Abstract
Objectives: The aim of this study was to evaluate the clinical application value of a 3D visualisation preoperative treatment planning system in microwave ablation for liver cancer.
Methods: From December 2011 to November 2012, 94 enrolment patients of liver cancer were divided into two groups. The 3D preoperative planning group included 36 patients with 44 lesions, who underwent microwave ablation with the aid of the self-developed 3D visualisation preoperative treatment planning system. The 2D preoperative planning group included 58 patients with 64 lesions, who underwent microwave ablation according to conventional 2D image preoperative planning methods. After microwave ablation, therapeutic efficacy was assessed by contrast-enhanced imaging during follow-up.
Results: The 3D preoperative planning group had a higher success rate of first ablation than the 2D preoperative planning group (p = 0.01). There were more sessions in the 2D preoperative planning group than in the 3D preoperative planning group (p = 0.002). There were no significant differences in technique effectiveness rate between the 2D preoperative planning group (96.55%) and the 3D preoperative planning group (100%) according to the contrast-enhanced imaging follow-up after microwave ablation (p = 0.64). There were no significant differences in the rate of LTP between the 2D preoperative planning group and the 3D preoperative planning group (p = 0.64) during 3–12 months follow up (median 6 months).
Conclusions: Compared with the 2D preoperative planning group, the 3D preoperative planning group had a higher success rate of first ablation and fewer sessions. Therefore, the 3D visualisation preoperative treatment planning system has a relatively high clinical application value.
Introduction
With the development of thermal ablation techniques, thermal ablation has been a curative method in the treatment of liver cancer [Citation1]. Preoperative treatment planning is defined in a virtual reality simulation based on patient-specific data from, for example, computed tomography (CT), magnetic resonance imaging (MRI), and medical imaging techniques involving surface segmentation. Preoperative treatment planning as the first step in the thermal ablation process acts to lower the rate of complications, ensure tumour-free safety margins after ablation, and improve long-term survival outcomes. Preoperative treatment planning is important to predict the time–temperature profile during ablation, helps guide thermal ablation therapy, and may improve the accuracy.
Preoperative treatment planning is usually performed using two-dimensional (2D) imaging data. The radiologist must then reconstruct a three-dimensional (3D) image in their own perception, but this is dependent on their spatial awareness and experience, which are highly subjective. The radiologist’s judgement is not consistent with the actual situation sometimes, and this may lead to treatment failure or major complications. Therefore, how to achieve scientific, objective, convenient, individualised treatment planning is one of the key issues for thermal ablation [Citation2].
In order to overcome the shortcomings of 2D imaging techniques for preoperative planning, 3D surgical planning has been developed over recent years. 3D image reconstruction can show the tumour volume data for surgical planning, and make up for the lack of 3D images, such as 3D ultrasound, 3D CT and 3D MRI [Citation3,Citation4]. So far, there have been a number of methods and software systems for 3D surgical planning for liver surgery [Citation5,Citation6]. In particular, the application of 3D visualisation technology allows physicians to perform various operations on the image, such as rotation, scaling and moving, to see more intuitively the internal complexity of the structure of the human tissue. The doctor may conduct repeated preoperative planning in the individual 3D model of surgical planning, to optimise the surgical programme, improve surgical skills, improve the safety of surgery, and reduce surgical complications [Citation7–10].
Due to the differences in ways of operating between image-guided percutaneous thermal ablation and surgical resection, the requirements of the 3D planning methods are quite different, so the 3D surgical planning method is not available for image-guided percutaneous thermal ablation. The surgical resection for liver tumour is larger, because the location of the tumour in liver segment needs to be considered; however, image-guided thermal ablation therapy requires quantitative calculation of the tumour volume and the distance between the tumour and surrounding vital structures, accurate simulation of 3D thermal field, and the planning of puncture path. A 3D visualisation preoperative planning method has been applied to thermal ablation [Citation11–13], which could intuitively display ablation planning through the segmentation of tumour and vascular and 3D volume reconstruction, and avoid thermal damage of surrounding structures. However, the preoperative planning system reported for the puncture path planning has only been based on CT images and/or 3D visual images, which is not enough for doctors who usually perform ablation under real-time ultrasound guidance. On the basis of the characteristics and clinical application requirements of image-guided percutaneous thermal ablation for liver tumour, we combined 3D image processing and analysis methods with navigation technology to establish a 3D visualisation preoperative treatment planning system in microwave ablation for liver tumours. Was the 3D preoperative planning better than 2D preoperative planning in microwave ablation for liver tumours? We compared 3D visualisation preoperative treatment planning with the conventional 2D image planning method in microwave ablation for liver tumours in the study. The purpose of this study is to evaluate the clinical application value of fhe 3D visualisation preoperative treatment planning system in microwave ablation for liver tumours.
Materials and methods
Design of a 3D visualisation preoperative treatment planning system
A computer-assisted 3D visualisation preoperative treatment planning system was developed that integrated electromagnetic tracking technology with guidance and planning software. The system enabled the operator to load the preprocedural images, perform offline tumour segmentation, and create a treatment plan. The integrated system consists of the components described in the following sections.
Electromagnetic navigation system
A 3D visualisation preoperative planning system was developed based on a commercially available electromagnetic tracking system (Aurora; Northern Digital, Ontario, Canada). The system also included a simulation model (Model071, CIRS, Elkhart, IN), an ultrasound probe and appropriate computer hardware. There were two sensors, one attached to the ultrasound probe, and the other attached to the simulation model ().
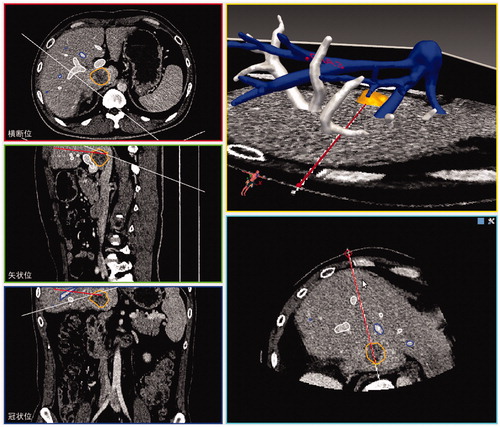
Figure 1. Physical set up and components of 3D visualisation preoperative treatment planning system. (1) A control unit of the electromagnetic tracking system, (2) two sensor interface devices of the electromagnetic tracking system, (3) a field generator of the electromagnetic tracking system, (4) tracked ultrasound probe, (5) a simulation model, (6) screen.

Image guidance software and tumour segmentation
The image guidance software package was developed by our research team. The software provides the basic components needed for rapid prototyping and the development of image-guided microwave ablation applications. The graphical user interface () displayed the real-time simulation ultrasound 2D guided planning and the 3D visualisation planning, as well as the planning path from the transverse, coronal and sagittal plane. The tumour was segmented in a semiautomatic manner using the open source program FITME (http://www.fitme.org). The same user interface was also employed to allow the interventional radiologist to contour the tumour. Additionally, the system could visually display the tumour size and the distance between the tumour and the surrounding pipeline structure and organisation.
Image registration
The Aurora electromagnetic tracking system was positioned and enabled, and image registration of the CT space and electromagnetic space was performed with the use of inner fiducial point registration. Briefly, four inner fiducial markers were selected in the simulation ultrasound images and then the corresponding points in CT space were selected by the radiologist with the mouse on the workstation. The image registration based on least-squares method was performed.
Interactive puncture path planning
Ultrasound guidance had advantages of convenience, flexibility, and being real-time. We first performed the puncture path planning under simulation ultrasound guidance, then adjusted the puncture path planning according to a 3D visualisation model which accorded with the operating habit of the surgeon, using the advantages of 3D ultrasound guidance, avoiding the shortcomings of the simple 2D ultrasound guidance. Our approach to covering the entire tumour with a series of ablation spheres was based on the following guiding objectives: (1) covering the entire tumour plus a predefined ablative margin with a composite of spherical thermal fields, (2) minimising the number of ablations, (3) minimising the number of electrode insertion trajectories, (4) avoiding any critical organ transgression or ablation. The planning software was designed to iterate through these goals until a complete treatment plan was finalised.
Clinical studies
The clinical data
From December 2011 to November 2012, 94 enrolment patients of liver cancer were divided into two groups. The 3D preoperative planning group included 36 patients with 44 lesions, who underwent microwave ablation with the aid of the self-developed 3D visualisation preoperative treatment planning system. The 2D preoperative planning group included 58 patients with 64 lesions, who underwent microwave ablation according to the conventional 2D image preoperative planning method. There was no significant difference in clinical backgrounds between the two groups (). Enrolment conditions for the patients were as follows: liver cancer patients who met the indications for microwave ablation, and the close relationship between the tumour and the surrounding organs on 2D images. This investigation was approved by our Institutional Ethics Committee. Written informed consent was obtained from all patients.
Table I. Clinical characteristics of patients who underwent microwave ablation assisted by 3D preoperative planning and conventional 2D image preoperative planning methods.
Establishment of preoperative planning
Contrast-enhanced CT scanning was performed before microwave ablation for each patient (slice thickness less than 0.5 mm). The contrast-enhanced CT with DICOM format data of 3D preoperative planning group patients was transformed into a 3D visualisation preoperative planning system for processing and analysis, and a 3D visualisation model and the quantitative distance between the tumour and surrounding vital structures were acquired. According to our previous study [Citation14–16], the thermal ablation zone was more suitable for clinical application when the power was 50 W than that at other power levels, so we set the power at only 50 W during preoperative planning. The spherical thermal field was estimated based on our previous experimental studies [2,15] and using canonical shape (ellipsoids) to represent the ablation zone (for example, when the emission time was 10 min, the short ellipse diameter (S) was 3 cm, and the long ellipse diameter (L) was 3.5 cm). We inputted the data of the short ellipse diameter and long ellipse diameter, the volume of the ablation zone was calculated based on the equation of V = πLS2/6 in the 3D preoperative planning system. For the 3D preoperative planning group of patients, the preoperative treatment planning, including the puncture path, the number of insertions and the energy, was finalised using a 3D visualisation preoperative planning system. For the 2D preoperative planning group of patients, the preoperative treatment planning was finalised by the radiologist just based on the 2D images.
Microwave ablation with preoperative planning
All treatments were performed in our institution and were carried out under US guidance with the patients under unconscious intravenous anaesthesia (propofol, 6–12 mg/kg/h, ketamine, 1–2 mg/kg) in the operating room. The microwave unit (KY-2000, Kangyou Medical, Nanjing, China) consists of three independent microwave generators, three flexible coaxial cables and three water pumps which can drive three cool-tip needle antennas. The generator is capable of producing 1∼100 W of power at 2,450 MHz. The antenna was 15-gauge in diameter with a cooled shaft. All therapy was performed by two experienced radiologists according to the preoperative planning. During the therapy we monitored the hyper-echoic area of ablation using grey-scale sonography and thermal monitoring to decide the end point of treatment. Within 3 days after ablation, every patient received contrast-enhanced sonography to evaluate the ablation area. If residual tumour or unablated therapeutic margin was detected, additional MWA treatment was performed.
Thermal monitoring procedure
A thermal monitoring system attached to the microwave unit was used during treatment for the study group. With US guidance, one or two 21-gauge thermal monitoring needles (Kangyou Medical) were placed into marginal tissue of the tumour or liver proximal to the surrounding tissues or organs for real-time temperature monitoring during the ablation to protect the surrounding tissues or organs from thermally mediated injury. Based on our experimental evidence and clinical experience, the temperature cut-off for ablation therapy was set at 60 °C in the patients. If the measured temperature reached 60 °C, emission of microwave antenna was stopped immediately and was only reactivated again after the temperature had decreased to 50 °C.
Therapeutic efficacy assessment and follow-up
Therapeutic efficacy assessment and follow-up was assessed by contrast enhanced imaging and serum tumour marker levels after the treatment. The success rate of the first ablation was defined as the ‘complete ablation’ of the macroscopic tumour proved by the contrast-enhanced ultrasound within 3 days after the first ablation. The technique effectiveness was defined as the ‘complete ablation’ of the macroscopic tumour proved by imaging follow-up after ablation. Local tumour progression (LTP) was defined as incompletely treated viable tumour continuing to grow, or a new tumour (or in the case of hepatocellular carcinoma, ‘daughter’ or ‘satellite’ tumours) growing at the original site during follow-up. The number of antenna insertions was defined as the total number of antenna placements for each tumour until this tumour was ablated completely. The follow-up period was calculated starting from the beginning of MW ablation in all patients. Contrast-enhanced US and contrast-enhanced CT or MRI were repeated at 1-month and 3-month intervals within 1 year and then at 6-month intervals after MW ablation. If abnormal peripheral nodular enhancement of the ablation area was found during follow-up and it was presumed to be LTP, further MW ablation was performed. The follow-up time was 3–12 months (median, 6 months) in our study.
Statistical analysis
Data analysis was performed using SPSS 11.0 for Windows (Chicago, IL) and the continuous data were expressed as mean ± standard deviation (SD). Independent samples t-test was used to compare the means between two groups, and Fisher’s exact test was undertaken to compare the success rate of the first ablation, the technique effectiveness rate, the LTP rate, and other ratios between two groups. The level of statistical significance was set at P-value less than 0.05.
Results
For a total of 44 tumours in 36 cases of liver cancer in the 3D preoperative planning group, the preoperative planning was achieved successfully. The distances between the tumours and surrounding structures were acquired by quantitative analysis of 3D visualisation preoperative planning system. The distances between the tumours and the grade three portal branches were less than 5 mm in 18 cases; the distances between the tumours and the hepatic vein and inferior vena cava were less than 5 mm in 13 cases, the distances between the tumours and the gallbladder wall were less than 5 mm in 4 cases; the distances between the tumours and the gastrointestinal tract were less than 5 mm in 3 cases.
In the 3D preoperative planning group 43 of 44 tumours (97.72%) and in 2D preoperative planning group 51 of 64 tumours (79.69%) achieved complete ablation confirmed by contrast-enhanced ultrasound within 3 days after the first ablation. The 3D preoperative planning group had a higher success rate of first ablation than the 2D preoperative planning group (p = 0.01). One case in the 3D preoperative planning group and 13 cases in the 2D preoperative planning group underwent the second microwave ablation. There were more sessions in the 2D preoperative planning group than in the 3D preoperative planning group (p = 0.002). All tumours in the 3D preoperative planning group (100%) and 62 of 64 tumours in 2D preoperative planning group (96.88%) were completely ablated according to the contrast-enhanced image follow-up after microwave ablation (). There were no significant differences in the technique effectiveness rate between the 2D preoperative planning group and the 3D preoperative planning group (p = 0.64).
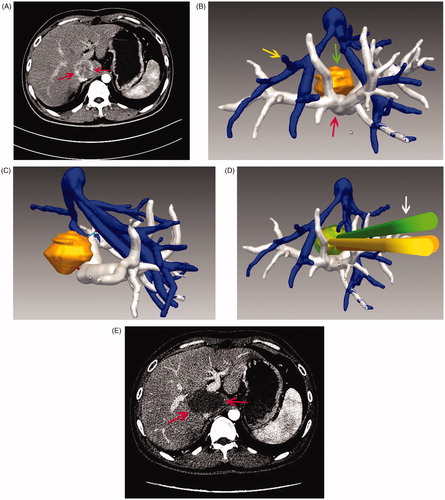
Figure 3. Images in a 45-year-old patient with a tumour in the caudate lobe. (A) Preoperative CT imaging showed that the tumour was close to the portal vein. (B) The 3D visualisation images visualised the spatial relationship of tumour and the surrounding pipe in multi-angle. (C) The 3D visualisation images visualised the shortest distance between the tumour and surrounding vessels. (D) The preoperative planning was achieved through 3D visualisation of preoperative planning system, and three needles were needed to ablate the tumour completely. (E) The contrast-enhanced CT showed complete tumour necrosis a month after microwave ablation.
LTP was found in two of 64 tumours (3.12%) in the 2D preoperative planning group on follow-up contrast-enhanced imaging. One case was found at 3 months after MW ablation, the other was found at 6 months after MW ablation. All cases of LTP were confirmed by biopsy which was performed simultaneously with re-treatment. There were no significant differences in the LTP rate between the 2D preoperative planning group and the 3D preoperative planning group (p = 0.64) ().
Table II. Comparison of ablation results in the 3D preoperative planning group and 2D preoperative planning group.
After MW ablation, mild pain and fever were noted. One case in the 3D preoperative planning group had intermittent fever and chills for 1 week, and the patient’s symptoms gradually disappeared after receiving anti-inflammatory treatment for a week. In one case in the 2D preoperative planning group slim thrombus of the portal vein occurred in the second day after microwave ablation, which did not enlarge during follow-up. No serious complications occurred in other patients during follow-up.
Discussion
The main content of the preoperative treatment planning is the selection of a reasonable puncture path and establishment of an effective thermal field in order to ablate the tumour completely and avoid damage to surrounding tissues and organs. Numerical modelling based on temperature calculations was used to provide treatment planning guidance in order to address the ablation of larger tumours [Citation17–18]. In recent years, 3D preoperative planning has been applied to thermal ablation [Citation11–13]. Sato et al. [Citation19] and Morikawa et al. [Citation20] reported a preoperative planning method which is feasible for MR-guided microwave ablation of liver tumours, while complete 3D visualisation information was not included. Keserci et al. [Citation21] reported a dedicated temperature imaging feedback control system to guide and assist in a thermal liver ablation procedure; although target visualisation, treatment planning and monitoring, and temperature and thermal dose visualisation with the graphical user interface of the thermal ablation software were demonstrated, the system can only be applied in MR imaging-guided liver thermal ablation therapy.
A novel 3D visualisation preoperative surgery planning method was proposed for percutaneous hepatic microwave ablation by Zhai et al. [Citation22]; however, the preoperative planning system reported for the puncture path planning was only based on CT images or/and 3D visual imaging, which was not enough for doctors who usually performed ablation under real-time ultrasound guidance. We have developed a 3D visualisation preoperative planning system which can visualise the spatial relationship of the tumour and surrounding structures in a 3D manner, calculate the distance from the tumour to the surrounding vital structures or organs, and provide the minimum number of insertions and the best needle path and other parameters of the treatment required under a simulated environment. The preoperative planning system we used for the puncture path planning was based on a method of combination of simulation ultrasound and 3D visual image, for doctors who usually perform ablation under real-time ultrasound guidance, and is conducive to the implementation of the plan. Preoperative planning can be repeated in the 3D visualisation system to optimise treatment planning, which may improve the success rate of ablation therapy and reduce the incidence of complications.
In our study, although there were no significant differences in the technique effectiveness rate and LTP rate between the 2D preoperative planning group and 3D preoperative planning group (p = 0.64), the 3D preoperative planning group has a higher success rate of first ablation than the 2D preoperative planning group (p = 0.01). Accounting for the high risk location of the tumours, doctors may have worried about thermal damage to the surrounding tissues and organs, an underestimated planning may have been performed in the 2D preoperative planning group, and this maybe one reason for the lower first ablation success rate in the 2D preoperative planning group. The application of the 3D visualisation preoperative planning system reduced the number of sessions compared with the conventional 2D imaging preoperative planning method. Fewer ablation sessions means less risk, lower hospitalisation costs and shorter hospital stays, so the application of 3D visualisation preoperative planning system is of benefit to patients with liver tumours. Meanwhile, based on the results, the 3D preoperative planning improved the accuracy of the positioning and may have strengthened doctors’ confidence in ablation therapy.
There was no difference in the incidence rate of complications between the two groups in our study. One case in the 3D preoperative planning group had intermittent fever and chills for 1 week, due to lesions near the bile duct, and the patient had undergone biliary-enteric anastomosis. Patients were prone to infection and fever after biliary-enteric anastomosis [Citation14]. In the 2D preoperative planning group, one case of slim thrombus of the portal vein occurred in the second day after microwave ablation due to the tumour being adjacent to the portal vein, with local thermal injuries to portal vein; the thrombus did not enlarge during follow-up. The reason for achieving good results in our study, aside from the experience of the doctors, was the application of temperature monitoring technology. We monitored the temperature at the point of tumour margin adjacent to the surrounding tissues or organs, which could both ensure the success of ablation therapy and prevent the incidence of complications caused by thermal damage [Citation15,Citation16].
There are some limitations in our study. First, the present study was a preliminary study; a further study, such as a randomised controlled study and longer follow-up period may be needed. Second, we just selected the point of tumour margin adjacent to the surrounding tissues or organs, in order to ensure the success of ablation therapy and prevent the incidence of complications caused by thermal damage, while temperature fields are 3D and non-linear, it is difficult to characterise a tumour temperature or tumour margin temperature with one or two temperature probes. Third, the treatment planning system does not account for cooling due to large vessel heat sinks. Fourth, this was only a single centre study; a multi-centre study would be more convincing.
Conclusions
Compared with the 2D preoperative planning group, the 3D preoperative planning group had a higher success rate of first ablation and fewer sessions. Therefore, the 3D visualisation preoperative treatment planning system has a relatively high clinical application value.
Declaration of interest
This study was supported by the National Scientific Foundation Committee of China (grant numbers 81201167 & 30825010) and the Beijing Nova Program (XX2013108). The authors alone are responsible for the content and writing of the paper.
References
- Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 2006;25:3848–56
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72:S124–31
- Sindram D, Swan RZ, Lau KN, McKillop IH, Iannitti DA, Martinie JB. Real-time three-dimensional guided ultrasound targeting system for microwave ablation of liver tumours: A human pilot study. HPB (Oxford) 2011;13:185–91
- Xu J, Jia ZZ, Song ZJ, Yang XD, Chen K, Liang P. Three-dimensional ultrasound image-guided robotic system for accurate microwave coagulation of malignant liver tumours. Int J Med Robot 2010;6:256–68
- Conversano F, Franchini R, Demitri C, Massoptier L, Montagna F, Maffezzoli A, et al. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol 2011;18:461–70
- Radtke A, Sotiropoulos GC, Molmenti EP, Schroeder T, Peitgen HO, Frilling A, et al. Computer-assisted surgery planning for complex liver resections: When is it helpful? A single-center experience over an 8-year period. Ann Surg 2010;252:876–83
- Lang H, Radtke A, Hindennach M, Schroeder T, Frühauf NR, Malagó M, et al. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg 2005;140:629–38 , discussion: 638
- Delingette H, Ayache N. Hepatic surgery simulation. Commun ACM, 2005;48:31–6
- Hansen C, Wieferich J, Ritter F, Rieder C, Peitgen HO. Illustrative visualization of 3D planning models for augmented reality in liver surgery. Int J Comput Assist Radiol Surg 2010;5:133–41
- Schwier M, Dicken V, Peitgen H. 3D visualization of vascular structures around liver tumors using fuzzy clustering. Int J Comput Assist Radiol Surg 2008;3:403–4
- Rieder C, Schwier M, Weihusen A, Zidowitz S, Peitgen HO. Visualization of risk structures for interactive planning of image guided radiofrequency ablation of liver tumors. Proc SPIE 2009;7261:1–9
- Knowles BR, Caulfield D, Cooklin M, Rinaldi CA, Gill J, Bostock J, et al. 3-D visualization of acute RF ablation lesions using MRI for the simultaneous determination of the patterns of necrosis and edema. IEEE Trans Biomed Eng 2010;57:1467–75
- Schumann C, Bieberstein J, Braunewell S, Niethammer M, Peitgen HO. Visualization support for the planning of hepatic needle placement. Int J Comput Assist Radiol Surg 2012;7:191–7
- Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol 2011;80:548–52
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009;13:318–24
- Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28:218–26
- Ryan TP, Turner PF, Hamilton B. Interstitial microwave transition from hyperthermia to ablation: Historical perspectives and current trends in thermal therapy. Int J Hyperthermia 2010;26:415–33
- Fuentes D, Cardan R, Stafford RJ, Yung J, Dodd GD III, Feng Y. High-fidelity computer models for prospective treatment planning of radiofrequency ablation with in vitro experimental correlation. J Vasc Interv Radiol 2010;21:1725–32
- Sato K, Morikawa S, Inubushi T, Kurumi Y, Naka S, Haque HA, et al. Alternate biplanar MR navigation for microwave ablation of liver tumors. Magn Reson Med Sci 2005;4:89–94
- Morikawa S, Inubushi T, Kurumi Y, Naka S, Sato K, Demura K, et al. Feasibility of respiratory triggering for MR-guided microwave ablation of liver tumors under general anesthesia. Cardiovasc Intervent Radiol 2004;27:370–3
- Keserci BM, Kokuryo D, Suzuki K, Kumamoto E, Okada A, Khankan AA, et al. Near-real-time feedback control system for liver thermal ablations based on self-referenced temperature imaging. Eur J Radiol 2006;59:175–82
- Zhai W, Xu J, Zhao Y, Song Y, Sheng L, Jia P. Preoperative surgery planning for percutaneous hepatic microwave ablation. Med Image Comput Comput Assist Interv 2008;11:569–77