Abstract
Purpose: Approximately 2–13% of the world population suffers from onychomycosis. Recently, lasers have been introduced for treatment. However, no effect was found with in vitro laser irradiation of pathogens on agar plates. This study aimed to investigate the efficacy of laser irradiation against fungi using an alternative in vitro approach.
Materials and methods: Lasers of 808, 980 and 1064 nm were used to heat cell culture media and a nail clipping. Trichophyton rubrum. T. interdigitale. Microsporum gypseum. Candida albicans. C. parapsilosis, and C. guilliermondii species were subcultured and subjected to laser treatments (808/980 nm: 9–27 J/cm2, 6 ms, 12 × 12 or 12 × 50 mm and 1064 nm: 50–240 J/cm2, 90 ms, 5–10 mm). After irradiation, the fungal elements were transferred onto agar plates using conventional and Drigalski spatulas and were incubated for 6 days.
Results: The highest increase in temperature was found using a 980-nm laser with a pulse duration of 6 ms and a fluence of 27 J/cm2. The histology work-up revealed a dissection of the nail plate from the nail bed tissue after laser irradiation. Growth inhibition was only found for C. guilliermondii and T. interdigitale. All other pathogens presented only reduced growth, and C. albicans growth was unaffected.
Conclusions: This study demonstrates a clear thermal effect for linear scanning 980-nm and long-pulsed 1064-nm laser systems on either nail clippings or cell culture media. Complete pathogen growth impairment was achieved if temperatures were measured above 50 °C. The results for the 1064-nm system were almost comparable to 980 nm results.
Introduction
Dermatophytes are a group of filamentous pathological fungi that can inhabit human skin, hair and nail plates. They are ubiquitous in distribution and produce superficial infections called dermatophytosis [Citation1]. Dermatophytosis is found in approximately 20–25% of the world population [Citation2]. An estimated 2–13% of the population suffers from onychomycosis (OM) [Citation3–5], which is the most common disease of nails worldwide and is responsible for approximately half of all nail abnormalities [Citation6]. The condition produces a huge impact on quality of life [Citation7,Citation8]. The primary age group affected consists of elderly patients.
Frequently, dermatophytes are causative agents for OM in temperate western countries [Citation2,Citation9]. These fungi include the genera Epidermophyton. Microsporum and Trichophyton [Citation1]. Among these fungi, T. rubrum and T. interdigitale (formerly T. mentagrophytes) are the most often (90–93%) detected in OM [Citation2,Citation6,Citation10]. The transmission of these fungi occurs by contact with hyphal fragments and arthroconidia. Household dust enables the preservation of spores for years [Citation2].
The typical clinical diagnosis is confirmed by mycological examination using direct microscopy with a potassium hydroxide preparation, cultivation on Sabouraud’s dextrose agar, and molecular methods such as PCR (polymerase chain reaction) [Citation6,Citation11]. However, because of the high frequency of potential false negative laboratory results accompanying typical clinical symptoms, antifungal treatment is advisable [Citation4]. Topical antifungal formulations are available but are only helpful in distal subungual onychomycosis cases affecting up to one third of the nail plate. Systemic treatment in combination with topical therapeutics is advisable if larger areas of the nails or more than 3 out of 10 nails are affected [Citation12]. Despite a variety of available compounds (e.g. terbinafine, fluconazole, and itraconazole), standard systemic terbinafine administration achieves a disease-free nail in only approximately 35–76% of cases [Citation13–15]. In addition, up to 22.3% relapse rates are found within 3 years after completed treatment [Citation16]. Furthermore, in geriatric patient cohorts, the regularity and efficacy of topical application is often reduced, and systemic medications may affect the liver or interfere with other drugs [Citation17]. Therefore, the long-term outcome of systemic treatments is unsatisfactory because of therapeutic failure, relapse, reinfection, and the resulting adverse events [Citation18].
Studies of alternative treatment options such as surgery [Citation19], high frequency waves [Citation20], photodynamic therapy [Citation21,Citation22], and CO2 laser-based therapy [Citation23] yielded discouraging results. The huge amount of heat applied with early CO2 lasers was helpful in managing hyperkeratotic conditions such as onychogryphosis [Citation24] and in performing matricectomies [Citation23,Citation25]. In contrast, the rather cold ablation effect of 2940-nm Er:YAG lasers has been shown to be unsuitable for the inhibition of fungal growth [Citation26]. In addition, the use of intense pulsed light (695–1000 nm), flashlamp pulsed dye laser (585 nm) and the KTP laser (532 nm) have also been shown to be ineffective [Citation26]. As CO2 lasers were found to be effective but unpredictable, q-switched [Citation26] and longer pulsed near-infrared lasers [Citation27,Citation28] with no ablation should have a much better side-effect profile while maintaining their efficacy. It has been postulated that multiplexed wavelengths of 870 and 930 nm produce their lethal effects against fungi via a decrease in mitochondrial membrane potential together with a simultaneous increase of ROS (reactive oxygen species) [Citation29]. Operating at physiological temperatures, a clinical improvement was observed in OM after four treatments [Citation30]. To destroy fungi in a highly selective way, an 800-nm femtosecond Ti:sapphire laser has been used to apply 200 × 10−15 s pulses at 76 MHz pulse frequencies. In addition, 7 × 1031 photons m−2 s−1 intensities or above have successfully eliminated fungi in nail clippings after a single treatment, and this has been managed without any destruction of the nail plate at up to intensities of 1.7 × 1032 photons m−2 s−1 [Citation31]. Because femtosecond lasers are not easily available, and lasers operating at a 1064-nm wavelength are widely used for other indications, the latter type of laser has also been investigated with respect to its suitability for the treatment of nail fungi. Nd:YAG systems at this wavelength have been shown to be effective if operated in a q-switched manner [Citation26]. Using a 0.65-ms pulse duration while applying a 2-mm spot with the energy fluence set at 223 J/cm2 in the absence of any cooling sprays, gels or topical anesthetics produces an 87.5% clearing rate after three treatments every 3 weeks [Citation32]. In clinical studies it has been shown that a rise of temperature within the nail plate over a given time might be crucial for the clearing of onychomycosis. Using a 1064-nm Nd:YAG laser set to a pulse duration of 35 ms to deliver 35–40 J/cm2, 45° ± 5 °C was achieved at the nail plate after being irradiated three times. The procedure was repeated four times weekly, resulting in a 95.8% fungal clearing rate [Citation33]. However, to date, many systems are available that operate at rather diverse parameter settings, although most often, the 1064-nm wavelength is chosen (). Recently, the efficacy of a 1064/532-nm Nd:YAG-system was tested in vitro using q-switched pulses as well as long pulses (1064 nm only) on T. rubrum. Unfortunately, no effect was observed from the irradiation of pathogens on agar plates after incubation at 30 °C for 3–6 days [Citation34]. However, the experimental design might not have been ideal, and contrasting findings might have been found.
Table I. Common laser systems available for treatment of onychomycosis. Applied laser parameters for the treatment of onychomycosis using a Nd:YAG laser and the laser parameter range used.
As fungal infection of the human skin and its appendages are so common and difficult to treat on both sides, and a variety of laser systems using different wavelengths and settings that have been shown to be effective against causative fungi, this study aimed to investigate the efficacy of laser irradiation in vitro against common pathogens using an alternative approach of pathogen culture post-irradiation.
Materials and methods
The objective of this study was to define the impact of 808-, 980-, and 1064-nm lasers using various parameter settings to deliver heat to cell culture media and to destroy common species and different strains of fungi in in vitro culture conditions.
Temperature measurements
Six-well ELISA reader plates (83.1839.300, Sarstedt, Newton, NC, USA) were used for irradiation of pure cell culture media to measure the heating effect of the tested laser systems. The temperature was evaluated by replicate measurement using a contact-free laser thermometer (MiniTemp4, Raytek, Berlin, Germany).
Pathogens
T. rubrum. T. interdigitale. M. gypseum. C. albicans. C. parapsilosis, and C. guilliermondii species serving as reference pathogens at the laboratory for medical microbiology were taken and subcultured into a Sabouraud’s broth before being further subjected to laser treatment. Although T. rubrum and T. interdigitale are the most common pathogens, the intentions to integrate the other pathogens were to see if there are possible differences in heat susceptibility. Liquid cultures of those pathogens were incubated at 37 °C and 27 °C, respectively, for 3 days to a maximum of 12 days until the Sabouraud’s broth became murky and clouded as an optical indicator of fungal growth.
In an initial pathogen eradication experiment, 2 mL of the fungal broth was transferred into glass Petri dishes or each well of the 6-well plastic ELISA plates for further laser treatment. To achieve a clinically relevant temperature scenario the samples were kept at room temperature before processing. After laser irradiation, the fungal broth samples were transferred in three sections onto agar plates (). For the third experiment, 10 µL and 50 µL of Sabouraud’s broth were transferred after laser treatment onto agar plates using Drigalski spatulas. Next, 25 µL of the same Sabouraud’s broth served as a control. The colonies were counted on day 6 post treatment.
Laser treatment
Laser treatment was performed using three different systems: an 808-nm linear scanning diode laser, a 980-nm linear scanning diode laser, and a long pulsed 1064-nm Nd:YAG-laser (all Alma Lasers, formerly Quantel-Derma and Wavelight, Erlangen, Germany). The latter is mainly indicated for hair removal, vascular lesion treatments and achieving non-ablative skin rejuvenation, and the first two systems are routinely used for hair removal.
Laser treatment was performed in three experiments: (1) the evaluation of the ability of a 980-nm laser to heat water together with the evaluation of 808 and 980 nm linear scanning diode laser to heat fungi in media (Sabouraud’s broth, ), (2) the testing of the ability of the 980-nm linear scanning diode laser as well as a long pulsed 1064-nm Nd:YAG-laser to efficiently destroy the entire range of pathogens of interest at the laser parameters with an improved read out, and (3) to evaluate the 1064 nm effect on nail clippings in vitro.
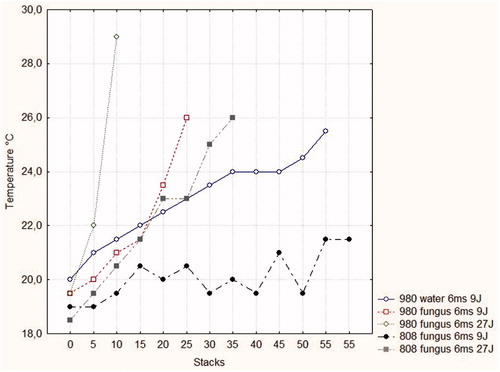
Figure 2. Temperatures within water and cell culture media containing pathogens in response to repetitive laser treatments (stacks) at various parameter settings.
The parameter settings used in the latter two experiments were selected according to the results of the first experiment: 808 nm, a fluence of 9–27 J/cm2 and a pulse duration of 6 ms at a spot size of 12 × 12 (6-well plates) or 12 × 50 mm (glass Petri dishes); 980 nm, a fluence of 9–27 J/cm2 and a pulse duration of 6 ms at a spot size of 12 × 12 (6-well plates) or 12 × 50 mm (glass Petri dishes); and 1064 nm, a fluence of 50–240 J/cm2, a pulse duration of 90 ms and a varying spot size of 5 to 10 mm. All experiments were conducted using glass Petri dishes or plastic 6-well ELISA plates.
Histology
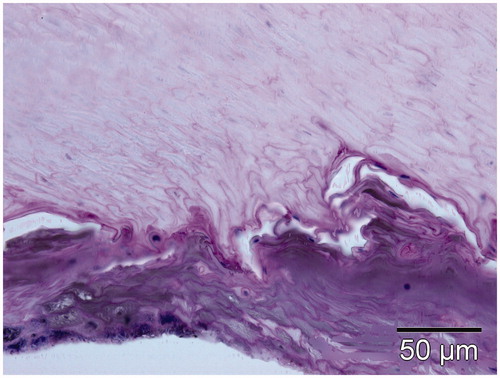
A routine histology work-up was performed on the nail clippings to evaluate the effect of the 1064-nm Nd:YAG laser. After removing the nail parts, because of other indications and ensuring informed consent from the patient, the nail parts were subjected to laser irradiation using a fluence of 70 J/cm2 and a pulse duration of 40 ms (three passes). The tissue block was embedded in paraffin, cut into 5–8-µm slices and stained with haematoxylin and eosin (H&E) and periodic acid Schiff (PAS) according to laboratory standard procedures. The biopsies were evaluated under a calibrated microscope (BX41, Olympus Germany, Hamburg) equipped with a digital camera (DP70, Olympus Germany). The specimen was measured using calibrated CellF software (Olympus Germany).
Statistics
Statistical analysis was performed using Statistica 7.0 software for Windows (StatSoft, Tulsa, OK, USA). A Mann-Whitney U test was performed to investigate the differences between the groups. P values <0.05 were considered statistically significant.
Results
Heating of cell culture media
When using the 808-nm system at a pulse duration of 6 ms and a fluence of 9 J/cm2, the temperature curve did not reach 22 °C, even after a long series of stacks were applied (). A temperature of 26 °C could only be achieved if the fluence was raised to 27 J/cm2 with 30 stacks. When the 980-nm system was used, there was a slow increase in the temperature of cell culture medium without pathogens when using a pulse duration of 6 ms and a fluence of 9 J/cm2. The increase in temperature was higher if pathogens were added to the cell culture medium. The most pronounced increase in temperature was found if a 980-nm system was applied using a 6-ms pulse duration to apply a fluence of 27 J/cm2. Overall, the time needed to heat the medium with and without pathogens was clearly dependent on the amount of stacks, and therefore exposure time at a given wavelength, fluence and pulse duration. While the 808-nm system did not produce sufficient heating at low fluences and delayed at higher fluences, the 980-nm system was proven to be efficient in a short time period. Higher fluences resulted in a more rapid heating.
Efficacy of laser treatment in eradication of common and rare medically important fungi
Efficacy of laser irradiation on fungal pathogens cultured in liquid media
The 980-nm system was used to heat the fungi within the culture media over time. During the application of a fluence of 27 J/cm2 at a 6-ms pulse duration (318 pulses) during a 9-min time period, a temperature of approximately 45 °C was reached at steady state using the 12 × 12 mm spot. Using the 12 × 50 mm spot resulted in a longer time period (21 min) until a temperature steady state was achieved with 318 pulses. After transferring the pathogens onto agar plates, at day 6 after treatment, the colonies were counted and growth inhibition was found for only one run of C. guilliermondii and T. interdigitale. All other runs demonstrated reduced growth compared with untreated controls (C. guilliermondii, C. parapsilosis, T. interdigitale, and M. gypseum). The growth of the yeast C. albicans was never disturbed (). The results indicated that complete clearance was achieved only if the measured temperatures exceeded 50 °C. This is in line with information that spores will survive temperatures up to 60 °C [Citation35].
Table II. Efficacy of 980-nm irradiation of pathogens in liquid culture media as measured 6 days post-treatment on culture plates.
The in vitro effect of the 1064-nm system was even worse. Pathogen growth was inhibited only temporarily using high fluences ().
Table III. Efficacy of 1064-nm irradiation of pathogens in liquid culture media as measured 6 days post-treatment on culture plates.
Histology
The routine histology work-up performed on nail clippings to evaluate the effect of the 1064-nm long pulsed laser showed a clear dissection of the nail plate from the nail bed, indicating a heat action at the target tissues as known from other lasers ().
Discussion
Onychomycosis is a worldwide emerging problem with no tendency for self-healing and the potential to affect surrounding tissues, as well as the possibility of predisposing the patient to secondary bacterial infections. In conjunction with a wide variety of topical but less effective treatment options, standard systemic terbinafine administration achieves a disease-free nail in only approximately 35–76% of cases [Citation13–15]. In addition, up to 22.3% relapse rates are found within 3 years after the completion of treatment [Citation16]. Recently, the option of laser nail fungus treatment has appeared. This therapy modality seems to be especially appropriate for the elderly patient. Typically, a decrease in flexibility and an increase in co-medication are observed in this cohort of patients. Thus, using a local therapy with no systemic effects from drug interference or metabolic effects would be beneficial for the elderly. To date, a variety of laser systems have been promoted to treat onychomycosis, and some of them already carry FDA approval for nail clearance. Most often, 1064-nm systems are used, and superior clearing rates from 87.5% [Citation32] up to 95.8% [Citation33,Citation36] have been published. However, the latter cure rates were published without any peer review. In contrast, no effect at all has been found by in vitro irradiation of pathogens on agar plates [Citation34]. As fungal infection of the human skin and its appendages is so common and a variety of laser systems using different wavelengths and settings are available, this study aimed to investigate laser irradiation efficacy against common and rare pathogens in vitro by performing three in vitro experiments.
For the long pulsed near-infrared lasers, heat is the most likely mechanism of action. Here, we clearly demonstrate that lasers emitting at 808 nm and 980 nm are effective in heating cell culture media with and without (neat cell culture medium) pathogens. The use of higher wavelengths and higher fluences are advantageous. However, with both there is an increase in pain if applied clinically. Basically, these results might direct us to use linear scanning hair removal lasers for experimental treatment of fungal pathogens, although clinically effective, safe and painless treatment protocols still need to be established. In addition, this study revealed that rather long stacking at a higher fluence is required to heat up cell culture media even in small volumes. Unfortunately, this might not really reflect the situation in vivo where the pathogens are not kept in a liquid environment. In contrast, a clinical treatment over a longer time using more stacks might be beneficial for homogeneous nail plate heating and to ensure definitive pathogen eradication since there is no specific absorber carried by the fungi to be destroyed. In comparison, the application of a 1064-nm long pulsed Nd:YAG using a fluence of 70 J/cm2 and a pulse duration of 40 ms (three passes) resulted in a clear dissection (clefting) of the nail plate from the nail bed, indicating a heat action at the target area or tissues. Clefting is often found at the junction between epidermis and dermis in response to other laser interventions, indicating a cumulative temperature effect. To what extent this effect is purely coincidental or a necessity for effective treatment needs to be further elaborated.
During the latter two experiments we tested the efficacy of the lasers in destroying pathogens in vitro. Other researchers have been unable to demonstrate the eradication of pathogens by direct irradiation of agar plates [Citation34]. Using our alternative approach of transferring irradiated pathogens to agar plates it was also demonstrated that lasers do not always have a harmful effect on fungi. This was especially the case in the two most common species T. rubrum and T. interdigitale. Indeed, in some cases, growth appears to have been induced. Most likely, the temperatures achieved were not high enough or were unevenly distributed, which might result in an occasional stimulation sometimes described as ‘low-level laser stimulation’. With this taken into account, an on-time temperature measurement might be helpful in evaluating the effects of the lasers and temperature. Otherwise, it remains to be proven if effective temperatures above 50 °C are really necessary, and if so, how they can be applied in vivo with no or low pain.
Conclusion
Dermatophytosis is found in approximately 20–25% of the worldwide population [Citation2]. Out of the worldwide population, an estimated 2–13% of the population suffers from onychomycosis [Citation3–5], which is the most common disease of nails worldwide and constitutes approximately half of all nail abnormalities [Citation6]. The disease has a huge impact on quality of life [Citation7,Citation8]. Recently, the option of laser nail fungus treatment has appeared. The reported superior treatment effects of 87.5% [Citation32] up to 95.8% [Citation33,Citation36] clearance are in contrast to in vitro studies where no effect has been found following the irradiation of pathogens [Citation34]. This study demonstrates a clear thermal effect of linear scanning 808-, 980 - and long pulsed 1064-nm laser systems on cell culture media or nail clippings. With shorter wavelengths, higher fluences and more stacks were needed. However, the post-irradiation growth of fungi was only occasionally inhibited. Finally, growth inhibition appears to be strongly dependent on the temperature reached. Most likely, homogeneous heat distribution is crucial, although nothing is currently known about the minimal time needed for a given plateau temperature. Further studies with on-time in vivo temperature evaluation, e.g. during the treatment, and clinical long-term follow-ups are needed to evaluate the in vivo laser and thermal effect for each specific system.
Declaration of interest
The 808-nm and 980-nm laser systems were loaned from the manufacturer to run a separate hair removal study. Uwe Paasch and Marc Bodendorf are working as an honorarium speaker for Alma Lasers. Uwe Paasch and Sonja Grunewald served as consultants for Quantel-Derma, now Alma Lasers. Uwe Paasch and Jan C. Simon received unrestricted research grants from Quantel-Derma, now Alma Lasers. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors wish to thank Cornelia Schneider for her expert technical assistance.
References
- Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol 2010;28:197–201
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008;51:S2–15
- Hamnerius N, Berglund J, Faergemann J. Pedal dermatophyte infection in psoriasis. Br J Dermatol 2004;150:1125–1128
- Amichai B, Davidovici B, Trau H, Lyakhovitsky A, Grunwald MH, Shemer A. A rationale for systemic treatment in onychomycosis with negative results on fungal examination. Clin Exp Dermatol 2011;36:724–727
- Scher RK, Tavakkol A, Sigurgeirsson B, Hay RJ, Joseph WS, Tosti A, et al. Onychomycosis: Diagnosis and definition of cure. J Am Acad Dermatol 2007;56:939–944
- Nenoff P, Ginter-Hanselmayer G, Tietz HJ. Onychomykose – ein Update: Teil 1 – Prävalenz, Epidemiologie, disponierende Faktoren und Differenzialdiagnose. [Fungal nail infections – an update: Part 1 – Prevalence, epidemiology, predisposing conditions, and differential diagnosis]. Hautarzt 2012;63:30–38
- Gupta AK, Jain HC, Lynde CW, Macdonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15 000 patients. J Am Acad Dermatol 2000;43:244–248
- Elewski BE. Onychomycosis. Treatment, quality of life, and economic issues. Am J Clin Dermatol 2000;1:19–26
- Elewski BE. Onychomycosis: Pathogenesis, diagnosis, and management. Clin Microbiol Rev 1998;11:415–429
- Elewski BE, Leyden J, Rinaldi MG, Atillasoy E. Office practice-based confirmation of onychomycosis: A US nationwide prospective survey. Arch Intern Med 2002;162:2133–2138
- Nenoff P, Ginter-Hanselmayer G, Tietz HJ. Onychomykose – ein Update: Teil 2 – Vom Erreger zur Diagnose – konventionelle und molekularbiologische mykologische Diagnostik. [Fungal nail infections – An update: Part 2: From the causative agent to diagnosis – Conventional and molecular procedures]. Hautarzt 2012;63:130–138
- Finch JJ, Warshaw EM. Toenail onychomycosis: Current and future treatment options. Dermatol Ther 2007;20:31–46
- Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? An analysis of published data. Arch Dermatol 1998;134:1551–1554
- Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol 2004;150:537–544
- Van Duyn GL, Elewski BE. Recent updates in oral terbinafine: Its use in onychomycosis and tinea capitis in the US. Mycoses 2011;54:e679–e685
- Tosti A, Piraccini BM, Stinchi C, Colombo MD. Relapses of onychomycosis after successful treatment with systemic antifungals: A three-year follow-up. Dermatology 1998;197:162–166
- Tchernev G, Penev PK, Nenoff P, Zisova LG, Cardoso JC, Taneva T, et al. Onychomycosis: Modern diagnostic and treatment approaches. Wien Med Wochenschr 2013;163:1–12
- Singal A, Khanna D. Onychomycosis: Diagnosis and management. Indian J Dermatol Venereol Leprol 2011;77:659–672
- Grover C, Bansal S, Nanda S, Reddy BS, Kumar V. Combination of surgical avulsion and topical therapy for single nail onychomycosis: A randomized controlled trial. Br J Dermatol 2007;157:364–368
- Silva JL, Doimo G, Faria DP. The use of high frequency waves to treat onychomycosis: Preliminary communication of three cases. An Bras Dermatol 2011;86:598–600
- Aspiroz C, Fortuno CB, Rezusta A, Paz-Cristobal P, Dominguez-Luzon F, Gene DJ, et al. Terapia fotodinámica aplicada al tratamiento de las onicomicosis. Presentación de un caso y revisión de la literatura. [Photodynamic therapy for onychomycosis. Case report and review of the literature]. Rev Iberoam Micol 2011;28:191–193
- Kamp H, Tietz HJ, Lutz M, Piazena H, Sowyrda P, Lademann J, et al. Antifungal effect of 5-aminolevulinic acid PDT in Trichophyton rubrum. Mycoses 2005;48:101–107
- Rothermel E, Apfelberg DB. Carbon dioxide laser use for certain diseases of the toenails. Clin Podiatr Med Surg 1987;4:809–821
- Kaplan I, Labandter H. Onychogryphosis treated with the CO2 surgical laser. Br J Plast Surg 1976;29:102–103
- Apfelberg DB, Rothermel E, Widtfeldt A, Maser MR, Lash H. Preliminary report on use of carbon dioxide laser in podiatry. J Am Podiatry Assoc 1984;74:509–513
- Vural E, Winfield HL, Shingleton AW, Horn TD, Shafirstein G. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci 2008;23:349–353
- Kimura U, Takeuchi K, Kinoshita A, Takamori K, Hiruma M, Suga Y. Treating onychomycoses of the toenail: Clinical efficacy of the sub-millisecond 1064 nm Nd: YAG laser using a 5 mm spot diameter. J Drugs Dermatol 2012;11:496–504
- Hiruma M, Kawada A, Noguchi H, Ishibashi A, Conti Diaz IA. Hyperthermic treatment of sporotrichosis: Experimental use of infrared and far infrared rays. Mycoses 1992;35:293–299
- Bornstein E, Hermans W, Gridley S, Manni J. Near-infrared photoinactivation of bacteria and fungi at physiologic temperatures. Photochem Photobiol 2009;85:1364–1374
- Landsman AS, Robbins AH, Angelini PF, Wu CC, Cook J, Oster M, et al. Treatment of mild, moderate, and severe onychomycosis using 870- and 930-nm light exposure. J Am Podiatr Med Assoc 2010;100:166–177
- Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD. Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis. Photochem Photobiol 2010;86:476–479
- Hochman LG. Laser treatment of onychomycosis using a novel 0.65-millisecond pulsed Nd:YAG 1064-nm laser. J Cosmet Laser Ther 2011;13:2–5
- Kozarev J, Vizintin Z. Novel laser therapy in treatment of onychomycosis. J Laser Health Acad 2010;1:1–8
- Hees H, Raulin C, Baumler W. Laser treatment of onychomycosis: An in vitro pilot study. J Dtsch Dermatol Ges 2012;10:913–918
- Tietz HJ, Nenoff P. Die Onychomykose – ein Kronjuwel der Dermatologie. [Onychomycosis: A crown jewel of dermatology]. Hautarzt 2012;63:842–847
- Kozarev J. ClearSteps – Laser onychomycosis treatment: Assessment of efficacy 12 months after treatment and beyond. J Laser Health Acad 2012;2011:S07