treat our patient weekly, and for as long as possible.
Because the patient displayed progression of metastatic disease on a repeated thoracic radiograph completed in week 30 of treatment, an additional one-time immunostimulatory treatment was added to her protocol in that week as an intradermal injection in the flank vaccination site prior to CRCL injection. Bacillus Calmette-Guerin (BCG) was administered for its immunostimulatory effects against numerous cancer types [Citation19–25]. The BCG vaccine is an attenuated live vaccine prepared from Mycobacterium bovis – a strain of bovine tuberculin bacilli. Historically, it has been utilised to inoculate humans against tuberculosis. It has also been approved as an adjunctive therapy for superficial bladder cancers in humans when administered intravesically, and has also undergone human trials in colorectal and lung cancers, as well as for treatment of melanoma. Mechanistically, this vaccine has been found to enhance the anti-cancer activities of currently available therapies by causing release of immunostimulatory cytokines specifically targeted against cancer cells. Unfortunately this immunotherapy has produced adverse local effects in human patients due to strain variability, including the development of severe eschars at injection sites. The BCG injection was initially well tolerated by the patient in this study. However, she developed a large 11 cm × 9 cm × 3 cm area of painful inflammation at the flank site 12 days post-injection. Pathological examination of the area after humane euthanasia revealed severe tissue necrosis penetrating through the dermal and muscle layers – nearly extending to the level of the peritoneum. In addition, a subcutaneous haemangiosarcoma had formed under the site, potentially due to the inflammation amidst tissue necrosis, exacerbated by an already-activated immune response from the CRCL vaccinations. Thus, BCG administration provoked the most adverse effect seen in this trial, with development of severe, painful tissue inflammation, which resolved in a relatively short time frame and at no point impeded the dog’s mobility. The necropsy results displayed extensive underlying tissue damage from this treatment, which has displayed variable effects across species. While we have no direct evidence of immune benefit from the BCG treatment, the patient continued with a high quality of life despite metastatic disease. Nonetheless, further use of such immune adjuvants will have to be carefully considered.
Since we were only able to obtain blood from this patient when she was under anaesthesia, immune monitoring was limited to only three low-volume blood draws over the course of therapy. Thus, we could not obtain data concerning T cell activities, but we were able to monitor humoral responses by western blotting, using her sera to probe immunoblots of the primary and metastatic tumours. We did see an increase in anti-tumour titres as gauged by intensified reactivity in westerns on an increasing range of tumour proteins over time. This does suggest that immune responses were engaged, and CRCL has been shown to generate bioactive antibodies against a known tumour antigen [Citation6]. HSP110 has also been demonstrated to lead to both T- and B-cell responses against chaperoned antigen, indicating that HSP-based vaccines may drive both TH1 and TH2 responses with favourable outcomes [Citation41–46]. Future trials will need to be incorporated to improve capabilities for obtaining sufficient blood for better cellular-based immune monitoring.
This patient developed progressive leucopenia over the course of her treatment; the significance of this is unclear. However, increased lymphocyte counts in canines with tumours have been associated with advanced tumour stages [Citation47] and worse prognosis (at least in canines with osteosarcoma) [Citation48]. Thus, the lowered WBC counts may reflect a more positive outcome due to treatment, or may imply that lymphocytes were infiltrating the tumour and were thus removed from circulation. Alternatively, it may be that the tumour ‘counterattacks’ and kills CD95-expressing lymphocytes via CD95L (Fas ligand) [Citation49]. While the mechanisms and physiology behind this leucopenia remain unknown, these clinical parameters should also be monitored in future trials.
In this case report we show that weekly CRCL interdermal vaccination was simple, safe, and well tolerated by the patient, and no overtly detrimental signs were seen on necropsy. The patient responded extremely well with prolonged survival of over 10 months past the literature median survival time (MST). Previously, reports on this tumour type showed metastatic tropism only to the lung; however, this patient’s metastatic disease was also found in the spleen, indicative of either an extremely aggressive disease, or the natural history of the tumour if survival is prolonged far beyond the typical time span. We clearly need to extend the patient repertoire for further validation, and more extensive immune monitoring would clarify the mechanism of how this treatment leads to such positive results beyond the model setting, with a spontaneous tumour in a large animal.
Conclusion
Historically, very few studies have been completed on primary canine pulmonary tumours. For one, they are quite rare in comparison to the incidence in humans. Many cases that present to veterinarians are too advanced to treat, often ending in short palliative care or immediate euthanasia given the extremely rapid progression and ensuing decline in quality of life. Additionally, very few treatments are available for these types of tumours, and chemotherapeutic trials almost always have detrimental side effects that compromise quality of life in these patients. These tumours also display robust chemoresistance to the treatment options available, culminating in a limited window of therapeutic benefit. The few epidemiological studies that do exist connote a very poor prognosis with survival times of less than a month for this stage and grade of tumour. Given these statistics, a study with this autologous vaccine that prolonged survival time of this patient to 11 months is an extremely encouraging finding. The lack of clinical side effects and ease of administration in comparison to some standard chemotherapeutics also makes it an attractive option worth additional study. Another benefit to further developing this immunotherapeutic agent, even if used in an adjunctive sense, is that it can be produced from small, 100 mg samples of any solid type of tumour – vastly increasing its utility. Finally, the costs associated with production and administration of this autologous vaccine are much lower than with other chemotherapeutic regimens. A more thorough multi-patient study is required to confirm the safety and efficacy of this treatment, and to delve deeper into immunological monitoring, mechanisms of action, and overall feasibility.
Declaration of interest
This work was supported by the University of Colorado Cancer Center (anonymous gift to M.W.G.), and the Anschutz Foundation (to K.O.L. and M.W.G.). L.M.E. is a Skippy Frank Fund Scholar. M.W.G. has served as a consultant for Immunovative Therapies, which has licensed CRCL for clinical use. The authors alone are responsible for the content and writing of the paper.
Supplementary Material
Download PDF (31.3 KB)Acknowledgements
The authors wish to thank the staff at VCA Alameda East for care and support, Colin Catel for additional assistance, and Jason Cordeiro for help in the end.
References
- Chen X, Zeng Y, Li G, Larmonier N, Graner MW, Katsanis E. Peritransplantation vaccination with chaperone-rich cell lysate induces antileukemia immunity. Biol Blood Marrow Transplant 2006;12:275–83
- Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother 2000;49:476–84
- Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res 2000;6:909–15
- Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003;52:226–34
- Kislin KL, Marron MT, Li G, Graner MW, Katsanis E. Chaperone-rich cell lysate embedded with BCR-ABL peptide demonstrates enhanced anti-tumor activity against a murine BCR-ABL positive leukemia. FASEB J 2007;21:2173–84
- Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol 2007;148:136–45
- Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood 2003;101:4485–91
- Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother 2006;55:329–38
- Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood 2002;100:4108–15
- Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood 2003;101:245–52
- Graner MW, Romanoski A, Katsanis E. The ‘peptidome’ of tumour-derived chaperone-rich cell lysate anti-cancer vaccines reveals potential tumour antigens that stimulate tumour immunity. Int J Hyperthermia, epub ahead of print. DOI: 10.3109/02656736.2013.793406
- Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood 2001;97:3505–12
- Larmonier N, Cantrell J, Lacasse C, Li G, Janikashvili N, Situ E, et al. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol 2008;83:1049–59
- Li G, Andreansky S, Helguera G, Sepassi M, Janikashvili N, Cantrell J, et al. A chaperone protein-enriched tumor cell lysate vaccine generates protective humoral immunity in a mouse breast cancer model. Mol Cancer Ther 2008;7:721–9
- Smith DF, Whitesell L, Katsanis E. Molecular chaperones: Biology and prospects for pharmacological intervention. Pharmacol Rev 1998;50:493–514
- Zeng Y, Chen X, Larmonier N, Li G, Sepassi M, Marron M, et al. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer 2006;119:2624–31
- Schon M, Schon MP. The antitumoral mode of action of imiquimod and other imidazoquinolines. Curr Med Chem 2007;14:681–7
- Shi C, Xiong Z, Chittepu P, Aldrich CC, Ohlfest JR, Ferguson DM. Discovery of imidazoquinolines with Toll-like receptor 7/8 independent cytokine induction. ACS Med Chem Lett 2012;3:501–4
- Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Zitvogel L, et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 2012;1:894–907
- Darieva ZA, Lasunskaia EB, Kipnis TL, Dias Da Silva W. Two BCG vaccine formulations prepared from the same strain with different J774 macrophage activation capacities and patterns of NF-kappaB induction. Int J Mol Med 2000;6:575–80
- Magno C, Melloni D, Gali A, Mucciardi G, Nicocia G, Morandi B, et al. The anti-tumor activity of bacillus Calmette-Guerin in bladder cancer is associated with an increase in the circulating level of interleukin-2. Immunol Lett 2002;81:235–8
- Makino M, Mukai T. [Enhanced activation of T lymphocytes by urease-deficient recombinant bacillus Calmette-Guerin producing heat shock protein 70-major membrane protein-II fusion protein]. Nihon Hansenbyo Gakkai Zasshi 2012;81:199–203
- Saban MR, Hellmich HL, Simpson C, Davis CA, Lang ML, Ihnat MA, et al. Repeated BCG treatment of mouse bladder selectively stimulates small GTPases and HLA antigens and inhibits single-spanning uroplakins. BMC Cancer 2007;7:204
- Segawa N, Iwamoto Y, Azuma H, Yamamoto K, Ueda H, Katsuoka Y. [Intravesical BCG therapy for superficial bladder cancer]. Hinyokika Kiyo 1998;44:627–31
- Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL-2 secreting recombinant bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol 2000;164:526–31
- LaRue SM, Withrow SJ, Wykes PM. Lung resection using surgical staples in dogs and cats. Vet Surg 1987;16:238–40
- Polton GA, Brearley MJ, Powell SM, Burton CA. Impact of primary tumour stage on survival in dogs with solitary lung tumours. J Small Anim Pract 2008;49:66–71
- McNiel EA, Ogilvie GK, Powers BE, Hutchison JM, Salman MD, Withrow SJ. Evaluation of prognostic factors for dogs with primary lung tumors: 67 cases (1985–1992). J Am Vet Med Assoc 1997;211:1422–7
- Ogilvie GK, Haschek WM, Withrow SJ, Richardson RC, Harvey HJ, Henderson RA, et al. Classification of primary lung tumors in dogs: 210 cases (1975–1985). J Am Vet Med Assoc 1989;195:106–8
- Ogilvie GK, Weigel RM, Haschek WM, Withrow SJ, Richardson RC, Harvey HJ, et al. Prognostic factors for tumor remission and survival in dogs after surgery for primary lung tumor: 76 cases (1975–1985). J Am Vet Med Assoc 1989;195:109–12
- Poirier VJ, Burgess KE, Adams WM, Vail DM. Toxicity, dosage, and efficacy of vinorelbine (Navelbine) in dogs with spontaneous neoplasia. J Vet Intern Med 2004;18:536–9
- Zeng Y, Graner MW, Feng H, Li G, Katsanis E. Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat BCR-ABL+ murine leukemia. Int J Cancer 2004;110:251–9
- Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette, RJ, et al. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS One 2012;7:e42064
- Vegran F, Rebucci M, Chevrier S, Cadouot M, Boidot R, Lizard-Nacol S. Only missense mutations affecting the DNA binding domain of p53 influence outcomes in patients with breast carcinoma. PLoS One 2013;8:e55103
- Velculescu VE, El-Deiry WS. Biological and clinical importance of the p53 tumor suppressor gene. Clin Chem 1996;42:858–68
- Lee CH, Kim WH, Lim JH, Kang MS, Kim DY, Kweon OK. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J Vet Sci 2004;5:63–9
- Lee CH, Kweon OK. Mutations of p53 tumor suppressor gene in spontaneous canine mammary tumors. J Vet Sci 2002;3:321–5
- York D, Higgins RJ, LeCouteur RA, Wolfe AN, Grahn R, Olby N, et al. TP53 mutations in canine brain tumors. Vet Pathol 2012;49:796–801
- Kirpensteijn J, Kik M, Teske E, Rutteman GR. TP53 gene mutations in canine osteosarcoma. Vet Surg 2008;37:454–60
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: The C-100-21 Study Group. J Clin Oncol 2008;26:955–62
- Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res 2002;62:1737–42
- Manjili MH, Park J, Facciponte JG, Subjeck JR. HSP110 induces ‘danger signals’ upon interaction with antigen presenting cells and mouse mammary carcinoma. Immunobiology 2005;210:295–303
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol 2001;166:490–7
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer 2003;105:226–31
- Wang XY, Manjili MH, Park J, Chen X, Repasky E, Subjeck JR. Development of cancer vaccines using autologous and recombinant high molecular weight stress proteins. Methods 2004;32:13–20
- Wang XY, Yi H, Yu X, Zuo D, Subjeck JR. Enhancing antigen cross-presentation and T-cell priming by complexing protein antigen to recombinant large heat-shock protein. Methods Mol Biol 2011;787:277–87
- Itoh H, Horiuchi Y, Nagasaki T, Sakonju I, Kakuta T, Fukushima U, et al. Evaluation of immunological status in tumor-bearing dogs. Vet Immunol Immunopathol 2009;132:85–90
- Sottnik JL, Rao S, Lafferty MH, Thamm DH, Morley PS, Withrow SJ, et al. Association of blood monocyte and lymphocyte count and disease-free interval in dogs with osteosarcoma. J Vet Intern Med 2010;24:1439–44
- Igney FH, Krammer PH. Immune escape of tumors: Apoptosis resistance and tumor counterattack. J Leukoc Biol 2002;71:907–20