Abstract
In this review article we present basic principles of magnetically induced heat generation of magnetic nanoparticles for application in magnetic particle hyperthermia. After explanation of heating mechanisms, the role of particle–particle as well as particle–tissue interactions is discussed with respect to achievable heating power of the particles inside the tumour. On the basis of heat transfer theory at the micro-scale, the balance between generated and dissipated heat inside the tumour and the resulting damaging effects for biological tissue is examined. The heating behaviour as a function of tumour size is examined in combination with feasible field strength and frequency. Numerical calculations and experimental investigations are used to show the lower tumour size limit for tumour heating to therapeutically suitable temperatures. In summary, this article illuminates practical aspects, limitations, and the state of the art for the application of magnetic heating in magnetic particle hyperthermia as thermal treatment of small tumours.
Introduction
The healing effect of heat has been well known since ancient times. In one of the first scientific publications on this issue, Busch found that cancer growth ceased at temperatures above 42 °C whilst healthy tissue can tolerate such high temperatures [Citation1]. Nowadays, hyperthermia of tumour tissue is an approved therapy for cancer [Citation2,Citation3] and is defined as local temperature increase in tumours aiming to achieve selective damage of tumour tissue.
The heating of biological tissue can be achieved by several physical mechanisms including microwave irradiation applied via radiofrequency antennas (dielectric heating) [Citation4–6], Ohmic heating via electrode-applied high frequency currents [Citation7–11], optical laser irradiation via fibres [Citation12] or water bath heating such as whole body hyperthermia [Citation13,Citation14]. With these heating methods, it remains a challenge to control the spatial extent of heating in tissue.
Magnetic particle hyperthermia improves the precision of heating by embedding the heating source (magnetic particles) into the tumour tissue and heating it using an external alternating magnetic field. First investigations on this topic were presented more than 50 years ago [Citation15]. In the early years phantom studies and animal experiments were used to investigate this method [Citation16–19]. More recently, magnetic particle hyperthermia has shown success in the first clinical trials [Citation20–24]. These clinical trials have demonstrated the efficacy of magnetic hyperthermia for prostate cancer and gliomas and showed that patients can tolerate this therapy without discomfort or serious side effects. An excellent review article about different aspects of hyperthermia was recently published by Ortega and Pankhurst [Citation25].
The recent success of magnetic hyperthermia in cancer therapy is very promising but the method still needs further improvement before it can become a standard medical procedure [Citation26]. In particular, two main tasks need to be addressed: First, a safe, comfortable, and reproducible application of particles to the tumour region is needed. This includes, besides the realisation of a reliable intratumoral injection, the examination of questions concerning antibody targeting and the resulting cellular uptake as well as the toxicity of different formulations or the particles themselves. Second, absorbing materials capable of reaching and maintaining therapeutic temperatures inside tumour tissue need to be improved.
The aim of the present article is to discuss the physical and materials science aspects of magnetic nanoparticle (MNP) development for hyperthermia. In particular, this discussion will take into account framework conditions for magnetic heating as they occur during real hyperthermia therapy experiments. The prospects as well as the limitations of this promising cancer therapy will be shown.
Basic principles of magnetic particle heating
Magnetic hysteresis
If magnetic materials such as dry magnetic nanoparticles are exposed to an external magnetic field, their magnetisation undergoes a closed loop during reversal of orientation: the hysteresis loop. This loop is characterised by three material-dependent parameters: saturation magnetisation Ms, remanent magnetisation (remanence) MR, and coercivity Hc [Citation27]. The area within the loop measures the magnetic energy delivered in the form of heat to the material of the magnetic particles during reversal of magnetisation. The energy conversion to heat is caused by the coupling of the atomic magnetic moments to the crystal lattice.
In macroscopic samples of magnetic materials, magnetic domains which are separated by domain walls usually exist [Citation28]. In the so-called multi-domain state, reversal of the magnetisation direction takes place via domain wall displacement and hysteresis energy is comparatively low. Below a defined particle size, the multi-domain state becomes energetically unfavourable and each particle represents a single magnetic domain. For magnetite, for example, the transition between the states at room temperature occurs at about 30 nm particle diameter [Citation29]. Single domain particles with uniaxial anisotropy, which were theoretically treated in the classical paper of Stoner and Wohlfarth [Citation30], may show the highest amount of hysteresis energy that can be expected. With the field parallel to the aligned particle axes, a rectangular hysteresis loop is observed which gives maximum hysteresis loss Q and specific loss power (SLP):
These losses occur only if the external field exceeds the coercivity field, otherwise no reversal of the magnetic moments and consequently no losses occur. The measure ‘coercivity’ contains the contribution of magnetic anisotropy to the magnetic behaviour of nanoparticles. This magnetic anisotropy is mainly determined by effects of crystal anisotropy, shape anisotropy, and surface anisotropy.
For a rough estimate of maximum SLP, let us consider magnetite (Fe3O4) which is one of the most useful candidates for hyperthermia applications. For a saturation magnetisation MS = 480 kA/m, a typically achievable coercivity of Hc =30 kA/m, a density ρ = 5240 kg/m3 and the feasible frequency f = 500 kHz, according to Equation 1 one may expect a rather large magnetic specific loss power of about 7 kW/g. The saturation magnetisation was handled here as constant but in reality it also shows dependencies, including a dependence on particle size [Citation31,Citation32] or coating materials [Citation33]. However, in practice this maximum amount of heating power is rarely realised due to the occurrence of relaxation processes.
Magnetic relaxation (superparamagnetism)
Magnetic hysteresis is a non-equilibrium process. This means that after shutting off the external field the macroscopic remanent magnetisation will tend to vanish with a typical relaxation time τ due to activation by thermal energy (k = Boltzmann constant). For magnetic single domain nanoparticles, the energy barrier
(against reversal of the magnetisation) decreases with decreasing particle volume V and the relaxation time is given by
where K is the magnetic anisotropy and the characteristic flipping time τ0 is of the order of 10−9 s. The process is commonly called Néel relaxation [Citation34]. It is the only relaxation process occurring in nanoparticles being immobilised, for instance in tumour tissue [Citation35]. However, as shown by Hergt et al. [Citation36], the Néel relaxation model presents no optimum conditions for hyperthermia.
In fluid magnetic particle suspensions, a relaxation path other than Néel relaxation may occur due to the ability of particles to rotate freely which commonly is termed Brownian relaxation [Citation37]. With that rotation the comparatively high magnetic anisotropy barrier may be avoided when the external field changes direction. The relaxation time of that rotation may be approximated by
where η is the viscosity of the carrier liquid and Vh is the effective hydrodynamic volume. Vh may differ from the geometric volume of the particle, for instance due to adhering fluid layers.
Particles choose the simplest path to achieve reversal of magnetisation with respect to the external magnetic field. In other words, reversal occurs by the process with the smallest relaxation time constant [Citation38]. The transition between the active relaxation modes depends, according to Equations 2 and 3, on the particle volume. Closer inspection of the equations reveals that for small diameters Néel relaxation is more favourable than Brownian relaxation. In contrast to what is sometimes stated in the literature, the occurrence of Brownian relaxation is not only connected to superparamagnetic particles, but rather can also be observed for particles of any size if the viscosity of the surrounding medium is low enough to allow rotation of the complete particle. A very comprehensive treatment of relaxation effects is given by Coffey and Kalmykov [Citation39].
When magnetic particles are injected into tissue and are taken up by tumour cells, they are fixed within the cells and no Brownian relaxation may occur when an alternating magnetic field is applied [Citation35,Citation40]. In consequence, contrary to some discussions in the literature, Brownian relaxation in fluid suspensions is not useful for hyperthermia, as discussed later in this article in more detail.
For discussions of the magnetisation process in alternating magnetic fields, linearity of the magnetisation process is often assumed [Citation41]; that is, the magnetic susceptibility of the particle ensemble is taken to be independent of the external magnetic field H. But in reality this independence is fulfilled only for small field amplitudes. For an approximation of real behaviour, the relaxation problem in the case of electrical polarisation of molecules has already been treated by Debye [Citation42]. The principles discussed by Debye hold for magnetisation relaxation also, and one of his basic formulas is commonly used for the description of the frequency dependence of the imaginary component [Citation43,Citation44] of susceptibility χ″: [Citation45]
The heating power during relaxation [Citation46] results from
or by using the mass density ρ the specific loss power results:
According to Equation 5, rising field amplitude or frequency or both seem to be promising for hyperthermia because of the resulting higher SLP. Continued increases, however, lead beyond the validity region of the Néel relaxation model. This is true for two reasons: First, rise of frequency may extend beyond the regime of superparamagnetism, and second, rise of field amplitude leads out of the validity range of linear approximation. Furthermore, the advantage of higher frequencies may be severely diminished by the drawback of only small field amplitudes realisable at high frequencies, in particular considering the relatively large field volume needed for a whole body application. Moreover, as criticised by Hergt and co-workers [Citation36], many authors overlook the limiting suppositions of the Néel relaxation model valid for higher frequencies. In particular, magnetic energy must be smaller than thermal energy
which does not hold for large field amplitude. Thus, to achieve large SLP, one has to take into consideration larger, thermally stable single domain particles which show magnetic hysteresis [Citation47–51].
Summarising the considerations and formulas above, it becomes obvious that specific heating power (SHP) depends on magnetic properties of applied MNP (which show a strong dependency on particle size and size distribution, besides other materials parameters) and amplitude and frequency of alternating magnetic field. The measure ‘SHP’ provides the real heating potential of MNP determined experimentally in an alternating magnetic field by means of caloric measurements – contrary to the calculated SLP on the basis of particle and field parameters. It was shown experimentally [Citation52] and in numerical simulations [Citation50] that certain combinations of particle size, size distribution as well as field frequency and amplitude lead to maximum in SHP/SLP. This means that for each applied magnetic field, tailor-made MNP are necessary to obtain maximum SHP or vice versa, the applied field has to be adapted on the magnetic properties of the MNP used. General advice to exploit the heating potential of the particles is to use a field frequency of several hundred kHz in combination with rather low field amplitude (few kA/m) for superparamagnetic particles and a relatively high field amplitude (a few tens of kA/m) in combination with a frequency of a few hundred kHz for MNP with hysteretic behaviour.
Here we constrain the treatment of the wide-ranging issue of correlation between particle parameters and resulting magnetic losses to the necessary facts to understand the following sections. Additional details to mechanisms of nanoparticle heating are given by Dennis et al. in this issue (pp. 715–29).
Influence of particle interactions
Particle–particle interactions (agglomeration)
In the literature, ferrofluids are often described as coated magnetic single cores, homogeneously dispersed in a carrier medium and showing constant distances between single cores. But in reality, especially when injected into the body, agglomeration of the single cores takes place due to biological effects, magnetic attraction or van der Waals forces. Using Monte Carlo simulations, Chantrell [Citation53] showed that dipolar chains and flux closure configurations might form in a magnetic particle system of higher concentrations. Castro et al. [Citation54] found that at volume fractions of about 1%, a relatively small part of agglomerated particles are only dimers. Above about 10% volume fraction, more than half of the particles are bound in agglomerations larger than trimers. The chain formation for relatively large particles (about 30 nm diameter) is well known from magnetotactic bacteria synthesising fine magnetite crystals [Citation55].
The dependency of magnetic properties on packing density for a system of identical uniaxial particles was predicted by numerical methods used to simulate quasistatic remagnetisation processes in fine magnetic particle systems [Citation56]. In experimental investigations [Citation57–59], the alteration of magnetic properties by changing the packing density of ferrimagnetic particles was confirmed. Dutz and Hergt [Citation57] found an exponential decrease of hysteresis losses down to 50% when changing packing density from 0.24% up to 67.6%. Urtizberea et al. [Citation59] described an increase in heating power of about 100% when the concentration was decreased by a factor of four.
From the found dependency of magnetic properties on particle packing density as shown in the results above, it can be stated that for ferrimagnetic particles an increasing packing density (decreasing distance between particles) leads to decreasing coercivity, remanence, and hysteresis losses of the single particle. These decreases are due to increasing dipole–dipole interactions which disturb the energy landscape by occurrence of local energy minima [Citation60]. A controversial behaviour was expected for superparamagnetic particles which show weak dipole–dipole interactions. Similar to ferrimagnetic particles, for the case of higher concentrations a stronger tendency to form agglomerates can be observed. Due to weak dipole–dipole interactions, the exchange interactions dominate and the agglomerates behave like a particle with an effective volume larger than the single core showing ferromagnetic behaviour with hysteresis. This agglomeration hypothesis is supported by experimental studies where increasing coercivity and heating power were found for agglomerated superparamagnetic nanoparticles in comparison to single cores [Citation61–63].
Multicore nanoparticles are a special type of particles with behaviour that cannot be described in the classical sense by superparamagnetic or ferrimagnetic theory. These particles consist of a number of superparamagnetic cores of around 10 nm each which form clusters a few tens of nanometers large [Citation64,Citation65]. The overall behaviour of these particles is ferrimagnetic, but coercivity and remanence are significantly lower than for single core particles of comparable size. Surprisingly, the heating power of multicore nanoparticles is higher than that of single core particles of comparable coercivity. Their special magnetic behaviour makes such particles very interesting for hyperthermia [Citation35] and other medical applications (e.g. magnetic particle imaging [Citation66]) despite the fact that their remagnetisation behaviour is not completely understood [Citation67,Citation68].
Particle–tissue interactions
When using magnetic nanoparticles as a heating source for magnetic particle hyperthermia, it is of particular interest to know whether the particles are free to move in the interstitial fluid or whether they are fixed to the tumour tissue. The immobilisation state determines which relaxation mechanism of the administered particles occurs, which in turn determines the attainable SHP of the particles inside the body. Many authors consider the loss mechanisms to be due to Néel relaxation [Citation69] for smaller particles, or Brownian relaxation [Citation41] for larger ones, assuming the particles are able to show Brownian rotation inside the tumour. However, if there is a hindrance of Brownian rotation in the tumour tissue, only Néel relaxation takes place for superparamagnetic particles, or hysteretic reversal for ferrimagnetic ones.
To clarify the immobilisation state, in animal experiments MNP were injected directly into experimentally grown subcutaneous mouse tumours and the magnetic behaviour of the particles inside the tumour tissue was investigated by magnetorelaxometry [Citation40] and vibrating sample magneto-metry [Citation35] immediately after sacrifice of the animals. For the investigated combinations of particle and tumour types, immobilisation of the injected particles immediately after application was found. The bonding between particle and tissue is rather strong () and hampers a rotation of the particles even for relatively high magnetic fields of up to 25 kA/m, which is an upper limit for the application of a feasible magnetic field strength in hyperthermia.
Figure 1. Comparison of the normalised minor loops for mobile MNPs (fluid), immobilised MNPs (gelatine), and MNPs inside the tumour (tumour) at a magnetic field amplitude of 25 kA/m measured under quasi static conditions, (from [35], with permission from IoP).
![Figure 1. Comparison of the normalised minor loops for mobile MNPs (fluid), immobilised MNPs (gelatine), and MNPs inside the tumour (tumour) at a magnetic field amplitude of 25 kA/m measured under quasi static conditions, (from [35], with permission from IoP).](/cms/asset/a1952764-922a-4c07-a5a1-59f34cc53c75/ihyt_a_822993_f0001_b.jpg)
The results shown in , where particles in tumour tissue show a hysteresis curve similar to immobilised particles, mean that for particles administered directly into tumour tissue, the contribution of Brownian relaxation to reversal losses can be neglected. Only a small proportion of Brownian relaxation losses due to oscillation (rotation of very limited torsion due to the elasticity of the tissue) is conceivable. Thus, for a reliable hyperthermia with therapeutically suitable temperatures inside the tumour, particles showing high heating power due to hysteresis or Néel relaxation have to be used.
Typically, in hyperthermia community heating rates of particles are determined on liquid samples considering Néel and Brownian losses which might not be appropriate for hyperthermia treatment as discussed above. For a reliable determination of heating potential of MNP measurement, conditions similar to those of particles in tissue have to be used. This includes consideration of the same magnetic field amplitude and frequency as at the place of tumour tissue inside the body as well as an immobilisation state of particles similar to the immobilisation state in tumour tissue. To obtain representative values and results comparable to these of other groups, standards for preparation of MNP for SHP measurements have to be defined, for example the definition of ‘intrinsic loss power’ (ILP) discussed below.
The results from Dutz et al. [Citation35] show a strong fixation of particle to tissue. But in their investigation only one type of particle and one type of tumour tissue was used. Since it was shown in previous investigations that cellular uptake is strongly influenced by coating material [Citation70], this aspect has to be expanded in further investigations to obtain more information about particle–tissue interactions and their influence on heating power of MNP. The reasons for observed differences in tissue heating are very complex. For sure, particle–tissue interactions play a key role for success of hyperthermia and need detailed investigation in further studies.
Heat transfer in tumour tissue
Temperature elevation
The magnetic energy supplied by the external alternating magnetic field is converted into heat within the magnetic nanoparticles, which is depleted into the surrounding medium. The attainable temperature in the particle-containing tissue is determined by the balance between heat generation in the particles and the depletion of heat into the tissue. The latter depletion process is mainly due to heat conduction, which depends on the distribution of particles located in the cell plasma or in the interstitial volume between tumour cells. Though the particle-containing tissue is inhomogeneous (e.g. cell membranes, varying cell size, cell shape and packing), one spatially constant heat conductivity (nearly that of water) is assumed in most calculations. The heat conduction problem resulting from balance of generated and depleted heat was treated analytically and experimentally by Andrä et al. [Citation71] and was confirmed in [Citation72]. Odenbach and co-workers [Citation73] recently presented a phantom design for closer investigation of such questions and confirmed the theory of Andrä et al. for a broader range of heat conductivity. The configurations considered in the Andrä et al. study apply to any spherical region of internal constant heat production; irrespective of whether that region contains many homogeneously distributed small particles or only one large particle.
Results of the calculations from Andrä et al. are summarised in . They show how rapidly the temperature increases within the heated zone after turning on the magnetic field and how steeply it declines outside of the spherical heat generation zone. After the initial steep rise in temperature, temperature reaches a saturation value (steady state) and a nearly constant temperature is approached within the heated zone. Reasonably, the results scale with the specific heating power of the particles.
Figure 2. Initial temporal development of the spatial dependence of temperature elevation around a spherical region of heat generation; radius of the heat generation zone 3.15 mm.
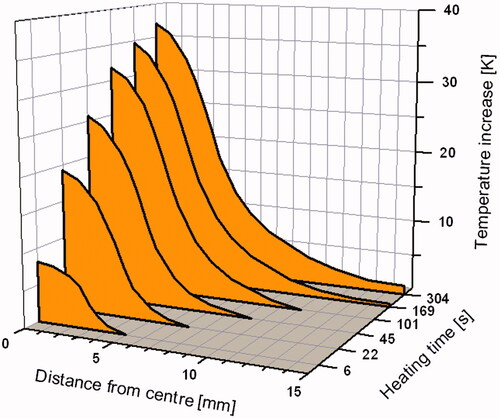
Furthermore, there is an influence of size of heat generation zone on the spatial dependence of temperature elevation around this zone. Due to the scaling of the heat production with r3, but heat depletion with r2, the decreasing heated volume will become very unfavourable. In particular, a single tumour cell, even filled completely with magnetic nanoparticles, would experience a temperature increase only under unrealistically high values of specific heating power (see the next section). This size dependency has been treated comprehensively by Rabin [Citation74]. He critically considered the possibility of nanoscale heat transfer. In particular, he showed that the high efficiency of so-called ‘intracellular’ hyperthermia that is occasionally claimed (e.g. [Citation75,Citation76]) has no biophysical basis. He pointed out that cell membranes present no essential resistance to heat conduction. Results of classical theory were mainly confirmed by Keblinski et al. [Citation77], while Chen et al. [Citation78] treat non-equilibrium heat conduction in the vicinity of nanoparticles. New optical probing techniques offer the possibility of nanoscale temperature measurements close to particle surface [Citation79–81]. In particular, Riedinger et al. [Citation82] found near the surface of magnetic nanoparticles heated by an alternating magnetic field, a temperature gradient which was much steeper than predicted by simulations of thermal conduction. The method uses high molecular spacers (polyethylene glycol) and dye-thermolabile groups coupled to the particle coating. Both determination of temperature as well as distance from particle surface need complicated calibration procedures, and further studies seem desirable.
Continuous heat conduction may, however, be disturbed by blood vessels which may result in an increase of the depletion of heat from the heated tumour region – and consequently may cause a decrease of the attainable peak temperature. The blood perfusion of the tumour may be modelled by means of the so-called bio-heat equation [Citation83–88] which has shown a strong dependency of tumour temperature on the special vessel configuration, in particular if larger blood vessels are involved.
Biological effect of temperature increase
The damaging effect of temperatures above the natural physiological level on living cells has been the subject of many investigations presented in the Journal of Hyperthermia. In 2003, a special issue was devoted to this topic. From this large body of work, a few representative reviews should be mentioned: Falk and Issels [Citation2], Moroz [Citation89], and Dewhirst [Citation90].
In these articles, two regimes of temperature treatment are differentiated: The first of these, thermoablation, refers to the application of elevated temperatures above about 48 °C which lead to cell death due to irreparable destruction of molecular constituents of the living cells (mainly protein denaturation). Damage at such elevated temperatures is irreversible and cell death occurs after just a few minutes of exposure to heating. This therapy modality is aimed at replacing surgery but the selectivity of this method depends on the success of placing the heat source preferentially in tumour tissue. In contrast, the second regime is hyperthermia where temperatures typically up to 44 °C are applied in human patients. In this temperature range, cytotoxic effects which depend on physiological cell parameters (e.g. acidity or hypoxia) are observed. An increased thermal sensitivity of tumour cells in comparison to healthy cells is expected due to their different metabolism. The death rate of cells in the narrow transition range between physiologically optimum and clearly lethal temperatures depends on nearly all the parameters of the cell environment that influence cell metabolism. In particular, there are several therapeutic attempts which combine cell damaging agents such as irradiation or chemotherapeutic drugs with temperature elevation [Citation24,Citation91–93]. For a combination of irradiation and heat, Dewhirst et al. [Citation94] found a greater reduction in tumour volume when using a few large thermal doses with higher temperatures than when using a larger number of doses with lower temperatures.
The degree of damage obtained only by heating is usually proved by determining surviving fractions in experiments with cell cultures. In such experiments it was found that in addition to the absolute value of temperature, the duration of the exposure to the elevated temperature plays a crucial role. This has led to suggestions that a ‘thermal dose’ be defined, which is analogous to what is done for irradiation damage. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia were discussed by Dewhirst [Citation90,Citation95] who reported that above 43 °C the effective exposure time may be halved for each 1 °C temperature elevation.
Attempts have been made to apply the concepts of thermal activation to hyperthermia [Citation96]. However, one should be aware that there is a superposition of different reaction paths of cell damage. A survey on the cellular and molecular basis of hyperthermia given by Hildebrandt et al. [Citation97] shows a complex picture due the superposition of effects of different physiological parameters. In a series of first therapeutic trials of hyperthermia, the concept of the thermal dose applied in clinical practice is described by Johannsen et al. [Citation21].
Besides the thermal damaging of tumour cells, there exist observations of ‘non-thermal’ damaging effects [Citation98] which show that magnetic nanoparticles oscillating under the influence of an external alternating magnetic field may induce mechanical damage in the cell interior. Typically, such cell damage was observed at low frequencies in the order of <100 Hz where thermal effects may surely be excluded.
Limitations and prospects
Though alternating magnetic fields induce eddy currents in any conducting medium which leads to heating of the medium, this mechanism of heat production is not suitable for nanoparticle heating for two reasons. First, the conductivity of magnetic oxides which proved to be very useful for MNP is rather low. Second, even for electrically well conducting metal particles, the induced electrical field is too small due to the smallness of the possible eddy current loops in nanoparticles. Thus the term ‘induction heating’ sometimes found in the literature with respect to the application of magnetic nanoparticles in hyperthermia is misleading.
However, the second argument considering current loops that are too small does not apply to the body of a human patient as a whole. The alternating magnetic field for heating up particles inside the body not only damages cancerous tissue but also causes an unwanted non-selective heating of healthy tissue due to eddy currents. The absorbed power due to eddy currents is given by
where σ is the electrical conductivity of the tissue, G a geometric coefficient and r the radius of the exposed area (coil size).
In an experimental study, it was found that for a coil of 30 cm in diameter, test patients were able to ‘withstand the treatment for more than 1 h without major discomfort' if the product of frequency and field strength did not exceed 4.85·108 Am−1s−1 [Citation99]. Taking into account the relationship between the exposed area and absorbed power for hyperthermia treatment of a serious illness with a smaller coil (typically around 10 cm), the given limit might be exceeded by a factor of 10. Therefore we suggest a limit for acceptable product of frequency and amplitude of 5·109 Am−1s−1. Resulting from these findings, it is recommended that the smallest possible coil dimensions be used to generate the alternating field and/or focused fields be applied to minimise the eddy currents in healthy or in tumour tissue.
In addition to eddy current effects, spontaneous nerve stimulations also occur in living tissue [Citation100]. These stimulations are due to induced potentials at neural cells and can lead to uncontrolled movement of limbs and phantom pain, especially when the frequency is below 100 kHz [Citation16]. In the last 15 years a lot of time was spent investigating such effects and stimulation thresholds for different types of tissue were determined [Citation101,Citation102]. Pankhurst et al. [Citation103] summarised these investigations and stated that the eddy current threshold is the limiting threshold for frequencies beyond several hundred kHz, the typical frequency range of alternating magnetic fields for hyperthermia.
In theory, the heating power of nanoparticles is an increasing function of f and H in a wide parameter range. In practice, however, due to medical reasons, enhancement of heating power is restricted by the limited product of f and H. Therefore a suitable combination of H and f resulting in maximum heating power has to be chosen taking into account the magnetic properties of the nanoparticles used.
In addition to the limitations of achievable SHP of MNP due to medical restrictions for the exposure of the body to alternating magnetic fields, the performance of hyperthermia is limited to tumours that are not too small in size. The reason for this limitation is the increasing surface-to-volume ratio with decreasing tumour size which leads to a stronger dissipation of the generated heat into healthy tissue and thus a lower temperature inside the tumour. Taking this size effect into account, the SHP needed to heat up a spherical tumour loaded with a MNP concentration c and with diameter R to a desired temperature increase ΔT is given by
where the heat conductivity of tissue λ is assumed to be 0.64 WK−1 m−1 [Citation104]. Equation 8 is based on the assumption that the cell membrane is not a significant barrier to diffusive heat transfer [Citation74].
shows how the SHP needed to obtain a temperature increase of 15 K (which represents the thermoablation mode) depends on tumour size for three different absorber concentrations: For direct injection of particles into tumours, we assumed concentrations of 100 and 10 mg MNP per mL of tumour tissue [Citation89] and for antibody targeting a typical MNP concentration of 1 mg MNP per mL [Citation105]. It can be seen that by intratumoral injection of available MNP with SHP of a few 100 W/g, the thermoablation treatment of tumours larger than 5 mm seems promising, but a heating effect of MNP applied by antibody targeting is unrealistic for tumours smaller than 15 mm. This situation seems to be more favourable for hyperthermia with a temperature increase of only 5 K, where tumours larger than 3 mm can be treated after direct injection and the minimum tumour size for antibody targeting is 10 mm.
Figure 3. SHP demand in dependence on tumour size to realise a temperature increase of 15 K for different MNP concentrations in the tumour tissue. (Data partially taken from [104], with permission from Elsevier).
![Figure 3. SHP demand in dependence on tumour size to realise a temperature increase of 15 K for different MNP concentrations in the tumour tissue. (Data partially taken from [104], with permission from Elsevier).](/cms/asset/e1378a15-188f-4dfc-b0cb-878dff011d7a/ihyt_a_822993_f0003_b.jpg)
The visionary idea behind the antibody targeting is the automatic loading of tumours smaller than the diagnostic limit of 3 mm with magnetic particles. This loading will be obtained by specific binding of systemically applied antibody-labelled MNP to the corresponding tumour tissue [Citation106,Citation107] with the aim of eliminating undetected metastases down to vagabonding tumour cells with the help of local hyperthermia. The demand of SHP for heating targets in this size range is shown in . There, one can see that hyperthermia treatment (ΔT = 5 K) of metastasis (3 mm) can become reality, but the treatment of single cells (15 µm) or even cell clusters (100 µm) is illusory. From these findings we conclude that so-called ‘intracellular hyperthermia’ [Citation75,Citation76] seems to be unachievable due to the unrealistically large amount of heating power necessary to obtain a suitable temperature increase. Even for the metastasis, heating with MNP concentration of 5 mg/mL (obtained from an improved antibody targeting) particles with a SHP of almost 1,000 W/g are required.
Figure 4. SHP demand in dependence on MNP concentration in the tumour tissue for heating of small biological objects about 5 K. (Data partially taken from [104], with permission from Elsevier).
![Figure 4. SHP demand in dependence on MNP concentration in the tumour tissue for heating of small biological objects about 5 K. (Data partially taken from [104], with permission from Elsevier).](/cms/asset/deea00c7-e85d-4467-993b-029a1f5485b7/ihyt_a_822993_f0004_b.jpg)
Similar analytical results for the investigation of this power demand were found by several groups [Citation77,Citation83,Citation108–110]. In the experimental work of Yamada and co-workers [Citation110], cancer cell pellets of different sizes (1 to 40 mm) were heated up to 50 °C for 10 min to obtain a complete cell killing. It was confirmed that larger tumours require a smaller heat dose to achieve the target temperature. Taking into account an available maximum SHP of 1,000 W/g and a MNP concentration of 2 mg/mL, the treatment of 10 mm tumours seems to be possible but tumours less than 5 mm require MNP with substantially higher SHP. Hedayati et al. [Citation111] extended the lower pellet size down to clusters of a few thousand cells. They found (when using particles showing a SHP of 480 W/g) a minimum pellet size of 1.2 mm (250,000 cells and each loaded with 200 pg Fe) was needed to obtain the minimum necessary temperature of 41.3 °C to cause a cytotoxic effect within the cells. This finding is in good accordance with the prediction of Rabin [Citation74].
Nevertheless, recently, some groups [Citation80,Citation81] found remarkable temperature elevations on the nanoscale which may stimulate a reinvestigation of problems of thermal transport barriers in the immediate neighbourhood of magnetically heated MNP. Zhang et al. [Citation112] found cell damaging effects using Herceptin-guided MNP to malignant human mammary epithelial cells without observing significant temperature increase. DeNardo and co-workers [Citation107] observed a statistically significant tumour growth delay for only minor estimated energy deposition into the tumour and significant differences in the growth delay as a function of total heat dose. Creixell et al. [Citation113] even claim killing of cancer cells ‘without a perceptible temperature rise’ by magnetic particle heating. However, it seems erroneous to exclude local nanoscale temperature rise by holding the incubator temperature of the samples constant since in [Citation80,Citation82] a temperature increase on particles’ surface was observed without a significant increase of the solution temperature.
Anyway, most investigations show that hyperthermia treatment of relatively small tumours in the lower mm range seems to be promising when using MNP with a high SHP of 500 W/g or more. In the last 5 to 10 years, a notable enhancement of the SHP of MNP was achieved. Ten years ago, SHP of synthetic MNP was typically in the range of 50 to 200 W/g and the bacterial magnetosomes with a SHP of around 1,000 W/g [Citation114] gave the benchmark for achievable heating power. Nowadays, absolute SHP values of more than 1,500 W/g can be reached. A SHP of 1,650 W/g (measured at f = 700 kHz and H = 24.8 kA/m) was reported by Fortin et al. [Citation69] for particles of 16.5 nm obtained from size-dependent fractionation of a sample showing a broader size distribution. The reported value was obtained for field parameters clearly out of range of feasible combinations for clinical hyperthermia.
While the used field parameters are crucial for the resulting SHP, a direct comparison of SHP values from different groups measured at varying field parameters is difficult. To allow a better comparison, the measure ILP was introduced by Pankhurst et al. [Citation115]. The ILP is defined as the SHP normalised to H2 and f assuming a corresponding dependence of SHP on these parameters. Despite the fact that this dependence is not appropriate for the whole range of MNP sizes and all applied field strengths and frequencies [Citation62,Citation116–118], the ILP gives a good measure for the heating performance of MNP. However, when looking at ILP values, it is absolutely necessary to assess these values together with the absolute values for SHP. For instance, a relatively low SHP measured for small field strength and frequency might result in a high ILP, whereas the particles are not suitable for heating since the absolute SHP is too low and the assumed dependency on H and f is not guaranteed for all particles. Typical ILP values suitable for heating are in the range from 3 to 4 nHm2kg−1 or more and the highest reported value ever is 23.4 nHm2kg−1 for magnetosomes [Citation114]. Obviously, the natural synthesis by those bacteria is favourable to all technical preparation methods.
Reviews of state of the art of particle development are given by Barry [Citation119], Kallumadil [Citation115], and Pollert [Citation120] and colleagues. Newest magnetic iron oxide particle developments reach SHP values of up to 1650 W/g (ILP =3.8 nHm2kg−1) [Citation69], 1500 W/g (ILP = 4.6 nHm2kg−1) [Citation117], 1179 W/g (ILP = 6.1 nHm2kg−1) [Citation121], 537 W/g (ILP =0.4 nHm2kg−1) [Citation116], and 332 W/g (ILP = 8.3 nHm2kg−1) [Citation65]. The last one is the highest ever reported ILP for synthetic particles [Citation120] and was obtained for fractionated multicore nanoparticles [Citation64,Citation65]. The multicore particles exhibit a magnetic behaviour different from single core particles [Citation117] and have shown promising results for hyperthermia treatment in animal experiments [Citation35]. For all the SHP values reported here, it must be kept in mind that these measurements were carried out on liquid samples and show a contribution of Brownian relaxation to heating losses. This relaxation might not be appropriate for the real magnetic behaviour of particles administered to tumours because particles bind to tissue immediately after injection [Citation35,Citation40] and this might lead to a reduced thermal dose applied to the tumour.
It has to be pointed out that even if the materials optimisation problem is solved satisfactorily, the problem of how to appropriately administer the nanoparticle suspension to the tumour remains. Typically it is difficult to achieve a nearly homogeneous distribution of nanoparticles within the tumour volume. The best method of administration seems to be slow multi-point injection into the tumour volume. An optimisation algorithm to determine the optimum heating patterns induced by multiple nanoparticle injections in tumour models with irregular geometries was presented by Salloum et al. [Citation122]. Results of clinical trials for treatment of prostate cancer under control of the three-dimensional temperature distribution in the tumour were reported by Johannsen et al. [Citation21].
Conclusions
Magnetic particle heating for thermal tumour therapy is based on several loss mechanisms that occur during reversal of magnetisation when magnetic nanoparticles are exposed to an external alternating field. Since the extrinsic rotation of particles inside tumour tissue is very improbable due to fixation of the particles in the tissue, only hysteresis and Néel relaxation contribute to this process. Hysteresis in thermally stable single domain particles principally provides higher reversal losses than Néel relaxation when the critical field amplitude of around twice the coercive field is exceeded.
To prevent harmful effects of electromagnetic fields on the human body when exposing the body to an alternating magnetic field, an upper limit for the product of frequency and amplitude should be considered. It is suggested that it be 5·109 Am−1s−1 with a frequency of at least 100 kHz when using solenoids with a diameter of about 100 mm as is typical for hyperthermia. Under these restrictions, tailor-made particles which show maximum reversal losses for feasible combinations of frequency and amplitude are necessary.
The magnetic properties of particles are affected by a plurality of factors but the main control elements are particle size and size distribution. When optimally adapting the particle diameter to the applied field amplitude and frequency, the highest hysteresis losses are obtained for monodisperse particles. However, if the field amplitude is below saturation, higher reversal losses are yielded for particles with broader size distributions than for monodisperse ones.
The administration of the particles to the body is a very sensitive point of hyperthermia. On the one hand, there are several medical risks due to toxic effects or embolisation, and on the other hand, the procedure of application is important for the success of the therapy with respect to magnetic heating. After a possible agglomeration of particles during administration, the heating power of hysteretic particles decreases, due to magnetic interactions between the single cores, while the heating power of superparamagnetic particles increases if the field amplitude is high enough to activate these agglomerates.
The magnetically induced heating of objects (tumours) inside a thermal conductible matrix (healthy tissue) requires more than a minimum volume of the tumour for the generation of heat. Taking into account an increasing surface-to-volume ratio for decreasing tumour size, particles with a very high heating power are necessary for the treatment of small tumours. Since there is a maximum obtainable heating power, there is also a limit in treatable tumour size of a few mm.
Despite many open questions and restrictions, it can be stated without a doubt that research on magnetic hyperthermia in the past decade has led to a much more profound understanding of the whole topic, and significant progress has occurred in all related aspects of this promising cancer therapy. The complexity of hyperthermia is obvious and demands a careful and comprehensive interpretation of the results and correlation of the findings to find strategies for further improvement of the present state of the art in hyperthermia. It is unlikely that an all-purpose therapy will be found, but instead each situation needs its own solution. In principle, magnetic particle hyperthermia has the potential to find its way into standard medical procedures as has already been shown by the first successful and promising clinical trials.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors thank Wilfried Andrä (Jena) and Urs Häfeli (University of British Columbia, Vancouver) for critical reading of the manuscript and helpful discussions. Their special thanks go to Lydia Cartar (Vancouver) for proofreading the article.
References
- Busch CJ. Einfluss heftiger Erysipeln auf organisierte Neubildungen [Influence of severe erysipelas on organised neoformation]. In: Andrä CJ, ed. Verhandlungen des naturhistorischen Vereins der preussischen Rheinlande und Westphalens [Proceedings of the Natural Historical Society of Prussian Rhineland and Westphalia]. Bonn: Max Cohen; 1866. pp 28–33
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001;17:1–18
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Douple EB, Strohbehn JW, Bowers ED, Walsh JE. Cancer-therapy with localized hyperthermia using an invasive microwave system. J Microw Power Electromagn Energy 1979;14:181–6
- Strohbehn JW, Bowers ED, Walsh JE, Douple EB. Invasive microwave antenna for locally-induced hyperthermia for cancer-therapy. J Microw Power Electromagn Energy 1979;14:339–50
- Taylor LS. Implantable radiators for cancer-therapy by microwave hyperthermia. Proc IEEE 1980;68:142–9
- Brezovich IA, Young JH. Hyperthermia with implanted electrodes. Med Phys 1981;8:79–84
- Cetas TC, Hevezi JM, Manning MR, Ozimek EJ. Dosimetry of interstitial thermoradio-therapy. In: Cancer Therapy by Hyperthermia, Drugs, and Radiation. Natl Cancer Inst Monogr, 1982;61:505–7
- Doss JD. Use of RF fields to produce hyperthermia in animal tumors. Proc Int Symp Cancer Ther Hyperthermia Radiat 1975;1:226–7
- Doss JD, McCabe CW. A technique for localized heating in tissue: An adjunct to tumor therapy. Med Instrum 1976;10:16–21
- Leveen HH, Wapnick S, Piccone V, Falk G, Ahmed N. Tumor eradication by radiofrequency therapy – Response in 21 patients. JAMA 1976;235:2198–2200
- Roggan A, Beuthan J, Schruender S, Mueller G. Diagnostik und Therapie mit dem Laser. Phys Bl 1999;55:25–30
- Ardenne MV. Principles and concept 1993 of the Systemic Cancer Multistep Therapy (SCMT). Strahlenther Onkol 1994;170:581–9
- Robins HI, Rushing D, Kutz M, Tutsch KD, Tiggelaar CL, Paul D, et al. Phase I clinical trial of melphalan and 41.8 °C whole-body hyperthermia in cancer patients. J Clin Oncol 1997;15:158–64
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg 1957;146:596–606
- Brezovich IA. Low frequency hyperthermia. Med Phys Monogr 1988;16:82–111
- Hilger I, Andra W, Hergt R, Hiergeist R, Schubert H, Kaiser WA. Electromagnetic heating of breast tumors in interventional radiology: In vitro and in vivo studies in human cadavers and mice. Radiology 2001;218:570–5
- Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, et al. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia 1997;13:587–605
- Rand RW, Snow HD, Elliott DG, Haskins GM. Induction heating method for use in causing necrosis of neoplasm. US Patent 4.545.368, 1985
- Gneveckow U, Jordan A, Scholz R, Eckelt L, Maier-Hauff K, Johannsen M, et al. Magnetic force nanotherapy. Biomed Tech (Berl) 2005;Supp 1, Part 1:92–3
- Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, et al. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 2007;52:1653–62
- Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldoefner N, Scholz R, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int J Hyperthermia 2007;23:315–23
- Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia 2010;26:790–5
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103:317–24
- Ortega D, Pankhurst QA. Magnetic hyperthermia. In: O’Brien P, ed. Nanoscience. Cambridge: Royal Society of Chemistry; 2013. pp 60–88
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–474
- Kronmueller H, Parkin SSP. Micromagnetism. New Jersey: Wiley; 2007
- Kronmueller H, Faehnle M. Micromagnetism and the Microstructure of Ferromagnetic Solids. Cambridge: Cambridge University Press; 2003
- Heider F, Dunlop DJ, Sugiura N. Magnetic properties of hydrothermally recrystallized magnetite crystals. Science 1987;236:1287–90
- Stoner EC, Wohlfarth EP. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos Trans R Soc London, A 1948;240:599–642
- Bakoglidis KD, Simeonidis K, Sakellari D, Stefanou G, Angelakeris M. Size-dependent mechanisms in AC magnetic hyperthermia response of iron-oxide nanoparticles. IEEE Trans Magn 2012;48:1320–3
- Muerbe J, Rechtenbach A, Toepfer J. Synthesis and physical characterization of magnetite nanoparticles for biomedical applications. Mater Chem Phys 2008;110:426–33
- Yuan Y, Rende D, Altan CL, Bucak S, Ozisik R, Borca-Tasciuc D-A. Effect of surface modification on magnetization of iron oxide nanoparticle colloids. Langmuir 2012;28:13051–13059
- Néel L. Influence des fluctuations thermiques a l’aimantation des particules ferromagnetiques. C R Acad Sci 1949;228:664–8
- Dutz S, Kettering M, Hilger I, Muller R, Zeisberger M. Magnetic multicore nanoparticles for hyperthermia – influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011;22:265102
- Hergt R, Dutz S, Zeisberger M. Validity limits of the Néel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology 2010;21:015706
- Brown WF. Thermal fluctuations of a single-domain particle. Phys Rev 1963;130:1677–1686
- Mamiya H, Jeyadevan B. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Sci Rep 2011;1:157
- Coffey WT, Kalmykov YP. Thermal fluctuations of magnetic nanoparticles: Fifty years after Brown. J Appl Phys 2012;112:121301
- Richter H, Kettering M, Wiekhorst F, Steinhoff U, Hilger I, Trahms L. Magnetorelaxometry for localization and quantification of magnetic nanoparticles for thermal ablation studies. Phys Med Biol 2010;55:623–33
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002;252:370–4
- Debye P. Polar Molecules. New York: Dover; 1929
- Ahrentorp F, Astalan AP, Jonasson C, Blomgren J, Qi B, Mefford OT, et al. Sensitive high frequency AC susceptometry in magnetic nanoparticle applications. AIP Conf Proc 2010;1311:213–23
- Astalan AP, Jonasson C, Petersson K, Blomgren J, Ilver D, Krozer A, et al. Magnetic response of thermally blocked magnetic nanoparticles in a pulsed magnetic field. J Magn Magn Mater 2007;311:166–70
- Kneller E. Ferromagnetism. Berlin: Springer, 1962
- Landau LD, Lifshitz EM. Electrodynamics of Continuous Media. London: Pergamon Press; 1960
- Dutz S, Hergt R, Muerbe J, Mueller R, Zeisberger M, Andrae W, et al. Hysteresis losses of magnetic nanoparticle powders in the single domain size range. J Magn Magn Mater 2007;308:305–12
- Dutz S, Hergt R, Murbe J, Topfer J, Muller R, Zeisberger M, et al. Magnetic nanoparticles for biomedical heating applications. Z Phys Chem 2006;220:145–51
- Hergt R, Dutz S, Mueller R, Zeisberger M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J Phys Condens Matter 2006;18:S2919–34
- Hergt R, Dutz S, Roeder M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J Phys: Condens Matter 2008;20:385214
- Mueller R, Hergt R, Dutz S, Zeisberger M, Gawalek W. Nanocrystalline iron oxide and Ba ferrite particles in the superparamagnetism-ferromagnetism transition range with ferrofluid applications. J Phys Condens Matter 2006;18:S2527–42
- Gloeckl G, Hergt R, Zeisberger M, Dutz S, Nagel S, Weitschies W. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J Phys Condens Matter 2006;18:S2935–49
- Chantrell RW, Bradbury A, Popplewell J, Charles SW. Particle cluster configuration in magnetic fluids. J Phys D Appl Phys 1980;13:L119–22
- Castro LL, da Silva MF, Bakuzis AF, Miotto R. Aggregate formation on polydisperse ferrofluids: A Monte Carlo analysis. J Magn Magn Mater 2005;293:553–8
- Dunin-Borkowski RE, McCartney MR, Posfai M, Frankel RB, Bazylinski DA, Buseck PR. Off-axis electron holography of magnetotactic bacteria: Magnetic microstructure of strains MV-1 and MS-1. Eur J Mineral 2001;13:671–84
- Berkov DV. Numerical simulations of quasistatic remagnetization processes in fine magnetic particle systems. J Magn Magn Mater 1996;161:337–56
- Dutz S, Hergt R. The role of interactions in systems of single domain ferrimagnetic iron oxide nanoparticles. J Nano- Electron Phys 2012;4:20101–7
- Serantes D, Baldomir D, Martinez-Boubeta C, Simeonidis K, Angelakeris M, Natividad E, et al. Influence of dipolar interactions on hyperthermia properties of ferromagnetic particles. J Appl Phys 2010;108:073918
- Urtizberea A, Natividad E, Arizaga A, Castro M, Mediano A. Specific absorption rates and magnetic properties of ferrofluids with interaction effects at low concentrations. J Phys Chem C 2010;114:4916–22
- Eberbeck D, Trahms L. Experimental investigation of dipolar interaction in suspensions of magnetic nanoparticles. J Magn Magn Mater 2011;323:1228–32
- Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, et al. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology 2009;20:395103
- Eggeman AS, Majetich SA, Farrell D, Pankhurst QA. Size and concentration effects on high frequency hysteresis of iron oxide nanoparticles. IEEE Trans Magn 2007;43:2451–3
- Yuan Y, Borca-Tasciuc D-A. Anomalously High Specific Absorption Rate In Bioaffine Ligand-Coated Iron Oxide Nanoparticle Suspensions. IEEE Trans Magn 2013;49:263–8
- Dutz S, Andrae W, Hergt R, Mueller R, Oestreich C, Schmidt C, et al. Influence of dextran coating on the magnetic behaviour of iron oxide nanoparticles. J Magn Magn Mater 2007;311:51–4
- Dutz S, Clement JH, Eberbeck D, Gelbrich T, Hergt R, Mueller R, et al. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J Magn Magn Mater 2009;321:1501–4
- Eberbeck D, Dennis CL, Huls NF, Krycka KL, Gruttner C, Westphal F. Multicore magnetic nanoparticles for magnetic particle imaging. IEEE Trans Magn 2013;49:269–74
- Schaller V, Wahnstrom G, Sanz-Velasco A, Enoksson P, Johansson C. Monte Carlo simulation of magnetic multi-core nanoparticles. J Magn Magn Mater 2009;321:1400–3
- Schaller V, Wahnstrom G, Sanz-Velasco A, Gustafsson S, Olsson E, Enoksson P, et al. Effective magnetic moment of magnetic multicore nanoparticles. Phys Rev B Condens Matter 2009;80: 924061–4
- Fortin J-P, Wilhelm C, Servais J, Menager C, Bacri J-C, Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 2007;129:2628–35
- Wotschadlo J, Liebert T, Heinze T, Wagner K, Schnabelrauch M, Dutz S, et al. Magnetic nanoparticles coated with carboxymethylated polysaccharide shells – Interaction with human cells. J Magn Magn Mater 2009;321:1469–73
- Andra W, d’Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J Magn Magn Mater 1999;194:197–203
- Attaluri A, Ma R, Qiu Y, Li W, Zhu L. Nanoparticle distribution and temperature elevations in prostatic tumours in mice during magnetic nanoparticle hyperthermia. Int J Hyperthermia 2011;27:491–502
- Rahn H, Schenk S, Engler H, Odenbach S. Tissue model for the study of heat transition during magnetic heating treatment. IEEE Trans Magn 2013;49:244–9
- Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int J Hyperthermia 2002;18:194–202
- Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia – Biophysical approach to cancer-treatment via intracellular temperature and biophysical alterations. Med Hypotheses 1979;5:83–102
- Jordan A, Scholz R, Wust P, Schirra H, Schiestel T, Schmidt H, et al. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J Magn Magn Mater 1999;194:185–96
- Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. J Appl Phys 2006;100:543051–55
- Chen G. Nonlocal and nonequilibrium heat conduction in the vicinity of nanoparticles. J Heat Transfer 1996;118:539–45
- Gupta A, Kane RS, Borca-Tasciuc D-A. Local temperature measurement in the vicinity of electromagnetically heated magnetite and gold nanoparticles. J Appl Phys 2010;108:649011–17
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotech 2010;5:602–6
- Yang J-M, Yang H, Lin L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 2011;5:5067–71
- Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, et al. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett 2013;13:2399–406
- Bagaria HG, Johnson DT. Transient solution to the bioheat equation and optimization for magnetic fluid hyperthermia treatment. Int J Hyperthermia 2005;21:57–75
- Giordano MA, Gutierrez G, Rinaldi C. Fundamental solutions to the bioheat equation and their application to magnetic fluid hyperthermia. Int J Hyperthermia 2010;26:475–84
- Golneshan AA, Lahonian M. The effect of magnetic nanoparticle dispersion on temperature distribution in a spherical tissue in magnetic fluid hyperthermia using the lattice Boltzmann method. Int J Hyperthermia 2011;27:266–74
- Hynynen K, Deyoung D, Kundrat M, Moros E. The effect of blood perfusion rate on the temperature distributions induced by multiple, scanned and focused ultrasonic beams in dogs kidneys in vivo. Int J Hyperthermia 1989;5:485–97
- Nyborg WL. Solutions of the bio-heat transfer equation. Phys Med Biol 1988;33:785–92
- Sonnenschein R, Gross J. Temperature field computation for radiofrequency heating of deep-seated tumors. Recent Results Cancer Res 1986;101:132–7
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hyperthermia 2002;18:267–284
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- Alvarez-Berrios MP, Castillo A, Mendez J, Soto O, Rinaldi C, Torres-Lugo M. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int J Nanomedicine 2013;8:1003–13
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, Seventh Edition. Philadelphia: Lippincott Williams & Wilkins; 2011
- Wang H, Li X, Xi X, Hu B, Zhao L, Liao Y, et al. Effects of magnetic induction hyperthermia and radiotherapy alone or combined on a murine 4T1 metastatic breast cancer model. Int J Hyperthermia 2011;27:563–72
- Thrall DE, Maccarini P, Stauffer P, Macfall J, Hauck M, Snyder S, et al. Thermal dose fractionation affects tumour physiological response. Int J Hyperthermia 2012;28:431–40
- van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994;10:457–83
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33–56
- Kim D-H, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat Mater 2010;9:165–71
- Atkinson WJ, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng 1984;31:70–75
- Borrelli NF, Luderer AA, Panzarino JN. Hysteresis heating for the treatment of tumors. Phys Med Biol 1984;29:487–94
- Harvey PR, Katznelson E. Modular gradient coil: A new concept in high-performance whole-body gradient coil design. Magn Reson Med 1999;42:561–70
- Reilly JP. Applied bioelectricity: From electrical stimulation to electro pathology. New York: Springer; 1998
- Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2009;42:224001
- Hergt R, Dutz S. Magnetic particle hyperthermia – Biophysical limitations of a visionary tumour therapy. J Magn Magn Mater 2007;311:187–92
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 2005;100:1–11
- DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, et al. Development of tumor targeting bioprobes (In-111-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clin Cancer Res 2005;11:7087S–92S
- DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, et al. Thermal dosimetry predictive of efficacy of In-111-ChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. J Nucl Med 2007;48:437–44
- Bellizzi G, Bucci OM. On the optimal choice of the exposure conditions and the nanoparticle features in magnetic nanoparticle hyperthermia. Int J Hyperthermia 2010;26:389–403
- Kalambur VS, Longmire EK, Bischof JC. Cellular level loading and heating of superparamagnetic iron oxide nanoparticles. Langmuir 2007;23:12329–36
- Yamada K, Oda T, Hashimoto S, Enomoto T, Ohkohchi N, Ikeda H, et al. Minimally required heat doses for various tumour sizes in induction heating cancer therapy determined by computer simulation using experimental data. Int J Hyperthermia 2010;26:465–74
- Hedayati M, Thomas O, Abubaker-Sharif B, Zhou H, Cornejo C, Zhang Y, et al. The effect of cell cluster size on intracellular nanoparticle-mediated hyperthermia: Is it possible to treat microscopic tumors? Nanomedicine 2013;8:29–41
- Zhang J, Dewilde AH, Chinn P, Foreman A, Barry S, Kanne D, et al. Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cells in vitro via hyperthermia. Int J Hyperthermia 2011;27:682–97
- Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011;5:7124–9
- Hergt R, Hiergeist R, Zeisberger M, Schuler D, Heyen U, Hilger I, et al. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J Magn Magn Mater 2005;293:80–86
- Kallumadil M, Tada M, Nakagawa T, Abe M, Southern P, Pankhurst QA. Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mater 2009;321:1509–13
- Bordelon DE, Cornejo C, Gruttner C, Westphal F, DeWeese TL, Ivkov R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl Phys 2011;109:1249041–8
- Lartigue L, Hugounenq P, Alloyeau D, Clarke SP, Levy M, Bacri J-C, et al. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012;6:10935–49
- Mueller R, Dutz S, Neeb A, Cato ACB, Zeisberger M. Magnetic heating effect of nanoparticles with different sizes and size distributions. J Magn Magn Mater 2013;328:80–85
- Barry SE. Challenges in the development of magnetic particles for therapeutic applications. Int J Hyperthermia 2008;24:451–66
- Pollert E, Záveta K. Nanocrystalline oxides in magnetic fluid hyperthermia. In: Thanh NTK, ed. Magnetic Nanoparticles: From Fabrication to Clinical Applications. London: CRC Press; 2012, pp. 449–79
- Thomas LA, Dekker L, Kallumadil M, Southern P, Wilson M, Nair SP, et al. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J Mater Chem 2009;19:6529–35
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimization of the heat absorption pattern. Int J Hyperthermia 2009;25:309–21
