Abstract
Purpose: The aim of this study was to evaluate the feasibility, safety, and efficiency of percutaneous microwave ablation (MWA) with artificial pleural effusion for liver tumours located in the hepatic dome.
Materials and methods: A total of 112 sessions of artificial pleural effusion performed on 102 liver tumour patients were summarised and analysed at our hospital. Among them, 31 hepatocellular carcinoma patients treated by percutaneous MWA were selected as the artificial pleural effusion group. The control group without artificial pleural effusion was matched with tumour size, tumour location and the histological grades of differentiation. The primary technique effectiveness rate, local tumour progression rate and tumour-free survival rate were compared.
Results: Artificial pleural effusion was achieved successfully in 110 of 112 sessions (98.2%), which helped to improve the visibility in 98.8% (82/83) and acquire safe puncture path in 96.3% (26/27). There were no statistical differences between the artificial pleural effusion group and the control group in the primary technique effectiveness rate (p = 1.000), the 1-, 2-, and 3-year local tumour progression rates (p = 0.669), and the 1-, 2-, and 3-year tumour-free survival rates (p = 0.979).
Conclusions: Percutaneous MWA with artificial pleural effusion could be a feasible, safe, and effective technique for liver tumours located in the hepatic dome.
Introduction
Image-guided percutaneous thermal ablation therapy, such as radiofrequency ablation (RFA) or microwave ablation (MWA), has been widely used for liver tumours, especially for small hepatocellular carcinoma (HCC) [Citation1–10]. For imaging guidance, ultrasound (US) is the most common guidance image because of easy availability and real-time monitoring capability [Citation11–15]. However, when the tumours are located in the hepatic dome, it is difficult to perform ablation because of poor visualisation or no safe puncture path due to the presence of pulmonary air which can obstruct the transmission of ultrasound [Citation16–20].
In order to overcome those drawbacks, artificial pleural effusion, or artificial ascites, which can act as an acoustic window, has been introduced during the ultrasound-guided percutaneous thermal ablation therapy in several previous reports [Citation18–28]. Although the artificial pleural effusion can improve the visibility of tumours or get a better electrode path, the effectiveness and complications are uncertain. Therefore, the problem is whether the percutaneous MWA with artificial pleural effusion treating the tumours located in the hepatic dome can achieve the same efficacy as the MWA of tumours which have good ultrasonic visibility. We designed a case-control study to evaluate the feasibility, safety, and efficacy of percutaneous MWA with artificial pleural effusion.
Materials and methods
Patients
This investigation is a retrospective case-control study in a single centre. The study was approved by our institutional review board. Written informed consent was obtained from all patients before therapy.
From October 2009 to October 2012, 112 sessions of artificial pleural effusion were performed on 102 consecutive patients with 119 liver tumours (17 patients had two tumours). The criteria for inducing artificial pleural effusion were as follows: one tumour at least could not be clearly visualised because of the obstruction of pulmonary air (in 84 sessions); no proper puncture path was identified (in 28 sessions). Liver tumour in this study contained HCC (n = 87), intrahepatic cholangiocarcinoma (n = 2), metastatic hepatic carcinoma (n = 11), hepatic haemangioma (n = 1), hepatic interstitialoma (n = 1). Because of the recurrence of liver tumour (five patients had local tumour progression, four patients had intrahepatic metastasis), eight patients were given two sessions of percutaneous MWA with artificial pleural effusion, and one patient was given three sessions. Patients were composed of 90 men and 12 women (age range 35–82 years, mean 57.8 years ± 10.2). The tumour size ranged from 0.7 to 5.0 cm (mean, 2.5 cm ± 1.0). Characteristics of the patients are summarised in .
Table I. Baseline characteristics of study population about artificial pleural effusion.
A total of 655 consecutive HCC patients were treated by percutaneous MWA in our institution from October 2009 to October 2012. Among them, 85 patients were treated by percutaneous MWA with artificial pleural effusion because the tumour could not be clearly visualised or did not have a safe puncture path by ultrasound. We selected 31 patients as the artificial pleural effusion group. The inclusion criteria were as follows: 1) patients were diagnosed HCC according to the criteria defined by the European Association for the Study of the Liver [Citation29], 2) a single tumour ≤5 cm in the maximum diameter; 3) liver cirrhosis classified as Child class A or B, 4) prothrombin time of less than 25 s, prothrombin activity higher than 40%, and platelet count higher than 40 × 109/L. Exclusion criteria included: (1) the presence of vascular invasion or extrahepatic metastases, (2) previous treatment for HCC ().
For patients in the control group, according to the same inclusion and exclusion criteria, 31 patients were selected from the remaining 570 HCC patients who did not take artificial pleural effusion (). In addition, the tumours in control group patients were clearly revealed by ultrasound. The artificial pleural effusion group and the control group were matched in tumour size, tumour location and the histological grades of differentiation. Tumour locations in the two groups were in the same liver segment. The histological grades of differentiation were defined as follows: well differentiated, corresponding to Edmondson’s grade I or I–II, moderately differentiated, corresponding to Edmondson’s grade I or II–III, or poorly differentiated, corresponding to Edmondson’s grade III or IV. The baseline characteristics of patients are reported in .
Table II. Baseline characteristics of patients in the two groups.
Artificial pleural effusion
Firstly, the position of patients was semi-erect and left lateral. We observed and identified the region of the pleural cavity on sonogram between the right anterior axillary line and the right posterior axillary line in patients. Sonography was performed by using an Acuson Sequoia 512 scanner (Signature 10.2; Siemens Medical Solutions, Mountain View, CA, USA) with a 6.0 MHz linear-array transducer. After local anaesthesia, a 16-gauge BD Angiocath™ needle (BD Medical, Sandy, UT) was inserted through the chest wall. We could monitor the process of puncture by US and felt the sudden reduction of resistance (Supplementary Video 1). Subsequently, 10 mL of 0.9% saline solution was injected to identify whether the tip of needle had entered into the pleural cavity. If the tip of the needle was in the pleural cavity, we pushed the outer soft sheath into the pleural cavity to avoid injuring lung and extracted the stylet of the needle. After the outer sheath was connected with the infusion set, the fluctuation of infusion with the same rhythm of breath could be observed. We injected a sufficient amount of 0.9% saline solution until the tumour was well revealed or the safe path for puncture was fully developed. At the same time we checked the vital signs and oxygen saturation of the blood. After the therapy, pleural effusion would be drawn through the sheath of the BD Angiocath needle. All procedures for the induction of artificial pleural effusion were performed by two interventional radiologists with more than 10 years’ experience in US-guided intervention.
Microwave ablation
The microwave unit (KY-2000; Kangyou Medical, Nanjing, China) was used for ablation with 100 W of power at 2450 MHz. The needle antenna had a diameter of 1.9 mm (15 gauge) and a length of 18 cm. In the antenna shaft there were dual channels through which distilled water was circulated by a peristaltic pump, continuously cooling the shaft to prevent overheating. After local anaesthesia with 1% lidocaine, the antenna was inserted into the tumour and placed at the desired location with US guidance. An Acuson Sequoia 512 scanner with 3.5–5.0 MHz curved array multi-frequency transducers was used. For some special cases which were poorly revealed on grey-scale US, contrast-enhanced US (Sonovue, Bracco, Milan, Italy) was used as guidance [Citation30] (Supplementary Video 2). For tumours less than 2.0 cm one antenna was inserted, for tumours measuring 2.0 cm or greater, two antennas were inserted with an inter-antenna distance of no more than 1.8 cm. After all insertions, intravenous anaesthesia was administered by a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE) and ketamine (Shuanghe Pharmaceuticals, Beijing, China) via the peripheral vein during standard haemodynamic monitoring. A power output of 50 W for 10 min was routinely used during MWA (Supplementary Videos 3,4). The aim of the treatment was to have the ablation zone covering an area 5 mm larger than the HCC. If the heat-generated hyper-echoic water vapour did not completely encompass the entire tumour and 5 mm ablative margin, prolonged microwave emission was applied until the desired temperature was reached. All MWA procedures were also performed by the same two interventional radiologists with more than 10 years’ experience in the performance of MWA.
Follow-up
Contrast-enhanced MR was performed 1 month after each MWA procedure. For the patients who could not take MR (because of heart stents or claustrophobia), we used contrast-enhanced CT to assess the therapeutic efficacy. Contrast-enhanced MR imaging was performed by using a 1.5-T unit (Signa Echo-Speed; GE Medical Systems, Milwaukee, WI) with the following sequences: spin-echo T1-weight (500/15 (repetition time ms/echo time ms), 256 × 192 matrix, and two signals acquired); fat suppressed T2-weighted respiratory-triggered fast spin-echo (3000–4000/102, 256 × 256 matrix, and three signals acquired); and fat-suppressed spin-echo T1-weighted (500/15, 256 × 192 matrix, and two signals acquired) sequence. Contrast-enhanced (iopromide, Ultravist 300; Schering, Berlin, Germany) multi-detector CT (Lightspeed 16; GE Medical) was performed using a 5-mm section thickness, a pitch of 1.35:1.0, 120 kV, and 250 mA.
To assess the feasibility of artificial pleural effusion, we summarised the clinical data of 102 patients mentioned above and calculated the technical success rate of artificial pleural effusion. The ability of artificial pleural effusion to improve the visibility of the tumours in the hepatic dome was assessed. We also recorded unsuccessful cases and analysed the reasons.
For evaluating the safety of artificial pleural effusion, we recorded the total amount of artificial pleural effusion infused and the complications related to procedures. Major complications were defined as permanent adverse sequelae or unexpected events increasing the level of care or prolonging hospital stay. Others were considered minor.
To evaluate the efficacy of percutaneous MWA with artificial pleural effusion, we compared the primary technique effectiveness rate, local tumour progression rate and tumour-free survival rate between the artificial pleural effusion group and the control group. The images before and after ablation were assessed side by side by the consensus of one radiologist with more than 10 years of experience and the operator of RFA. An ablative margin of more than 5 mm around the tumour was determined as curative [Citation31]. However, there were some cases where it was not possible to attain the 5-mm margin for the location of HCC which was under the liver capsule or due to obstructive structures such as adjacent vessels, intestines or gallbladder. In these cases where an ablative margin was formed around the tumour but was less than 5 mm in diameter in some places, elimination of vascularity of the tumour in the post-RFA imaging was determined as relatively curative. The primary technique effectiveness rate was defined as the percentage of tumours that had been evaluated as curative and relatively curative at 1-month follow-up [Citation32]. The primary end point was the recurrence of tumour tissue, which included local tumour progression, remote intrahepatic recurrence and extrahepatic recurrence. Patients who were lost to follow-up or had taken other therapies for HCC were excluded from this study. Local tumour progression was defined as the reappearance of tumour progression adjacent to the treated site, which included residual unablated tumour and new tumour.
Statistical analysis
Data analysis was performed using SPSS 17.0 for windows (Chicago, IL) and the continuous data were expressed as means ± standard deviations (SD). Continuous variables were compared by using Student t test. Chi squared test or Fisher exact test was applied to compare the primary technique effectiveness rate between the two groups. Local tumour progression and tumour-free survival rates were calculated using the Kaplan-Meier method. Differences in local tumour progression and tumour-free survival between the two groups were examined by using the log-rank test. The difference with a p value of less than 0.05 was considered statistically significant.
Results
A total of 112 sessions of MWA with artificial pleural effusion were performed on 102 consecutive patients with 119 liver tumours (17 patients had two tumours). Induction of artificial pleural effusion was achieved successfully in 110 of 112 sessions (98.2%). Two sessions of artificial pleural effusion failed. One session was stopped at inducing saline solution at the beginning of procedure because of dyspnoea. The other was a recurring case which had had induced artificial pleural effusion before. The saline solution could not be induced into the thorax and some liquid effused into the chest wall.
Among 110 successful artificial pleural effusion sessions, 98.8% (82/83) made tumours clear by grey-scale ultrasound (n = 74) and contrast-enhanced ultrasound (n = 8); 96.3% (26/27) of sessions secured a safe puncture path for percutaneous MWA. Two sessions failed to achieve the preoperative objective. One patient’s tumour was located at segment VII. The size of tumour was about 0.9 × 0.7 × 0.7 cm. After about 800 mL 0.9% saline solution injected, the tumour could not be revealed clearly by either method. The other patient’s tumour was located at segment II. After about 1500 mL 0.9% saline solution injected, there was still no safe puncture path to avoid injuring the the lung.
The volume of instilled fluid was 500–1500 mL (mean, 1061.3 ± 290.1 mL). After the procedure of percutaneous MWA, 240–1400 mL (mean 877.3 mL ± 322.5) fluid has been removed. The artificial pleural effusion disappeared in 102 (92.7%) of 110 sessions within 1 week and 5 (4.5%) of 110 sessions within 2 weeks. However, the pleural effusion emerged repeatedly in three cases, which were because of liver failure. No major complications related to the artificial pleural effusion procedure happened. There were some patients who had moderate pain. For a few patients, the pain could aggravate when they breathed deeply or changed position. The pain disappeared spontaneously without any special treatment within two weeks. Other minor complications including cough, mild dyspnoea, low-grade fever, transit elevation of liver transaminase, a few subcutaneous hydrops, also disappeared spontaneously within 1 week.
All patients in the artificial pleural effusion group and control group were given a 1-month follow-up contrast-enhanced MR or CT ( and ). The primary technique effectiveness rate was 96.8% (30/31) in the artificial pleural effusion group and 93.5% (29/31) in the control group (p = 1.000). One patient in the artificial pleural effusion group and two patients in the control group had residual viable tumour after first session. Therefore, another session of percutaneous MWA in the control group, and percutaneous MWA with artificial pleural effusion in the artificial pleural effusion group were performed.
Figure 2. Images of a 58-year-old man with a 1.8-cm HCC treated by percutaneous MWA with artificial pleural effusion. (a) Contrast-enhanced sonogram showing the hyper-enhancing neoplasm (large arrow) clearly with the artificial pleural effusion (thin arrows) as acoustic window. (b) Sonogram showing the procedure of microwave ablation. Note microwave antenna in index tumour (large arrow) and artificial pleural effusion (thin arrows). (c) Coronal preablation contrast-enhanced MR image shows the hypointense neoplasm in delayed phase (arrows). (d) Coronal contrast-enhanced MR image 1 month after MWA shows the ablation zone (arrows).
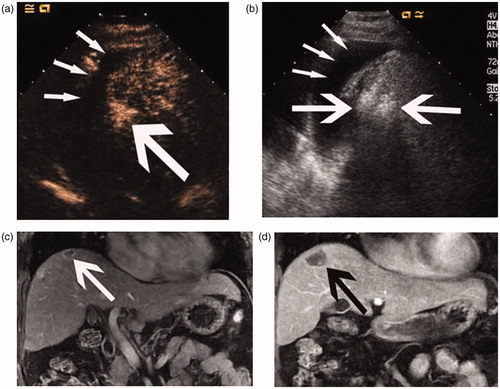
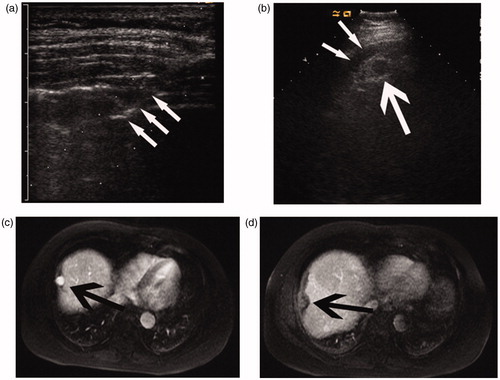
Figure 3. Images of a 48-year-old man with a 2.2-cm HCC treated by percutaneous MWA with artificial pleural effusion. (a) Sonogram shows the puncture of the pleural cavity. Note the 16-gauge BD Angiocath needle (arrow). (b) Sonogram shows the hypo-echoic nodule (large arrow) with the artificial pleural effusion (thin arrows). (c) Transverse preablation contrast-enhanced MR image shows the hyperintense neoplasm (arrows) located in the hepatic dome. (d) Transverse arterial phase MR image 1 month after MWA shows no enhancement in the ablation zone (arrow).
The median follow-up period was 21.1 ± 11.9 months (range 1.2–35.4 months) in the artificial pleural effusion group and 22.2 ± 15.8 months (range 0.5–65.5 months) in the control group. One patient in the artificial pleural effusion group who was lost to follow-up because of being out of contact after 15 months was excluded at that point. Another patient in the artificial pleural effusion group who was given a liver transplant after 3 months was excluded at that point.
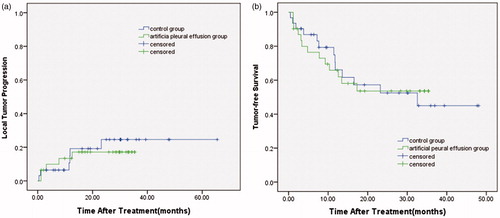
At the time of exclusion, five patients in the artificial pleural effusion group (16.1%) and six patients in the control group (19.4%) had developed local tumour progression. The median time to local tumour progression was 5.2 months in the artificial pleural effusion group and 9.9 months in the control group. The 1-, 2-, and 3-year local tumour progression rates in the artificial pleural effusion group were 13.4%, 17.1%, and 17.1%, respectively. The 1-, 2-, and 3-year local tumour progression rates in the control group were 19.2%, 24.6%, and 24.6%, respectively. There was no significant difference in the local tumour progression rates between the artificial pleural effusion group and the control group (p = 0.669) ().
Figure 4. (a) Local tumour progression and (b) Tumour-free survival for the artificial pleural effusion group and the control group.
Distant HCC recurrence occurred in eight patients in the artificial pleural effusion group and seven patients in the control group. Among these 15 recurrence patients in the artificial pleural effusion group and the control group, eleven patients had developed intrahepatic recurrence, two patients had developed pulmonary metastasis, one patient had developed renal metastasis, and one patient had developed peritoneal lymph nodes metastasis.
The 1-, 2-, and 3-year tumour-free survival rates in the artificial pleural effusion group were 65.8%, 53.6%, and 53.6%, respectively. The 1-, 2-, and 3-year tumour-free survival rates in the control group were 66.1%, 52.5%, and 45.0%, respectively. There was no significant difference in the tumour-free survival rates between the artificial pleural effusion group and the control group (p = 0.979) ().
Discussion
Ultrasound-guided percutaneous thermal ablation has become one of main treatments of early stages hepatic cancer [Citation15,Citation30,Citation33–37]. However, some lesions are inappropriate for thermal ablation because the lesions are sheltered by pulmonary tissue. Although several studies had reported the use of artificial pleural effusion or ascites with radiofrequency ablation to solve this problem [Citation18–20,Citation24,Citation26], there are no large-series studies and comparative controlled studies about the usefulness of artificial pleural effusion with percutaneous MWA treatment to liver tumours located in the hepatic dome. In this case-control study, we compared the therapeutic effectiveness between patients treated by percutaneous MWA with artificial pleural effusion and by percutaneous MWA only. We also summarised our experience about the use of percutaneous microwave ablation with artificial pleural effusion.
In our study, artificial pleural effusion had been induced successfully in 108 of 110 sessions (98.2%), which showed that it could be a feasible technique. We analysed the failed cases and inferred that the adhesion of pleurae might be the reason why artificial pleural effusion could not be induced into the thorax. Our view is similar with the point of Inyoung Song et al. [Citation28].who reported that perihepatic adhesion caused the failure of induction of artificial ascites. Therefore, when treating patients who had suspected adhesion of pleurae, the operator should be more careful.
Many studies have reported that artificial ascites could improve the visibility of tumour tissue located in the hepatic dome [Citation26–28]. However, in our initial study, we found that it was not always possible with artificial ascites to reveal tumour tissue under the diaphragm by ultrasound. Because the fluid often moved away from the subphrenic space, we could not have enough sonic windows to show the entire tumour. In contrast, artificial pleural effusion could be a good technique to improve the visibility of tumours located in the hepatic dome. The rates of improving the visibility or obtaining safe puncture path were 98.8% and 96.3% in this study. In our department, artificial ascites was used to separate intestines from liver and avoid ablation heat transmission to adjacent intestinal wall, when liver tumour was close to intestines.
Regarding the safety of artificial pleural effusion, potential complications related to the procedure are bleeding, tumour seeding, and pleuritis. No major complications or deaths related to the artificial pleural effusion procedure happened in our study. Minor complications disappeared spontaneously without any special treatment. Tae Wook Kang et al. [Citation22] reported thermal injury to the diaphragm and used CT to observe the thickness of the diaphragm. In this study, only a few patients had moderate pain, which aggravated when deeply breathing or changing position. We considered these patients might have thermal injury to the diaphragm. Because the pain disappeared spontaneously without any special treatment within two weeks, we did not monitor the thickness of the diaphragm on CT. Besides, in our previous study, we had reported the safety and effectiveness of percutaneous microwave ablation for liver cancer adjacent to the diaphragm [Citation33]. To avoid tumour seeding in the pleural cavity we never took puncture biopsy through the artificial pleural effusion. If we had to insert microwave antenna into a tumour through the artificial pleural effusion because of no other safe path, repeated punctures were forbidden and the applicator track had to be heated with sufficient microwave energy by stopping the cooling-shaft water dump. No tumour seeding in pleural cavity happened in this study.
Several previous studies have reported using 5% dextrose in water as artificial fluid [Citation21,Citation25]. Because the heating theory of radiofrequency ablation is the friction of ions, 5% dextrose in water which is isotonic and non-ionic could be an ideal buffer to infuse into the pleural cavity. However, we chose 0.9% saline solution as the artificial fluid for percutaneous MWA in this study because, firstly, our ablation technique was microwave, the heating theory of microwave ablation is the vibration of dipolar molecules rather than the friction of ions, secondly, the use of 5% dextrose in water is not suitable for diabetes patients, which restricts the use of 5% dextrose in water. In addition, 0.9% saline solution is cheap, safe, and easy to acquire. The results of this study showed 0.9% saline solution as an artificial fluid achieved good effectiveness without any complications. Therefore, we conclude that 0.9% saline solution is the appropriate fluid for artificial pleural effusion with MWA.
Considering the efficacy of percutaneous MWA with artificial pleural effusion, we designed the case-control study. In a previous study [Citation1,Citation3], tumour differentiation and tumour size could be the independent prognostic factors affecting recurrence of HCC after microwave ablation treatment. Therefore, tumour differentiation and tumour size were selected to match standard to make sure that the two groups were balanced as far as possible. In addition, we matched tumour location between the two groups to compare complications after treatment. The results of our study showed that the 1-, 2-, and 3-year local tumour progression rates and the 1-, 2-, and 3-year tumour-free survival rates in the two groups were not significantly different. We believe that MWA with artificial pleural effusion treating tumours located in the hepatic dome can achieve the same efficacy as with tumours which have good ultrasonic visibility.
There were several limitations in this study. First, there is selection bias in the control group. Because of the characteristics of the tumours located in the hepatic dome, a randomised controlled trial could not be performed. Therefore, we chose a case-control study. The selection bias cannot be avoided. Second, the size of the case-control study was a relatively small sample. A large sample study would be more convincing. Third, this was only a single-centre study.
In conclusion, percutaneous microwave ablation with artificial pleural effusion could be a feasible, safe, and effective technique for treating liver tumours located in the hepatic dome.
Declaration of interest
This paper was supported by the National Scientific Foundation Committee of China (grants 81127006 and 81171358) and the National Science and Technology Ministry of China (2013BAI01B01). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary Videos are available online at www.informahealthcare.com/hth
Supplementary Video 1 shows the procedure of the puncture of the pleural cavity.
Supplementary Video 2 shows the hyper-enhancing neoplasm clearly with the artificial pleural effusion on the contrast-enhanced sonogram.
Supplementary Video 3 shows the procedure of microwave ablation. You can see two heating microwave antennas.
Supplementary Video 4 shows the hypo-echo lesion with artificial pleural effusion on the grey-scale sonogram.
Supplementary Material
Download Microsoft Video (AVI) (12.8 MB)Supplementary Material
Download Microsoft Video (AVI) (883.9 KB)Supplementary Material
Download Microsoft Video (AVI) (14.2 MB)Supplementary Material
Download Microsoft Video (AVI) (14.4 MB)References
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299–307
- N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475–83
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003;180:1547–55
- Lencioni R, Cioni D, Crocetti L, Franchini C, Della Pina C, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961–7
- Yoon HM, Kim JH, Shin YM, Won HJ, Kim PN. Percutaneous radiofrequency ablation using internally cooled wet electrodes for treatment of colorectal liver metastases. Clin Radiol 2012;67:122–7
- Nishikawa H, Osaki Y, Iguchi E, Takeda H, Ohara Y, Sakamoto A, et al. Percutaneous radiofrequency ablation therapy for recurrent hepatocellular carcinoma. Anticancer Res 2012;32:5059–65
- Zacharoulis D, Asopa V, Navarra G, Nicholls JP, Jensen SL, Habib NA. Hepatectomy using intraoperative ultrasound-guided radiofrequency ablation. Int Surg 2003;88:80–82
- Yokoyama T, Egami K, Miyamoto M, Watanabe H, Hasegawa H, Iida S, et al. Percutaneous and laparoscopic approaches of radiofrequency ablation treatment for liver cancer. J Hepatobiliary Pancreat Surg 2003;10:425–7
- Crucitti A, Danza FM, Antinori A, Vincenzo A, Pirulli PG, Bock E, et al. Radiofrequency thermal ablation (RFA) of liver tumors: Percutaneous and open surgical approaches. J Exp Clin Cancer Res 2003;22:S191–5
- Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi A, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: Percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg 2001;5:477–89
- Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol 2012;85:1078–1084
- Carrafiello G, Fontana F, Cotta E, Petullà M, Brunese L, Mangini M, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med 2011;116:1059–66
- Hofer S, Oberholzer C, Beck S, Looser C, Ludwig C. Ultrasound-guided radiofrequency ablation (RFA) for inoperable gastrointestinal liver metastases. Ultraschall Med 2008;29:388–92
- Chiou YY, Hwang JI, Chou YH, Wang HK, Chiang JH, Chang CY. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci 2005;21:304–9
- Xu HX, Xie XY, Lu MD, Liu LN. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53–61
- Kim PN, Choi D, Rhim H, Rha SE, Hong HP, Lee J, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (≤ 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: A multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol 2012;23:627–34
- Nesher N, Ben Haim M, Pevni D, Kessler A, Paz Y. Ultrasound-guided, video-assisted transdiaphragmatic radiofrequency ablation for primary liver malignancy or metastatic nodules. Innovations (Phila) 2011;6:337–40
- Liu LN, Xu HX, Lu MD, Xie XY. Percutaneous ultrasound-guided thermal ablation for liver tumor with artificial pleural effusion or ascites. Chin J Cancer 2010;29:830–35
- Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol 2007;42:306–11
- Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol 2003;38:1066–70
- Hinshaw JL, Laeseke PF, Winter TC III, Kliewer MA, Fine JP, Lee FT Jr. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. Am J Roentgenol 2006;186:S306–310
- Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: Comparison of effects of thermal protection and therapeutic efficacy. Am J Roentgenol 2011;196:907–13
- Kapoor BS, Hunter DW. Injection of subphrenic saline during radiofrequency ablation to minimize diaphragmatic injury. Cardiovasc Intervent Radiol 2003;26:302–4
- Koda M, Ueki M, Maeda Y, Ken-ichi Mimura K-I, Okamoto K, Matsunaga Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. Am J Roentgenol 2004;183:583–8
- Laeseke PF, Sampson LA, Brace CL, Winter TC III, Fine JP, Lee FTJr . Unintended thermal injuries from radiofrequency ablation: Protection with 5% dextrose in water. Am J Roentgenol 2006;186:S249–54
- Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: The value of artificial ascites. Abdom Imaging 2009;34:371–80
- Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: Initial experience. Am J Roentgenol 2008;190:91–8
- Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: Safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630–40
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona–2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421–30
- Liu F, Yu X, Liang P, Cheng Z, Han Z, Dong B. Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia 2011;27:555–62
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol 2011;46:1418–26
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD III, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005;235:728–39
- Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28:218–26
- Sindram D, Swan RZ, Lau KN, McKillop IH, Iannitti DA, Martinie JB. Real-time three-dimensional guided ultrasound targeting system for microwave ablation of liver tumours: A human pilot study. HPB (Oxford) 2011;13:185–91
- Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol 2011;17:4952–9
- Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: Percutaneous and open approaches. J Surg Oncol 2009;100:619–34
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72:S124–31