Abstract
Purpose: We retrospectively analysed the long-term outcomes of cytoreductive surgery and post-operative heated pleural chemotherapy (HPC) for thoracic malignancies with pleural spread. Materials and methods: Between 1987 and 2010, 160 patients were enrolled. There were 101 patients with non-small cell lung cancer (NSCLC), 25 with malignant pleural mesothelioma (MPM), 12 with thymoma, and 22 with tumours metastatic to the lung and pleura. Immediately after intra-thoracic administration of cisplatin or carboplatin, hyperthermia was performed by using an 8.00 MHz radiofrequency capacitive heating device for 1 to 4 courses in each patient. Results: There was no systemic toxicity or treatment-related mortality. Five-year overall survival rates were 37.4% in NSCLC, 15.9% in MPM, 91.7% in thymoma, and 25.8% in metastatic lung tumour. Five-year local relapse-free survival (RFS) rates were 55.2% in NSCLC, 24.4% in MPM, 64.8% in thymoma, and 27.2% in tumours metastatic to the lung and pleura. When 101 NSCLCs were categorised into pleural lavage cytology positive (grade 1: n = 37), limited extent of carcinomatous pleuritis (grade 2: n = 21), and extensive carcinomatous pleuritis (grade 3: n = 43), 5-year overall survival rates were 62.5%, 49.2%, and 13.6%, respectively. The local RFS was significantly better in group 1/2 than in group 3. Conclusions: Although our study has some of the usual weaknesses of a single institution retrospective study, cytoreductive surgery and HPC are feasible and safe. It is suggested that HPC may have a potential role for local control as adjuvant treatment for cytoreductive surgery in patients with minor pleural spread.
Introduction
Pleural dissemination and malignant effusion are common manifestations of disease progression in lung cancer, malignant pleural mesothelioma (MPM), invasive thymoma, and tumours metastatic to the lung and pleura. The majority of these are regarded as having no surgical indication. Many multimodality treatment programmes combining cytoreductive surgery, radiotherapy, chemotherapy, and many experimental treatments such as photodynamic therapy, immunotherapy, and gene therapy have been applied for selected patients among them; however, there has been no standard treatment for pleural surface malignancy.
It is difficult to detect pleural surface malignancy in the early phase, even using modern radiological imaging techniques. Recently, several investigators reported that the early phase of pleural surface malignancy can be detected by pleural lavage cytology at the time of thoracotomy in patients with lung cancer [Citation1].
Hyperthermic intracavitary chemotherapy has been applied after cytoreductive surgery to enhance locoregional control for pleural [Citation2] or peritoneal malignancies [Citation3]. Intracavitary drug administration offers a theoretical advantage: the tumour is exposed directly to higher drug concentrations, whereas a lower incidence of toxic side effects may be expected compared with systemic chemotherapy. Several types of interaction of the heart with chemotherapeutic agents have been found [Citation4,Citation5].
Previously, we reported the results of a pilot study regarding heated pleural chemotherapy (HPC), designated ‘post-operative intra-thoracic chemo-thermotherapy’ (PICT) for carcinomatous pleuritis due to non-small cell lung cancer (NSCLC) with the objective of improving local cure [Citation6]. In the present work we extended the retrospective study to select the subgroup that achieved an advantage from post-operative HPC among the patients with pleural surface malignancy due to not only NSCLC but also MPM, thymoma, or tumours metastatic to the lung and pleura from other organs.
Materials and methods
Patients
Between November 1983 and August 2010, we performed post-operative HPC following cytoreductive surgery for 184 patients with pleural surface malignancy. Of these, 14 early patients who underwent HPC from 1983 to 1987 using equipment different from Thermotron-RF8 (Yamamoto Vinitor, Osaka, Japan) were excluded. In addition, five patients whose operation resulted in exploratory thoracotomy, three with insufficient heating lower than 400 W of radiofrequency (RF) power, one without peri-pleural temperature measurement, and one with an insufficient clinical record were also excluded. Consequently, 160 patients treated between 1987 and 2010 were enrolled in this study. Their average age at the time of cytoreductive surgery was 56 ± 12.9 (range: 16–81) years; 92 were male and 68 were female.
There were 101 patients with NSCLC, 25 with MPM, 12 with thymoma, and 22 with tumours metastatic to the lung and pleura from other organs. Of 2,923 NSCLC patients who underwent surgery, 152 were found to have pleural surface malignancy at the time of thoracotomy. Of these, 64 patients underwent HPC following cytoreductive surgery from August 1987. In addition, 37 patients with cytologically proven cluster cancer cells on pleural lavage cytology also underwent heated pleural chemotherapy. HPC was employed for 25 of 26 MPM patients from July 1990, and 12 of 78 thymoma patients from October 1989. This modality was employed for 17 of 31 patients with pleural dissemination discovered at thoracotomy and five patients with positive cytological findings on pleural lavage cytology among 729 patients who underwent metastasectomy for tumours metastatic to the lung from March 1988.
Twenty-five patients with MPM were histologically subdivided into nine patients with epithelial type and 16 with non-epithelial type. When patients with MPM was classified according to IMIG-TNM staging [Citation7], one patient had stage Ia, two patients stage Ib, two stage II, 13 stage III, and seven stage IV. Of 12 thymoma patients, four patients with de novo Masaoka stage IVa [Citation8] and one with mucoepidermoid carcinoma underwent thymo-thymectomy followed by HPC. Six other patients underwent cytoreductive surgery followed by HPC for thymoma with pleural relapse. An addition, one patient had ectopic thymoma on the pleura and underwent HPC after removal of the thymoma to prevent pleural recurrence. However, there were no patients complicated with myasthenia gravis. Of the 22 patients with tumours metastatic to the lung and pleura, 13 patients had soft tissue sarcoma, four osteosarcoma, four other organ cancer, and one melanoma.
Patients for whom post-operative HPC was planned provided written informed consent after fully talking over the risks and benefits with their surgeons. Since 1996, chemotherapy combined with hyperthermia has been accepted by the Japanese government such that the public medical insurance has covered 70% of the medical fee. The institutional review board of Osaka Medical Centre approved this study (approval no. 1207095022), and waived the individual consent requirement to use clinical and pathological data from the institutional database because of the retrospective nature of this study.
Cytoreductive surgery
Procedures employed for cytoreductive surgery were extrapleural pneumonectomy (EPP) in 27 patients, pneumonectomy in four, lobectomy in 77, sub-lobar resection in 48, and pleurectomy in four. We defined EPP or pneumonectomy as major resection, and lobectomy, sub-lobar resection, or pleurectomy as minor resection.
Post-operative heated pleural chemotherapy
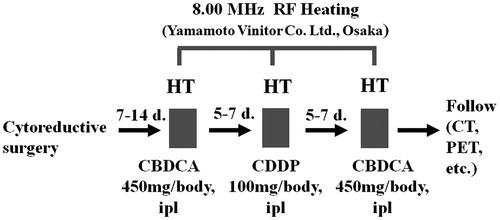
Cisplatin (50–100 mg/chest cavity) or carboplatin (450 mg/chest cavity) adjusted to a volume of 200 mL with saline was administered into the chest cavity through a narrow chest drainage tube(s) retained at the time of the surgery beside the regular chest drainage tube. Teflon-coated probe of copper-constantan micro-thermocouple (IT-18, Physitemp Instrument, Clifton, NJ) with a sterilised sheath was also inserted into the chest cavity through the same tube and the orifice of the tube was sealed using gentamicin ointment to block air inflow into the chest cavity. Immediately after injection, hyperthermia was performed for 1 h using an 8.00 MHz radiofrequency (RF) capacitive heating device (Thermotron RF-8, Yamamoto Vinita, Osaka). Heated pleural chemotherapy (HPC) was started between 7 and 14 post-surgical days and performed for one to four courses in each patient ().
The RF generator (Thermotron RF8) has a self-excited oscillation circuit at 8 MHz and 1,500 W maximum output power. The RF was applied through a pair of electrodes placed on opposite sides (anterior versus posterior) of the chest, and power was distributed locally through interaction of electric fields produced between the parallel-opposed electrodes. We employed a pair of electrodes with a large diameter of 25 cm in order to cover the whole hemithorax. The surface of the metal plate of the electrode was covered with a flexible water pad which was filled with 0.4% saline solution nearly electrically equivalent to muscle. In order to avoid excessive heating of the skin and subcutaneous fat, the overlay bolus connected to the water-cooled apparatus was sandwiched between the skin and the electrode pads. This device was developed in Japan [Citation9] and the distribution of its specific absorption rate (SAR) was investigated by Paliwal and co-workers [Citation10]. Basically, we employed the heating method described by Lee and co-workers [Citation11]. The Thermotron RF8 was approved as a medical device for thermotherapy of cancer by the Ministry of Health and Welfare Japan on 15 December 1984. The same 8-MHz RF-capacitive heating device is currently in use for deep regional hyperthermia for the whole thoracic region in other institutes [Citation12,Citation13]. After completion of the therapy, cisplatin or carboplatin was removed from the chest cavity, if possible.
Statistical analysis
In the analysis of baseline characteristics of four groups with different diseases, analysis of variance (ANOVA) was used for parametric comparisons, proportions were compared by chi-square test, and frequency analysis was performed with Fisher’s exact test. Pearson’s correlation coefficient (r) was used to study the relationship between RF power (W) and maximal peri-pleural temperature (°C) achieved during heating.
Survival analyses for all-cause death and local relapse-free survival (RFS) were performed by means of the Kaplan-Meier approach. For both events, the days of the start of observation and the termination event were defined as the dates of surgery and event occurrence, respectively. Cases that had no termination event were considered as censored cases at the final observation date. Local relapse was defined as the appearance of a new lesion on chest computerised tomography (CT) and/or positron emission CT (PET) scan or exacerbation of the symptom within the treated hemithorax (pleura, hilum, and/or ipsilateral mediastinum). Vital status as of 21 March 2012 was confirmed through the Osaka Medical Centre cancer registry database.
NSCLC patients were categorised into three subgroups regarding grade of pleural dissemination (D-factor) or pleural effusion (E-factor) in accordance with the sixth edition of General Rules for Clinical and Pathological Records for Lung Cancer [Citation14]. We categorised the patients in whom malignant cell clusters were detected only at the lavage cytology (D0E0) of the chest cavity as grade 1. Limited extent of carcinomatous pleuritis (D1E0 or D0E1) was categorised as grade 2, and extensive carcinomatous pleuritis (D1E1, D2E0-2, or D0-2E2) as grade 3. The survival rates were also compared among these three groups with different categories of pleural malignancy. The log-rank test was used for the comparison.
Statistical analyses were performed using IBM SPSS Statistics (version 19.0) software and Software R (version 2.12.0). Differences were considered significant when p < 0.05.
Results
shows baseline characteristics of the four groups classified according to the original diseases. There was a significant difference in age among the groups (p = 0.001). Surgical approaches employed as cytoreductive surgery were significantly different among the four groups (p < 0.0001). When these approaches were categorised into major and minor resections, major resection such as EPP was frequently employed in the MPM group compared with the other groups. In the NSCLC group and the MPM group, 25 of 101 patients (25%) and seven of 25 patients (28%) had mediastinal lymph node metastasis (N2), respectively, while this occurred in only one of 12 thymoma patients (8%) and no patients with tumours metastatic to the lung and pleura (p = 0.015). There was also a significant difference in D, E status when categorised into two groups, D0E0, D1E0, and D0E1 versus D1E1, D2E0-2, and D0-2E2 (p < 0.0001). Averages of maximal temperature reached in each patient were significantly different (p = 0.007) among the four groups, and the temperature was highest in the MPM group. A statistically significant positive correlation was observed between peri-pleural temperature and RF power. Pearson’s correlation coefficient (r) and the corresponding p value for the correlation were 0.443 and 0.0001, respectively ().
Figure 2. Correlation analysis between RF power and peri-pleural temperature in 160 patients with PICT.
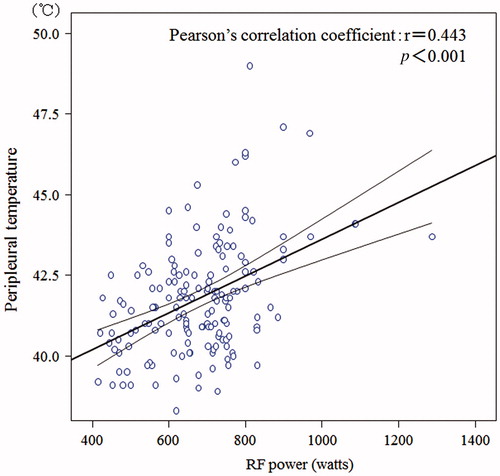
Table I. Baseline characteristics of the patients.
Post-operative HPC was performed for an average of 1.8 courses (range: 1–4 courses) for each patient. We generally administered a single course of HPC for patients with positive lavage cytology (D0E0). Another common reason for administering a single course of heated pleural chemotherapy was pleural adhesion of the residual lung to parietal pleura in minor resection; therefore, 2–4 courses were administered to patients with major resection such as pneumonectomy or EPP. There was neither surgery-related nor HPC-related mortality. The median follow-up periods after surgery were 37 months in NSCLC, 17 months in MPM, 68 months in thymoma, and 21 months in the metastatic lung tumour group. Overall survival rates for 3, 5 and 10 years were 59.7%, 37.4% and 13.9% in NSCLC, 21.3%, 15.9% and 8.0% in MPM, 91.7%, 91.7% and 73.3% in thymoma, and 36.1%, 25.8% and 10.3% in tumours metastatic to the lung and pleura, respectively. The median survival times (MST) of NSCLC, MPM, and tumours metastatic to the lung and pleura were 40 months, 19 months and 24 months, respectively. The MST in thymoma was not reached. The survival in thymoma was significantly better than those of the other three diseases (p = 0.0034, 0.0011 and 0.0002).
Local RFS rates for 3, 5 and 10 years were 61.5%, 55.2% and 47.8% in NSCLC, 45.7%, 24.4% and 24.4% (at 7 years) in MPM, 74.1%, 64.8% and 54.0% in thymoma, and 54.4%, 27.2% and 27.2% (at 9 years) in tumours metastatic to the lung and pleura, respectively.
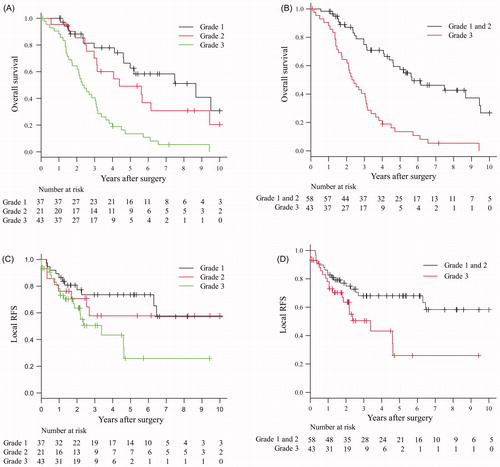
A total of 101 NSCLCs were categorised into pleural lavage cytology positive as grade 1 (D0E0: n = 37), limited extent of carcinomatous pleuritis as grade 2 (D1E0 or D0E1: n = 21), and extensive carcinomatous pleuritis as grade 3 (D1E1, D2E0-2 or D0-2E2: n = 43) according to their intraoperative findings. As a result, 3-, 5-, and 10-year overall survival rates were 78.0%, 62.5% and 30.6% in grade 1, 70.2%, 49.2% and 20.5% in grade 2, and 40.5%, 13.6% and 0% in grade 3, respectively (). There were significant differences in survival between grade 1 and grade 3 (p < 0.0001) and between grade 2 and grade 3 (p = 0.0029). However, there was no significant difference between grade 1 and grade 2 (p = 0.2190). When grade 1 and grade 2 were grouped together (n = 58), the 3-, 5- and 10-year overall survival rates were 74.9%, 57.2% and 26.7%, respectively, showing a clear significant difference (p < 0.0001) to grade 3 (). On the other hand, 3-, 5- and 10-year local RFS rates were 73.5%, 73.5% and 57.2% in grade 1, they were all the same rate of 57.9% in grade 2, and 50.5%, 26.0% and 26.0% (at 9 years) in grade 3, respectively (). A significant difference in local RFS curves was found between grades 1 and 3 (p = 0.041). When grade 1 and grade 2 were grouped together (n = 58), the 3-, 5- and 10-year local RFS rates were 68%, 68% and 58.3%, respectively, with a significant difference (p = 0.02) to grade 3 ().
Figure 3. Overall survival curves (A and B) and local PFS curves (C and D) for 101 NSCLC patients according to the grade of pleural malignancy.
When the 101 patients with NSCLC were classified into minor and major resection groups according to the type of resection, 3-, 5- and 10-year survival rates were 62.9%, 39.9% and 12.9% in minor resection and 38.5%, 23.1% and 23.1% in major resection, respectively. The overall survival in minor resection had a trend towards a better outcome than major resection, although this difference was not statistically significant (p = 0.1150) (). In contrast, 3-, 5- and 10-year local RFS rates were 58.9%, 52.5% and 44.4% in minor resection, respectively, and were all the same at 83.9% in major resection. The local RFS in major resection had a trend towards a better outcome than minor resection, although this difference was also not statistically significant (p = 0.1838) ().
Figure 4. Overall and local RFS curves of 101 NSCLC (A and C) patients and 25 MPM (B and D) patients according to the type of resection.
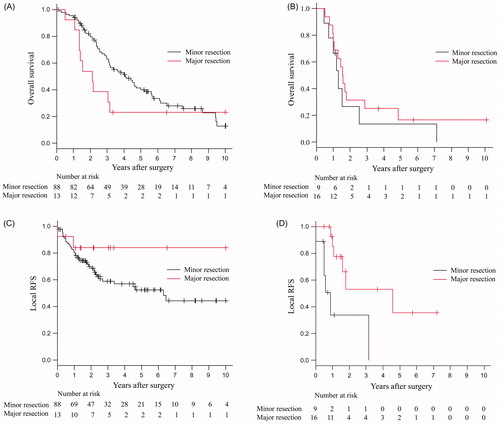
Similarly, MPM patients with major resection combined with HPC had better local RFS of 53.1% and 35.4% at 3 and 5 years than those with minor resection combined with HPC of 33.9% and 0% at 3 and 4 years (p = 0.0053) (). However, there was no significant difference in overall survival between the two groups (25% and 16.7% versus 13.3% and 13.3% at 3 and 5 years) ().
There was no systemic toxicity or treatment-related mortality. Adverse events related to HPC for 164 patients are presented in . No patients exhibited grade 2 or higher toxicity. The most common grade 1 toxicity was pain (53%) of the skin beneath the RF electrode on the anterior chest wall. As rare toxicities, two female patients (1.2%) exhibited induration in the breast, two patients (1.2%) a small spot of skin burn with bulla, one (0.6%) complained of anxiety, and one (0.6%) of vomiting. Of these 164 patients, four patients whose RF power did not exceed 400 W due to uncontrollable pain were excluded from the analysis.
Table II. Adverse events related to postoperative heated pleural chemotherapy (HPC) (NCI-CTC version 2.0).
Discussion
We started post-operative HPC in 1983 and experienced a case showing a dramatic response to it in 1988. In brief, a patient with multiple lung metastases and malignant pleurisy on both sides due to Ewing’s sarcoma was treated on the right side with intra-thoracic injection of 50 mg of cisplatin and local heating using Thermotron RF8 for 60 minutes, along with concomitant systemic administration of 50 mg of cisplatin. Three courses of HPC were administered. Although the patient died 3 months later due to advanced metastases in the left lung and malignant pleurisy on the left side, metastases in the right lung parenchyma were stable on radiographs, and autopsy results showed no malignant lesions in the right thoracic cavity. Since no effective response had been obtained clinically and histologically before starting the HPC, despite frequent systemic administration of anti-cancer drugs, we concluded that heat acted synergistically with cisplatin on drug-resistant cells in this patient [Citation15,Citation16]. The same result of in vitro chemo-thermosensitivity test in human Ewing’s sarcoma cell lines was reported by Debes and co-workers [Citation17]. They disclosed the synergistic enhancement of cisplatin cytotoxicity by heat application, which might predict chemo- and thermosensitivity. Our co-worker, Ohguchi [Citation18], also demonstrated by using a computer simulation of RF capacitive-type hyperthermia that heat transport from the body by air convection leads to reasonable temperature profiles in the body surface area, such as the pleural vicinity. On the basis of these lines of evidence, since 1988 we have extended HPC to pleural malignancy due to not only NSCLC but also MPM, thymoma and tumours metastatic to the lung and pleura. However, after we clarified the poor survival and local control of HPC in NSCLC patients with N2 disease and/or macroscopically residual disease in 1993 [Citation6], the indication of post-operative HPC was basically towards patients with N0–1 diseases, regardless of the type of original malignancy. Platinum compounds are the most common drugs showing temperature-dependent supra-additive effects on not only cancer but also refractory sarcoma [Citation19]. We reported that the free platinum concentration was kept at more than 10 µg/mL for 2 h immediately after a bolus injection of cisplatin (50–100 mg) in the pleural cavity [Citation20].
We searched for articles indicating the outcome of the treatment for patients with pleural surface malignancy due to thymoma [Citation21–28], MPM [Citation12,Citation29–34], or NSCLC [Citation1,Citation35–37] (). According to our literature survey there were no reports concerning cytoreductive surgery for pleural surface malignancy discovered at the time of thoracotomy for tumours metastatic to the lung.
Table III. Summary of studies concerning outcomes for the management of pleural surface malignancy arising from three different diseases.
Because of the indolent nature of thymoma, the overall survival in thymoma was significantly better than those of other malignant diseases. At present there is no standard approach to advanced thymoma apart from an operation. Refaely and co-workers [Citation21] reported that operation and perfusion thermochemotherapy are feasible and safe in patients with stage IVa thymoma with 3-year and 5-year actuarial survival rates of 70% and 55% (median follow-up: 34 months). According to a recent report by Ishikawa and co-workers [Citation25], multimodality therapy combined with induction chemotherapy, surgery, and post-operative radiation therapy for stage IVa thymoma offers a good outcome, especially in patients with EPP. In their analysis, overall survival was 81% at 5 years and 70% at 10 years after cytoreductive surgery. Recently, Yellin and co-workers [Citation28] reported that the 5-, 10- and 15-year overall survival rates for de novo stage IVa thymoma (DNT) and thymoma with pleural relapse (TPR) were 80.8%, 72.7% and 58.2% (DNT) and 66.7%, 55.6% and 27.8% (TPR) with a median follow-up of 62 months after resection and heated pleural chemoperfusion. They also suggested that completeness of resection and the perfusion temperature may play a role in local control. In our study 5- and 10-year overall survival rates were 91.7% and 73.3% for thymoma with pleural surface malignancy. The 10-year overall survival of 73% is among the best ever reported.
The goal of an operation in treating MPM is to remove all gross disease, achieving a macroscopically complete resection. Other modalities have been used to treat the residual microscopic disease that is always present. Monneuse and co-workers [Citation29] reported the long-term results of intra-thoracic chemohyperthermia (ITCH) combined with pleurectomy/decortication (P/D) as cytoreductive surgery. They concluded that their procedure may offer unexpected long-term survival in a selected group of patients with T1 and T2 MPM or fibrosarcoma. According to the results of a phase II study of EPP followed by intracavitary intraoperative hyperthermic cisplatin for MPM reported by Tilleman and co-workers [Citation32], recurrence of MPM was 51%, with ipsilateral recurrence in 17.4% of patients. Hospital mortality was 4.3%. About half of their patients had grade 3 or 4 morbidity, including atrial fibrillation in 23.9%. The median survival was 12.8 months. They concluded that hyperthermic intraoperative intracavitary cisplatin perfusion following EPP might enhance local control in the chest. It is noteworthy that they simultaneously carried out intracavitary cisplatin lavage of both ipsilateral chest cavity and abdominal cavities, which has high potential for inducing recurrence. We employed HPC to treat the residual microscopic disease in 25 MPM patients. Their 3-, 5-, and 10-year overall survival rates were 21.3%, 15.9% and 8%, respectively. The 3-, 5-, and 7-year local PFS rates were 45.7%, 24.4%, and 24.7%, respectively. When we restricted the analysis to patients with EPP (1995–2008), the median overall survival was 19 months, and the local recurrence rate was 36.4% [Citation33]. When major resection is performed, the pleural surface may be exposed homogeneously to a high temperature and a high concentration of free platinum. The benefits of post-operative HPC are to reduce the amount of drugs such as cisplatin or carboplatin [Citation12] and the ability to perform it repeatedly compared with intraoperative perfusion thermochemotherapy [Citation21,Citation28,Citation29,Citation32,Citation34]. In contrast, HPC in combination with minor resection may lead to heterogeneous exposure of heat and drug on the pleural surface compared with intraoperative perfusion chemotherapy.
Recently, Ueda and co-workers [Citation38] reported excellent outcomes of low dose chemotherapy and regional 8 MHz RF hyperthermia for multiple lung metastases of bladder cancer. Of note, in their presented case, all metastases showing complete response were close to the pleura, where there is an advantage of being well heated by RF. In this group of our study, the 3- and 5-year PFS rates were 54.4% and 27.2%, respectively. Most of the patients included in this group had pleural malignancy due to metastatic sarcoma. Recently, the effectiveness of neo-adjuvant chemotherapy combined with regional hyperthermia for the primary lesion of high risk soft tissue sarcoma was demonstrated in a randomised phase III trial (NCT 00003052) [Citation39].
In our pilot study [Citation2,Citation6], we reported that the survival of NSCLC patients with pleural dissemination and concomitant p-N2 was significantly poor compared with that of patients with dissemination and concomitant p-N0-1. In the present study we extended the indication for HPC to patients with proven cancer cells as clusters at pleural lavage cytology (D0E0). However, we never performed HPC for patients with cancer cell positivity but without cluster formation at the cytology because of the low incidence of pleural recurrence [Citation40]. Consequently, our survival analysis demonstrated that there were significant differences in both overall survival and RFS between patients with grade 1–2 and grade 3. Recently, the relationship between tissue platinum concentration and survival was assessed by Kim and co-workers [Citation41]. Their study demonstrated a relationship between tissue platinum concentration and response in NSCLC, and suggested that reduced platinum accumulation might be an important mechanism of platinum resistance in a clinical setting. Our result suggested that a relatively small amount of malignant tumour present at the pleural surface (grade 1–2) is exposed to an extremely high concentration of platinum and its uptake into the cancer cells is reinforced by heating. However, it might have limited platinum diffusion into the centre of solid lesions categorised as grade 3.
There was no ≥grade 2 morbidity. However, about half of patients (53%) had a complaint of skin pain under an electrode placed on the anterior chest wall during heating. As we performed HPC without premedication and anaesthesia, we were able immediately to block the RF wave focally at the site of pain with a vinyl sheet. Consequently, we were able to prevent skin burn of ≥grade 2.
In conclusion, HPC was shown to be feasible and safe, even when combined with major resection such as extra-pleural pneumonectomy. It is suggested that HPC may offer excellent local control for patients with free tumour cells in the chest cavity and micrometastases on the pleural surface. Although these findings are of interest, they should be replicated in independent prospective studies to validate the importance of HPC.
Declaration of interest
This work was supported in part by a grant from the Osaka Community Foundation. The authors alone are responsible for the content and writing of the paper.
References
- International Pleural Lavage Cytology Collaborators. Impact of positive pleural lavage cytology on survival in patients having lung resection for non-small cell lung cancer: An international individual patient data meta-analysis. J Thorac Cardiovasc Surg 2010;139:1441–6
- Kodama K, Doi O, Tatsuta M, Kuriyama K, Tateishi R. Development of postoperative intrathoracic chemo-thermotherapy for lung cancer with objective of improving local cure. Cancer 1989;64:1422–8
- Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529–34
- Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 1999;15:79–107
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003;10:463–8
- Kodama K, Doi O, Higashiyama M, Yokouchi H, Tatsuta M. Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer 1993;72:426–31
- International Mesothelioma Interest Group. A proposed new international TNM staging system for malignant pleural mesothelioma. Chest 1995;108:1122–8
- Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485–92
- Kato H, Hiraoka M, Nakajima T, Ishida T. Deep-heating characteristics of a RF capacitive heating device. Int J Hyperthermia 1985;1:15–28
- Paliwal BR, Gehring MA, Sanders C, Mackie TR, Raffety HM, Song CW. 3D rendering of SAR distributions from Thermotorn RF-8 using a ray casting technique. Int J Hyperthermia 1991;7:567–75
- Lee CK, Song CW, Rhee JG, Levitt SH. Clinical experience with Thermotron RF-8 capacitive heating for bulky tumors: University of Minnesota experience. Radiol Clin North Am 1989;27:543–8
- Xia H, Karasawa K, Hanyu N, Chang TC, Okamoto M, Kiguchi Y, et al. Hyperthermia combined with intra-thoracic chemotherapy and radiotherapy for malignant pleural mesothelioma. Int J Hyperthermia 2006;22:613–21
- Ohguri T, Yahara K, Moon SD, Yamaguchi S, Imada H, Terashima H, Korogi Y. Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: Relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia 2011;27:20–26
- Japan Lung Cancer Society. General Rule for Clinical and Pathological Record of Lung Cancer. 6th ed. Tokyo: Kanehara, 2003
- Doi O, Kodama K, Tatsuta M, Higashiyama M, Aoki Y, Kuriyama K, et al. Effectiveness of intrathoracic chemothermotherapy for malignant pleurisy due to Ewing’s sarcoma: A case report. Int J Hyperthermia 1990;6:963–9
- Kodama K, Higashiyama M, Tokunaga T. Postoperative intrathoracic chemo-thermotherapy (PICT) in pleural surface malignancy (in Japanese). Surgery Frontier 2012;19:159–67
- Debes A, Rommel F, Breise M, Willers R, Göbel U, Wessalowski R, et al. In vitro test-system for chemo- and thermosensitivity: An analysis of survival fraction and cell-cycle distributions in human Ewing’s sarcomas as a model for tumors in pediatric oncology. Klin Pediatr 2002;214:223–9
- Ohguchi Y, Watanabe N, Niitsu Y, Doi O, Kodama K. A simulation model of hyperthermia by RF capacitive heating. IEICE Trans Inf Syst 1992;E75-D:219–26
- Wiedemann GJ, d’Oleire F, Knop E, Eleftheriadis S, Bucsky P, Feddersen S, et al. Ifosfamide and carboplatin combined with 41.8 °C whole-body hyperthermia in patients with refractory sarcoma and malignant teratoma. Cancer Res 1994;54:5346–50
- Kodama K, Doi O, Kurokawa E, Kawasaki S, Terasawa T. Local chemo-thermotherapy for lung cancer [in Japanese]. Haigan 1987;27:133–40
- Refaely Y, Simansky DA, Paley M, Gottfried M, Yellin A. Resection and perfusion thermochemotherapy: A new approach for the treatment of thymic malignancies with pleural spread. Ann Thorac Surg 2001;72:366–70
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878–85
- Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 2006;82:1234–9
- Haung J, Rizk NP, Travis WD, Seahan VE, Bains MS, Dycoco J, et al. Feasibility of multimodality therapy including extended resections in stage IVa thymoma. J Thorac Cardiovasc Surg 2007;134:1477–84
- Ishikawa Y, Matsuguma H, Nakahara R, Suzuki H, Ui A, Kondo T, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: The potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009;88:952–7
- Lucchi M, Davini F, Ricciardi R, Duranti L, Boldrini L, Palmiero G, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185–9
- Siesling S, van der Zwan JM, Izarzugaza I, Jaal J, Treasure T, Foschi R, et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer 2012;48:949–60
- Yellin A, Simansky DA, Ben-Avi R, Perelman M, Zeitlin N, Refaely Y, et al. Resection and heated pleural chemoperfusion in patients with thymic epithelial malignant disease and pleural spread: A single-institution experience. J Thorac Cardiovasc Surg 2013;145:83–9
- Monneuse O, Beaujard AC, Guibert B, Gilly FN, Mulsant P, Carry PY, et al. Long-term results of intrathoracic chemohyperthermia (ITCH) for the treatment of pleural malignancies. Br J Cancer 2003;88:1839–43
- Richards WG, Zellos L, Bueno R, Jaklitsch MT, Jänne PA, Chirieac LR, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561–7
- Rice DC, Stevens CW, Correa AM, Vaporciyan AA, Tsao A, Forster KM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685–93
- Tilleman TR, Richards WG, Zellos L, Johnson BE, Jaklitsch MT, Mueller J, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405–11
- Tokunaga T, Higashiyama M, Okami J, Maeda J, Fujiwara A, Kodama K. Intrathoracic chemo-thermotherapy with radiofrequency waves after extrapleural pneumonectomy for malignant pleural mesothelioma. Interact Cardiovasc Thorac Surg 2011;13:267–70
- Sugerbaker DJ, Gill RR, Yeap BY, Wolf AS, DaSilva MC, Baldini EH, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955–63
- Sawabata N, Matsumura A, Motohiro A, Osaka Y, Gennga K, Fukai S, et al. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: Is tumor resection beneficial for prognosis? Ann Thorac Surg 2002;73:412–15
- Muraoka M, Oka T, Akamine S, Tagawa T, Morinaga M, Inoue M, et al. Modified intrapleural cisplatin treatment for lung cancer with positive pleural lavage cytology or malignant effusion. J Surg Oncol 2006;93:323–9
- Seto T, Ushijima S, Yamamoto H, Ito K, Araki J, Inoue Y, et al. Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: A multi-institutional phase II trial. Br J Cancer 2006;95:717–21
- Ueda K, Maeda F, Ito Y. Combined treatment with low dose chemotherapy and regional hyperthermia for progressive urothelial cancer. Thermal Med 2011;27:109–12
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localized high-risk soft-tissue sarcoma: A randomized phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Higashiyama M, Kodama K, Yokouchi H, Takami K, Nakayama T, Horai T. Clinical value of pleural lavage cytological positivity in lung cancer patients without intraoperative malignant pleuritis. Recurrent pattern based on semiquantitative analysis of tumor cells in pleural lavage. Jpn J Thorac Cardiovasc Surg 2000;48:611–17
- Kim ES, Lee JJ, He G, Chow CW, Fujimoto J, Kalhor N, et al. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol 2012;30:3345–52