Abstract
Hyperthermia is considered to be a promising tool for the treatment of tumours. Intensive research activities reveal a distinct impact not only on the cellular level but also on tumour physiology which favours the combination with the classical oncologic modalities radio- and chemotherapy. Different techniques have been established so far. Among them, magnetic hyperthermia exploits the intrinsic magnetic properties of iron oxide nanoparticles (magnetite and maghemite) which induce heating during the exposure to an alternating magnetic field. Beyond the advantage that heating is generated within the tumour and not from outside the body, the amounts of magnetic material and their intratumoral distribution patterns are key factors determining the therapeutic outcome. They can be influenced by the use of different application routes, which will be discussed in this paper.
Introduction
Hyperthermia basically describes the transient rise of temperature above 37 °C at the tumour site for therapeutic purposes. This procedure is generally known to induce changes of the tumour pathophysiology, which ultimately lead to cell death. In general, two different concepts of heating have been followed so far: (1) hyperthermia with temperatures between 41 and 45 °C, and (2) thermoablation when temperatures rise higher than 45 °C for the local induction of tissue necrosis.
Particularly for hyperthermia applications, the heating sources are mostly placed from outside the body (e.g. antennas) to locally illuminate the tumour region with electromagnetic waves (microwaves or radiowaves). These techniques require the previous identification of the tumour location in the respective organ by corresponding imaging modalities (CT, MRI). In contrast, when carcinomas with distant metastases are present, whole body hyperthermia is recommended. A very good summary of the different methodologies was given some time ago by Wust et al. [Citation1].
However, a high focusing of energy to the tumour region can be obtained by the use of magnetic nanoparticles or seeds. The magnetic material is usually made up of a core of biocompatible iron oxides (magnetite or maghemite) and a polysaccharide coating. When exposing them to an alternating magnetic field, specific magnetisation processes take place, which induce heating (magnetic hyperthermia) [Citation2]. The heating potential of nanoparticles (the specific absorption rate) is an important parameter, which mainly dictates the dosages which have to be applied to the tumour region in order to achieve a reliable inactivation of target cells. The heating potential is defined by the amount of heating delivered per unit mass and time as a consequence of the exposure of the nanoparticles to an alternating magnetic field.
Depending on the morphological features of the magnetic material (nanoparticle size, shape and microstructure), different mechanisms are responsible for the delivery of heating. In relation to multi-domain magnetic nanoparticles (sizes larger than approximately 40 nm depending on the magnetic field parameters), heating is delivered by displacements of the domain wall (hysteresis losses). In contrast, suspensions of nanoparticles with diameters lower than 40 nm are called ‘super-paramagnetic nanoparticles’. These single domain particles induce heating as result of loss processes during the reorientation of the magnetisation in the magnetic field, or frictional losses where the nanoparticle is able to rotate in the surrounding medium [Citation3]. Due to their suspension features and US Food and Drug Administration (FDA) approval for MRI applications, super-paramagnetic nanoparticles have been increasingly investigated in recent years for hyperthermic purposes.
With consideration of the known pathobiological effects of hyperthermia, the modification of the normal structure of phospholipids, proteins, and nucleic acids was demonstrated in in vitro experiments for temperatures higher than 42 °C. These effects ultimately lead to the deterioration of integrity of cellular structures, such as the cytoskeleton, the mitochondria, the synthesis of macromolecules, and the impairment of enzyme activity, particularly DNA repair systems, [Citation4–6]. Also, gene expression is affected. One prominent example is the heat-shock protein (HSP) family [Citation7]. It was hypothesised that HSP might not only be involved in thermotolerance but also in antigen presentation with MHC class I molecules, which could foster immunogenicity of tumour cells [Citation8]. Since the effects in the in vivo situation were rather ambiguous, it is being discussed whether they are attributed to HSP themselves or to the molecules bound to or chaperoned by them [Citation9].
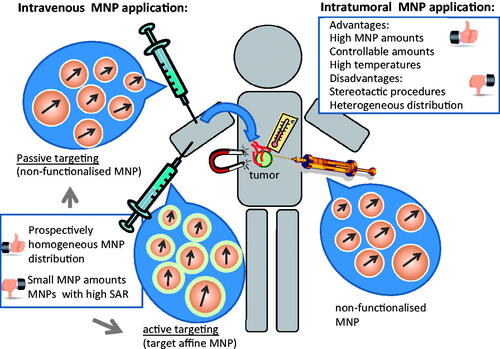
As a result of intensive research activities, hyperthermia has been proven to be an important adjunct to established oncological modalities but it has not yet achieved the status of a standard oncological therapy. Mainly, the deposition and monitoring of adequate temperatures at the tumour site is one of the unsolved problems. In this context magnetic hyperthermia has the advantage that the heat source is directly in contact with the target cells. Nevertheless, the control of the deposition of nanoparticle dosages and the handling of the intratumoral distribution patterns are key factors determining the therapeutic outcome. Therefore, in the present paper we will discuss the implications and parameters of different nanoparticle application routes for magnetic hyperthermia treatments of tumours ().
Pros and cons for magnetic hyperthermia after intratumoral application
One widely investigated technique to deposit the magnetic material into the tumour region is its intratumoral application. It has the advantage that the amounts to be deposited can be easily controlled and that comparatively high dosages can be achieved in relation to other nanoparticle application modalities (see below). For example, nanoparticle concentrations between 20 and 80 mg/cm3 [Citation10,Citation11] are able to induce thermoablative temperatures (over 50 °C). In consequence, even magnetic materials with comparatively low SAR could be suitable for magnetic hyperthermia as long as the required volumes of the nanoparticle suspensions are still several times lower than that of the tumour.
According to extensive in vitro investigations performed so far the cellular uptake of iron oxide nanoparticles is very complex. Cellular uptake not only depends on the cell type, but also on the size, shape, surface charge and chemistry of the materials. In general, the intracellular accumulation is typically of 20 to 30 pg/cell (e.g. human mesenchymal stem cells, [Citation12]). In order to increase the intracellular load particularly in non-phagocytizing cells, the use of commercial biocompatible transfection agents, functionalization of nanoparticles with cell membrane translocating peptides have been proposed. The mentioned relationships have been nicely summarized by Neoh & Kang [Citation13], Berman et al. [Citation14], and Tantra & Knight [Citation15] among others. Within the cells, nanoparticles were often shown to be accumulated in endo-lysosomes [e.g. Citation16, Citation17].
The intratumoral infiltration of the magnetic material leads commonly to irregular nanoparticle distribution patterns according to the morphology of the tumour. Hereto, cells at the injection area will be exposed to nanoparticles at comparatively high concentrations (e.g. 20 mg/cm3 tumour tissue, [Citation10]). According to the observations on cell uptake in isolated cells in vitro (see above) and due to the fact that tumour cells are rather non-phagocytizing ones, most of the injected nanoparticles will prospectively be confined to the extracellular space (tumour matrix).
In terms of the induced heating effects, protein denaturation was shown to occur at temperatures higher than 50 °C [Citation18]. If one combines the nanoparticle exposure with hyperthermia and mitomycin C treatments, a distinct modification of the MRP 1 and 3 expression levels take place, which are not associated to de novo mRNA expression, but rather to an altered translocation of MRP 1 and 3 to the cell membrane. This observation is attributed to be the result of reactive oxygen species production, e.g. shifting of intracellular MRP storage pools, changes in membrane fluidity at the protein level [Citation19]. The application of heating at temperatures above 55 °C for 5 min, leads to distinct DNA damages, which cannot be repaired by endogenous DNA repair systems [Citation10] as well as to gamma H2AX foci formation [Citation20], decrease of cellular ATP [Citation21] for example.
The intratumoral application of the magnetic material was shown to be an efficient tool for the induction of hyperthermic and thermoablative temperatures in several preclinical studies using laboratory animals. Hereto, similar effects on the cellular level were found as expected from experiments on isolated cells. Examples are the induction of pyknotic cell nuclei, an early sign of apoptosis [Citation22], and the reduction of tumour volumes with increasing time after treatment [Citation23], among others. Interestingly, no alteration of the intracellular magnetic nanoparticle accumulation was observed after therapy. This implicated the possibility of multiple treatment sessions [Citation24,Citation25]. Also by implanting stick type carboxymethyl-cellulose (CMC)-magnetite particles into T-90 gliomas in the brain of rat, an increased mean survival was found after three (44.2 ± 10.9 days) and two (17.0 ± 1.5 days) heating sessions compared to controls (14.4 ± 1.5 days, no therapy) [Citation26].
The long-term fate of intratumorally injected nanoparticles has not been extensively studied so far. Even after a magnetic hyperthermia treatment, systemic nanoparticle release from the tumour or uptake by macrophages is a rather slow process [Citation16,Citation27]. Expectedly, nanoparticles and tumour cell detritus containing nanoparticles will be transported to the liver via tissue macrophages, where nanoparticles are degraded and the released iron is incorporated into the normal iron metabolism.
Magnetic hyperthermia after intratumoral application of the magnetic material was also shown to induce host immune response in addition to local tumour cell killing. In this context, two T-9 rat gliomas were implanted in each animal, whereas only one was treated by magnetic hyperthermia using magnetite cationic liposomes as mediator. The tumours disappeared completely in many rats exposed to the magnetic field, even though only one was thermally treated. This effect was sought to be mediated by both CD8+ and CD4+ T cells and accompanied by a marked augmentation of tumour-selective cytotoxic T lymphocyte activity [Citation28].
Even though distinct therapeutic effects were observed, the aim of homogeneous intratumoral deposition of the magnetic material is still a challenging one, since the high interstitial pressures at the tumour area often lead to irregular distribution patterns even at slow infiltration rates of the magnetic material. This effect, in fact, produces inhomogeneous temperature dosages and areas which escape from exposure to lethal temperatures [Citation22]. For example, whereas hyperthermic temperatures of 47 °C over 30 min induced necrosis and some tumours elicited no evidence for regrowth at 50 days, others were shown to do so quite readily [Citation23].
The intratumoral application of the magnetic material has already been used for magnetic hyperthermia treatments in the clinical situation. Hereto, the magnetic nanoparticles were instilled by the use of specific stereotactic devices in combination with CT imaging. A total of 14 patients with glioblastoma multiforme received 4 to 10 thermotherapy treatments followed by single fractions of a radiotherapy series. The median maximum intratumoral temperatures were of 44.6 °C. In general the thermotherapy was tolerated well by all patients with minor or no side effects. In particular, no local bleeding, brain swelling or rise of inter-cranial pressure occurred [Citation29]. Interestingly, the mean time interval between primary diagnosis and first tumour recurrence after thermo-(magnetic heating) and radiotherapy was of 13.9 months [Citation30] compared to 6.2 months reported in a EORTC-NCIC trial on the treatment of primary glioblastomas with temozolomide [Citation31]. In post-mortem brain biopsies, dispersed or aggregated nanoparticles within the tumour tissue were found. Generally, the nanoparticle uptake by glioblastoma cells was very low. Instead, macrophages seem to phagocytize dispersed nanoparticles as a result of induction of tumour necrosis and subsequent infiltration of activated phagocytes [Citation32]. Also non-resectable and pre-treated prostate and cervix carcinoma, and soft tissue sarcomas have been treated with this methodology [Citation27,Citation33].
Taken together, magnetic hyperthermia with intratumorally administered nanoparticles was shown to be effective in preclinical and very promising in clinical investigations. Particular challenges are the requirement of stereotactic methods of application of the magnetic material, the implementation of non-invasive thermometry as well as homogeneous distribution of the magnetic material.
How can passive targeting favour the effectivity of magnetic hyperthermia?
Passive targeting is the local accumulation of nanoparticles as a result of the particular physiology and anatomy of the tumour after intravenous application. The accessibility is given by the presence of neovascularisation in tumours and by the faculty of the nanoparticles to extravasate to the tumour interstitium as a consequence of the specific tumour vessel architecture.
Tumour vessels differ from normal ones particularly because they are irregular in shape, leaky, defective (lack of basal membrane, endothelial cells poorly aligned) and dilated [Citation34]. An increased retention of the nanoparticles is attributed to the lack of lymphatic drainage in tumours. This effect is called the ‘enhanced permeability and retention (EPR) effect’. It was initially described by Maeda [Citation35] and has nowadays been shown to be influenced by a variety of factors such as bradykinin, prostaglandins, nitric oxide and other vascular mediators [Citation36]. To facilitate extravasation, nanoparticles should be small in size, e.g. lower than the diameter of vascular leakages of around 200 and 400 nm [Citation37]. Importantly, this requirement counteracts with the heating potential, for which larger nanoparticle core sizes are required [Citation38].
Passive targeting of magnetic nanoparticles to the tumour area could be favourable in terms of improving a homogeneous distribution throughout its vital and vascularised area (). Such a distribution pattern would explicitly include the destruction of tumour angiogenesis and prospectively complement anti-angiogenic therapies. Since the magnetic material is administered intravasally, there is no need to access the tumour by means of stereotactic methods. Nevertheless, the accumulation of nanoparticles at the tumour site can be controlled to a lesser extent compared to the intratumoral application. This makes this methodology particularly challenging and is one of the reasons why it has not been as intensively pursued compared to the intratumoral application of the magnetic material.
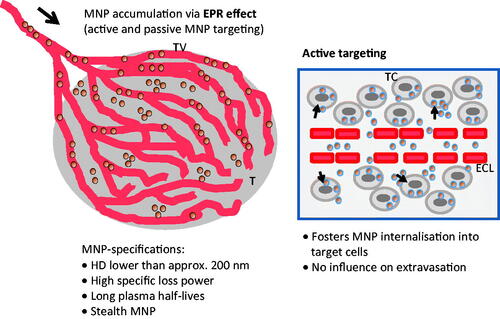
Figure 2. Intratumoral nanoparticle distribution patterns according to dependence upon different application routes. Intratumoral application: heterogeneous distribution as a result of the high interstitial pressure of tumour tissue. Active and passive targeting leads prospectively to a comparatively homogeneous distribution around the vessels of the vital tumour area. Nanoparticle retention during passive targeting is due to the lack of lymphatic drainage in tumours. Active targeting prospectively leads to increased retention compared to passive targeting on the basis of augmented internalisation into cells after specific ligand binding. There are ongoing discussions that only 1 to 10 % of the intravenously injected dose are will end up in the tumour region [Citation56] as well as on how active targeting favours nanoparticle accumulation in tumours. MNP, magnetic nanoparticles.
![Figure 2. Intratumoral nanoparticle distribution patterns according to dependence upon different application routes. Intratumoral application: heterogeneous distribution as a result of the high interstitial pressure of tumour tissue. Active and passive targeting leads prospectively to a comparatively homogeneous distribution around the vessels of the vital tumour area. Nanoparticle retention during passive targeting is due to the lack of lymphatic drainage in tumours. Active targeting prospectively leads to increased retention compared to passive targeting on the basis of augmented internalisation into cells after specific ligand binding. There are ongoing discussions that only 1 to 10 % of the intravenously injected dose are will end up in the tumour region [Citation56] as well as on how active targeting favours nanoparticle accumulation in tumours. MNP, magnetic nanoparticles.](/cms/asset/027cd06e-cb62-4de7-a28d-b45f9d724dcd/ihyt_a_832815_f0002_b.jpg)
Figure 3. The main rules governing active and passive targeting for magnetic hyperthermia. Both active and passive targeting are based on the EPR effect. Via active targeting nanoparticle internalisation into target cells in augmented. TV, tumour vessel; T, tumour; TC, tumour cell; ECL, endothelial cell layer; MNP, magnetic nanoparticle; HD, hydrodynamic diameter; MNP, magnetic nanoparticles.
Intravenously injected nanoparticles will mainly accumulate in the liver and spleen, and to a comparatively lower extent in brain, heart, kidney, and lung [Citation39]. Therefore, one important prerequisite to favour nanoparticle accumulation via passive targeting is the use of ‘stealth’ magnetic nanoparticles with increased circulation time and a reduced uptake by the monocyte phagocyte system (MPS) [Citation40]. Basically, longer plasma half-lives are obtained with small, neutral and hydrophilic nanoparticle surfaces. Therefore, the introduction of a barrier of hydrophilic oligosaccharide groups on the magnetic nanoparticle's surface to prevent opsonin adsorption (particularly immunoglobulins, albumin, and components of the complement system) and macrophage recognition have been suggested. Hereto, particularly the presence of high molecular weight PEG was seen to be very efficient and clearance rates of dextran-coated and PEG-coated nanoparticles depend on the density of the polysaccharides on the surface [Citation40]. This strategy has been efficiently exploited in terms of magnetic nanoparticle-based MRI [Citation41].
From the viewpoint that distinct amounts of magnetic material (several mg/cm3 of tumour tissue depending of the heating capabilities of the magnetic material) are necessary to achieve reliable treatment temperatures at the tumour site, one has to claim that, in principle, all injected nanoparticles should reach the tumour region. This aim has rarely been achieved so far. More likely, the accumulation of therapeutic amounts of MNP via passive targeting will be feasible in selected cases, for example on the basis of phagocyte activity as it has been reported in relation to oligomannose-coated liposomes accumulated in the omentum and other lymphoid tissues (up to 160 mg) [Citation42]. For non-phagocytic tumour cells, the use of external magnets capable of retaining the magnetic nanoparticles passing through the tumour and therefore increasing the local intratumoral concentration could be an efficient alternative [Citation43]. Nevertheless, rather large nanoparticle core diameters are needed [Citation44]. In this context, it is particularly known that the force the nanoparticle encounters in order to be retained by an external magnet will be highly dependent upon its size [Citation45,Citation46]. Also, the presence of adequate magnetic field gradients is detrimental in order to allow for a migration of the nanoparticle out of the vascular system. Other important physiological factors include the viscosity of circulating blood and of the tumour interstitium, the tumour interstitial pressure, the interaction of nanoparticles with molecular and fibrillar structures, and the shape of the nanoparticles [Citation45,Citation47]. Magnetic targeting has been already applied for the local delivery of drugs [Citation43,Citation48].
To overcome the problems associated with the passive targeting of nanoparticles, several suggestions have been made on how to increase the permeability of the tumour. Namely, it is known that mild hyperthermia (temperature increases between 40 to 43°C) leads to an increased blood flow and enhanced vascular permeability in tumours [Citation49] which favours the delivery of nano-carriers [Citation50]. The increased degree of extravasation of nanoparticles (e.g. 100 nm liposomes) seems to last up to 6 h after heating [Citation51]. Intensive extravasation occurs particularly in tumour areas where angiogenesis is most active [Citation52]. An excellent review on this topic has been given by Yudina & Moonen [Citation53]. In general, the tools focused ultrasound to deliver the heating stimulus and thermosensitive drug carrying liposomes made up of a lipid membrane which undergoes a temperature transition from a gel to a liquid phase have been efficiently combined. This means that the support of mild hyperthermia is twice: the nanoparticle accumulation at the tumour area and the release of its cargo. Newer investigations show that magnetic nanoparticles are able to release drugs in a temperature-dependent manner in a similar way. For example by utilisation of thermo-responsive polymers, hydrogels in which magnetic nanoparticles and defined drugs are encapsulated (e.g. [Citation54,Citation55]).
Taken together, passive targeting is essentially a beneficial tool to accumulate magnetic nanoparticles to the tumour site, because it can basically favour the homogeneous nanoparticle distribution pattern and therefore the therapeutic outcome of hyperthermia. Nevertheless, passive targeting tightly depends on the pathophysiology of the tumour, such as vascularisation degree, interstitial pressure, lack of lymphatic drainage, and the structural features of the magnetic material (size, surface coating). Interestingly, mild hyperthermia could also be used to precisely increase local accumulation and extravasation of magnetic nanoparticles to the tumour region. Nevertheless, no specific proofs in relation to magnetic hyperthermia have been reported so far, which is particularly due to fact that the accumulated amounts of magnetic material do not suffice for the induction of relevant temperatures.
What are the prospects for active-targeting magnetic hyperthermia?
Active targeting of magnetic nanoparticles for hyperthermia has ideally the advantage of a highly selective accumulation at cells within the tumour with a high affinity binding between the nanoparticle surface and a selective target structure at the tumour region. In analogy to passive targeting hyperthermia, the magnetic material is sought to accumulate particularly at the tumour interstitium surrounding the hyper-vascularised areas of the tumour (). Hereto, nanoparticle retention at the tumour site is not only modulated by the lack of lymphatic drainage but also by target-affinity binding and internalisation in specific cells. This effect should prospectively lead to comparatively higher nanoparticle amounts in target cells compared to passive targeting (). However up to now there are contradictory results on how active targeting can really increase the nanoparticle accumulation in tumours [Citation56], a topic which is currently being discussed in the scientific community.
This strategy implies the incorporation of functional groups and affinity ligands to the nanoparticle surface coating. Hereto, targets on endothelial cells of tumours are easily accessible. In this case nanoparticles could well be functionalised with target-affine antibodies, particularly recombinant antibodies [Citation57] in order to prevent immunogenic reactions in patients. Nevertheless, if the molecular structures to be addressed are localised in the tumour interstitium (e.g. tumour cells), the functionalised magnetic nanoparticles should not exceed in size the diameter of fenestrae of leaky vasculature in order not to hinder extravasation and diffusion into the interstitium (EPR-effect, see above). Additionally, the extent of target-affinity and avidity of ligands to molecular structures in the tumour region should be carefully designed because it influences the magnetic nanoparticle's migration in the interstitium [Citation58].
Even though initial in vivo attempts on tumour-bearing mice have shown that nanoparticles conjugated with an antibody fragment (not further specified [Citation59]) or with a chimeric L6 antibody [Citation60,Citation61] are able to induce tumour regression after exposure to an alternating magnetic field, active targeted hyperthermia has not reached the clinical situation so far. The reasons are: (1) the required structural nanoparticle specifications for magnetic hyperthermia and active nanoparticle targeting are opposed to each other. For example, large diameters favour heating capabilities but not nanoparticle targeting [Citation41]. (2) The efficacy of ‘active targeted hyperthermia’ tightly depends on the tumour physiology, such as vascularisation degree, selectivity of target expression, levels of target over-expression. (3) The specific absorption rates of all nanoparticles known so far are still too low to be able to achieve therapeutic temperatures at the amounts which can actually be accumulated via active targeting. (4) The active targeting efficacy of nanoparticles in the in vivo situation is lower compared to data from experimentation on isolated cell systems [Citation62], which is – among others – the result of opsonisation processes masking the ligand on the nanoparticle surface [Citation40]. (5) The targeting moieties on the nanoparticle surface seem to foster nanoparticle internalisation after binding to target cells but not its permeability into the tumour region [Citation63]. (6) ‘Active targeted hyperthermia’ requires the introduction of new components to the magnetic nanoparticle formulation which implicates costly and long-lasting procedures for authorisation by organisations such as the US FDA.
Conclusion
In principle, magnetic hyperthermia was demonstrated to be a promising adjunct therapeutic modality in oncology. Intensive research activities have shown that the therapeutic outcome is distinctly influenced not only by the structural features of the magnetic nanoparticle formulations but also by the amounts accumulated at the tumour site and the intratumoral distribution patterns. These parameters can basically be modulated by different application routes. The intratumoral application of the magnetic materials allows the deposition of comparatively high amounts of magnetic material but the distribution patterns are rather inhomogeneous due to the intrinsic properties of tumour tissue. Active and passive targeting prospectively offers the possibility of a homogeneous nanoparticle accumulation predominantly at the hypervascularised and highly perfused areas of the tumour. These two strategies mainly differ in the mechanisms of retention of the nanoparticles at the tumour site. However, the amounts to be deposited are fundamentally controlled by pharmacodynamics laws. The present knowledge shows that magnetic nanoparticles with high heating potential and adequate pharmacokinetic features are necessary to permit an appreciable intravasal accumulation in the tumour region.
Declaration of interest
This work was supported in parts by the European Seventh Framework Program (FP7/2007-2013), grant agreement no. 262943. The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3(8):487–97. Epub 2002/07/31
- Berkov D. Basic Physical Principles. In: Andra W, Nowak H, editors. Magnetism in Medicine, A Handbook. Berlin: Wiley-VCH Verlag GmbH & Co. KGaA; 2007. p. 26–64
- Hergt R, Andra W, d'Ambly CG, Hilger I, Kaiser WA, Richter U, et al. Physical limits of hyperthermia using magnetite fine particles. Ieee Transactions on Magnetics 1998;34(5):3745–54
- Dewey WC. Arrhenius Relationships from the Molecule and Cell to the Clinic. International Journal of Hyperthermia 1994;10(4):457–83
- Dikomey E, Franzke J. Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int J Radiat Biol 1992;61(2):221–33
- Roti Roti JL, Kampinga HH, Malyapa RS, Wright WD, vanderWaal RP, Xu M. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones 1998;3(4):245–55
- Li GC, Mivechi NF, Weitzel G. Heat-Shock Proteins, Thermotolerance, and Their Relevance to Clinical Hyperthermia. International Journal of Hyperthermia 1995;11(4):459–88
- Suto R, Srivastava PK. A Mechanism for the Specific Immunogenicity of Heat-Shock Protein-Chaperoned Peptides. Science 1995;269(5230):1585–8
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukocyte Biol 2009;85(6):905–10
- Hilger I, Rapp A, Greulich KO, Kaiser WA. Assessment of DNA damage in target tumor cells after thermoablation in mice. Radiology 2005;237(2):500–6
- Dutz S, Kettering M, Hilger I, Muller R, Zeisberger M. Magnetic multicore nanoparticles for hyperthermia--influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011;22(26):265102
- Horak D, Babic M, Jendelova P, Herynek V, Trchova M, Likavcanova K, et al. Effect of different magnetic nanoparticle coatings on the efficiency of stem cell labeling. J Magn Magn Mater 2009;321(10):1539–47
- Neoh KG, Kang ET. Surface modification of magnetic nanoparticles for stem cell labeling. The Royal Society of Chemistry 2012;8:2057–69
- Berman SMC, Walczak P, Bulte JWM. Tracking stem cells using magnetic nanoparticles. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 2011;3(4):343–55
- Tantra R, Knight A. Cellular uptake and intracellular fate of engineered nanoparticles: A review on the application of imaging techniques. Nanotoxicology 2011;5(3):381–92
- Kettering M, Richter H, Wiekhorst F, Bremer-Streck S, Trahms L, Kaiser WA, et al. Minimal-invasive magnetic heating of tumors does not alter intra-tumoral nanoparticle accumulation, allowing for repeated therapy sessions: an in vivo study in mice. Nanotechnology 2011;22(50):505102
- Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, et al. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 2009;20(11). Doi: 10.1088/0957-4484/20/11/115103
- Hilger I, Andra W, Hergt R, Hiergeist R, Schubert H, Kaiser WA. Electromagnetic heating of breast tumors in interventional radiology: In vitro and in vivo studies in human cadavers and mice. Radiology 2001;218(2):570–5
- Franke K, Kettering M, Lange K, Kaiser WA, Hilger I. The exposure of cancer cells to hyperthermia, iron oxide nanoparticles, and mitomycin C influences membrane multidrug resistance protein (MRP) expression levels. International journal of nanomedicine 2013;8:351–63
- Hori T, Kondo T, Lee H, Song CW, Park HJ. Hyperthermia enhances the effect of beta beta-lapachone to cause gamma gamma H2AX formations and cell death in human osteosarcoma cells. International Journal of Hyperthermia 2011;27(1):53–62
- Krupka TM, Dremann D, Exner AA. Time and Dose Dependence of Pluronic Bioactivity in Hyperthermia-Induced Tumor Cell Death. Exp Biol Med 2009;234(1):95–104
- Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles - An in vivo feasibility study. Investigative Radiology 2002;37(10):580–6
- Jordan A, Wust P, Fähling H. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. International Journal of Hyperthermia 1993;9:51–68
- Kettering M, Richter H, Wiekhorst F, Bremer-Streck S, Trahms L, Kaiser WA, et al. Minimal-invasive magnetic heating of tumors does not alter intra-tumoral nanoparticle accumulation, allowing for repeated therapy sessions: an in vivo study in mice. Nanotechnology 2011;22(50):505102
- Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. Journal of bioscience and bioengineering 2003;96(4):364–9
- Ohno T, Wakabayashi T, Takemura A, Yoshida J, Ito A, Shinkai M, et al. Effective solitary hyperthermia treatment of malignant glioma using stick type CMC-magnetite. In vivo study. Journal of Neuro-Oncology 2002;56(3):233–9
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. International Journal of Hyperthermia: the Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2005;21(7):637–47
- Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Japanese Journal of Cancer Research 1998;89(7):775–82
- Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. Journal of Neuro-Oncology 2007;81(1):53–60
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2010;103(2):317–24
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncology 2009;10(5):459–66
- van Landeghem FKH, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30(1):52–7
- Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, et al. Magnetic nanoparticles for interstitial thermotherapy - feasibility, tolerance and achieved temperatures. International Journal of Hyperthermia 2006;22(8):673–85
- Folkman J. Angiogenesis in Cancer, Vascular, Rheumatoid and Other Disease. Nature medicine 1995;1(1):27–31
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release 2000;65(1–2):271–84
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 2006;11(17–18):812–8
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular-Permeability in a Human Tumor Xenograft - Molecular-Size Dependence and Cutoff Size. Cancer Res 1995;55(17):3752–6
- Hergt R, Hiergeist R, Hilger I, Kaiser WA, Lapatnikov Y, Margel S, et al. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J Magn Magn Mater 2004;270(3):345–57
- Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Molecular pharmaceutics 2008;5(2):316–27
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev 2001;53(2):283–318
- Hilger I, Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine 2012;7(9):1443–59
- Ikehara Y, Niwa T, Biao L, Ikehara SK, Ohashi N, Kobayashi T, et al. A carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res 2006;66(17):8740–8
- Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, et al. Locoregional cancer treatment with magnetic drug targeting. Cancer Res 2000;60(23):6641–8
- Hergt R, Hiergeist R, Zeisberger M, Schuler D, Heyen U, Hilger I, et al. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J Magn Magn Mater 2005;293(1):80–6
- Nacev A, Beni C, Bruno O, Shapiro B. The Behaviors of Ferro-Magnetic Nano-Particles In and Around Blood Vessels under Applied Magnetic Fields. J Magn Magn Mater 2011;323(6):651–68
- Rosensweig RE. Directions in Ferrohydrodynamics. Journal of Applied Physics 1985;57(8):4259–64
- Kalambur VS, Han B, Hammer BE, Shield TW, Bischof JC. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology 2005;16(8):1221–33
- Mejias R, Perez-Yague S, Gutierrez L, Cabrera LI, Spada R, Acedo P, et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials 2011;32(11):2938–52
- Kong G, Dewhirst MW. Hyperthermia and liposomes. International Journal of Hyperthermia 1999;15(5):345–70
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. International Journal of Hyperthermia 2005;21(8):761–7
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001;61(7):3027–32
- Liu P, Zhang A, Xu Y, Xu LX. Study of non-uniform nanoparticle liposome extravasation in tumour. International Journal of Hyperthermia 2005;21(3):259–70
- Yudina A, Moonen C. Ultrasound-induced cell permeabilisation and hyperthermia: Strategies for local delivery of compounds with intracellular mode of action. International Journal of Hyperthermia 2012;28(4):311–9
- Satarkar NS, Hilt JZ. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. Journal of Controlled Release 2008;130(3):246–51
- Yin H, Yu S, Casey PS, Chow GM. Synthesis and properties of poly(D,L-lactide) drug carrier with maghemite nanoparticles. Mat Sci Eng C-Mater 2010;30(4):618–23
- Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng 2012;14:1–16
- Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother 2006;29(1):1–9
- Rudnick SI, Adams GP. Affinity and Avidity in Antibody-Based Tumor Targeting. Cancer Biotherapy and Radiopharmaceuticals 2009;24(2):155–61
- Le B, Shinkai M, Kitade T, Honda H, Yoshida J, Wakabayashi T, et al. Preparation of tumor-specific magnetoliposomes and their application for hyperthermia. J Chem Eng Jpn 2001;34(1):66–72
- DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, et al. Development of tumor targeting bioprobes (In-111-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clinical Cancer Research 2005;11(19):7087s-92s
- DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, et al. Thermal dosimetry predictive of efficacy of In-111-ChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. Journal of Nuclear Medicine 2007;48(3):437–44
- Hilger I, Leistner Y, Berndt A, Fritsche C, Haas KM, Kosmehl H, et al. Near-infrared fluorescence imaging of HER-2 protein over-expression in tumour cells. Eur Radiol 2004;14:1124 – 9
- Lee H, Fonge H, Hoang B, Reilly RM, Allen C. The Effects of Particle Size and Molecular Targeting on the Intratumoral and Subcellular Distribution of Polymeric Nanoparticles. Molecular pharmaceutics 2010;7(4):1195–208