Abstract
A summary of recent developments in the synthesis, stabilisation and coating of magnetic iron oxide nanoparticles for hyperthermia applications is presented. Methods for synthesis in aqueous, organic and microemulsion systems are reviewed together with the resulting heating rates of the nanoparticles. Different stabilisation mechanisms for iron oxide nanoparticles from aqueous and organic media are discussed as intermediates for further coating and functionalisation. Coating with silica and/or polysaccharides is mainly used for design of nanoparticles especially for targeted hyperthermia application. These coatings permit versatile functionalisation as a basis for conjugating biomolecules, e.g. antibodies or peptides. Various strategies to conjugate biomolecules on the particle surface are discussed, with emphasis on methods that preserve biofunctionality after immobilisation. The efficiency of established methods such as carbodiimide coupling and oriented conjugation strategies is compared with new developments such as the bioorthogonal approaches that are based on the cycloaddition of strain-promoted alkynes with azides or nitrones. For targeted hyperthermia applications the study of the formation of a protein corona around nanoparticles with site-specific biomolecules on the surface is essential to achieve improved circulation times in the blood and reduced non-specific uptake by non-targeted organs for a high specific accumulation in the target tissue.
Introduction
Therapeutic hyperthermia applications with alternating magnetic fields require magnetic nanoparticles (MNPs) having an effective heating rate to enable therapeutic applications. Furthermore, the particles have to be designed for sufficient particle accumulation and adequate distribution in the target area to allow the application of mild alternating magnetic fields (AMF) to prevent any side-effects of high magnetic field strengths on the patient. Recently, Laurent et al. [Citation1] provided a comprehensive review of magnetic fluid hyperthermia with superparamagnetic iron oxide (SPIO) nanoparticles. They provided the detailed physical background of magnetic heating and the resulting requirements for MNPs for thermotherapy. Therapeutic hyperthermia applications have become more diverse, necessitating new requirements for the design of suitable MNPs. While the established clinically applied hyperthermia treatment is based on the direct injection of MNPs into the target tumour [Citation2,Citation3] ongoing approaches develop active MNPs for the targeted accumulation of particles in tumour areas including metastases [Citation4,Citation5]. Another strategy uses dendritic cells as natural carriers of MNPs to mimic biological units and to elude the immune response of the body (‘Trojan horse’ strategy). The feasibility of this strategy was demonstrated by negligible cytotoxic effects of the MNPs and by the selective AMF-induced killing of MNP-loaded cells [Citation6,Citation7]. Furthermore, the application of localised hyperthermia of malignant lesions, using nanoparticles (NPs) and AMF, has the important potential to either directly kill the cancer cells or enhance their susceptibility to radiation or chemotherapy [Citation8–12]. Very recently hyperthermia with MNPs was suggested for drug-resistant bacteria that are known to be responsible for many therapeutic failures in current oncological treatments [Citation13,Citation14] also in combination with chemotherapy [Citation15]. Intracellular hyperthermia was shown to be a suitable technology to induce death of protozoan parasites bearing MNPs. These findings expand the possibilities for new therapeutic strategies combating parasitic infection [Citation16].
The design of optimal MNPs for these multifaceted hyperthermia applications remains a challenge for future research that determines the timeline for the introduction of the new strategies for hyperthermia applications into the clinic. Therefore, this review will consider the state-of-the-art of the synthesis and coating of MNPs with high heating rates and suitable surface modifications to improve colloidal stability, prevent aggregation of NPs, ensure non-toxic status in physiological conditions and introduce functional groups for binding of application-specific target molecules. We will focus on iron oxide based MNPs that are currently the only clinically approved magnetic materials, that can be used in humans. The biocompatibility and stability of magnetite or maghemite NPs is known from comprehensive clinical experience as magnetic resonance imaging (MRI) contrast agents [Citation17,Citation18] and as nanocarriers in clinical hyperthermia applications [Citation2,Citation3,Citation19].
Synthesis of iron oxide NPs
Synthesis of iron oxide NPs for various biological applications, including particle stabilisation with appropriate coatings and physico-chemical particle characterisation were summarised in several excellent reviews by Tartaj et al. [Citation20], Laurent et al. [Citation1,Citation21,Citation22], Pankhurst et al. [Citation23] and others [Citation24–31]. Thus we will focus on very recent developments in particle synthesis and functionalisation. For hyperthermia applications particles with iron oxide core sizes around the monodomain-multidomain transition, i.e. particles with diameters below 50 nm, are proven to exhibit the highest heating rates [Citation9,Citation32–35]. The common measure of heating rate, specific absorption rate (SAR), also called specific loss power (SLP), is the power loss associated by unit mass of the magnetic material involved:
where Cm is the specific heat capacity of the sample, msample and miron oxide are the masses of the ferrofluid and iron oxide, respectively, and ΔT is the temperature rise during the time interval Δt [Citation36].
The heating potential depends strongly on the iron oxide core size and also on the size distribution, the particle shape, the iron oxide morphology, the medium, and on the distribution of magnetic parameters, such as anisotropy. Hergt et al. [Citation37] have studied the dependence of the heating rates on the morphology and mean particle size over a broad size range from superparamagnetic to multidomain particles and bacterial magnetosomes. MNPs with a mean size of 18 nm and a small size distribution were identified as optimal candidates for hyperthermia applications. Many different strategies are described that adjust the synthesis parameters of iron oxide NPs for efficient hyperthermia applications according to these findings.
About 30 years ago Molday [Citation38] described the precipitation of iron oxide in the presence of biopolymers, such as dextran. This method leads to SPIO nanoparticles with a poorly defined morphology and large size distribution of the iron oxide domains in the dextran matrix. Good biocompatibility, colloidal stability, and well-developed surface functionalisation methods, however, initiated a large variety of improvements and variations of the original method to design MNPs that are suitable as contrast agents for magnetic resonance imaging [Citation18,Citation39,Citation40] and magnetic particle imaging [Citation41,Citation42]. The well-established surface chemistry of these superparamagnetic particles [Citation43] allowed their use in molecular imaging [Citation44], in bioassays [Citation45], and also as model particles for hyperthermia applications [Citation46,Citation47] with acceptable heating rates [Citation14,Citation46]. The dextran matrix particles have single-crystal morphologies with individual iron oxide crystals distributed throughout the dextran matrix. This leads to significant limitations in their heating rates especially in comparison to particles with multi-crystalline iron oxide cores. To enable the controlled formation of iron oxide crystals and multi-crystalline iron oxide cores, more sophisticated ‘core-shell’ methods were developed with the iron oxide core formation in the first step, followed by stabilisation and final coating. The main strategies for the controlled formation of iron oxide cores for hyperthermia applications can be classified into aqueous phase synthesis, organic phase synthesis and microemulsion processes. A summary of the main available size and morphology data of the iron oxide NPs that are described in the following sections, together with their SAR values and corresponding AMF parameters is given in and .
Table 1. Summary of morphology data and SAR values of MNPs from aqueous phase synthesis together with corresponding AMF parameters.
Table 2. Summary of morphology data and SAR values of MNPs from organic phase synthesis together with corresponding AMF parameters.
Aqueous phase synthesis of iron oxide cores
The wet chemical routes to MNPs are simple, tractable and efficient regarding the yield, with appreciable control over size, composition and sometimes even the shape of the NPs. Iron oxides (either Fe3O4 or γ-Fe2O3) can be synthesised through the co-precipitation of Fe2+ and Fe3+ aqueous salt solutions by addition of a base. The control of size, shape and composition of nanoparticles depends on the type of salts used (e.g. chlorides, sulphates), the Fe2+:Fe3+ ratio, pH, and ionic strength of the media. Reimers and Khalafalla [Citation48] and Massart [Citation49] were pioneers in preparing iron oxide NPs from ferric and ferrous chlorides by addition of alkaline media such as NaOH or ammonia. Massart et al. used a Fe2+:Fe3+ ratio of 1:2 and obtained magnetite NPs having diameters between 6 and 17 nm with a poor morphology and large size distributions. Fortin et al. [Citation50] modified and complemented the Massart method by a size-fractionation of citrate stabilised colloidal maghemite by an increase in ionic strength, that allowed a controlled size–dependent phase separation from water or glycerol–water mixtures. The highest heating rate was obtained for 17-nm γ-Fe2O3 particles, that was in good agreement with the findings of Hergt et al. [Citation37]. Other modifications of the Massart synthesis include an improved pH control and peptisation with HNO3 [Citation51,Citation52], a modified stoichiometry of Fe2+:Fe3+ to 1:1.3 combined with the use of 1 M NaHCO3 for precipitation of iron oxide particles until reaching pH = 8 [Citation53]. Cheraghipour et al. performed the iron oxide precipitation under sonication followed by citrate stabilisation [Citation54]. A more efficient energy input during the iron oxide precipitation was achieved by high pressure homogenisation. This method controls the formation of the individual crystals with mean diameters of 15–20 nm, and then creates aggregates to form a multi-crystalline particle core [Citation55]. After coating with dextran or starch, the magnetite NPs possess a hydrodynamic diameter of about 100 nm [Citation46,Citation56]. Verges et al. [Citation36] developed the FeSO4 precipitation in the presence of NaOH and a mild oxidant (KNO3) with subsequent ageing. Magnetite particles with diameters >30 nm and different shapes (spherical, cuboid or octahedral) were obtained depending upon the FeSO4 concentration. In a similar approach of oxidation precipitation, Li et al. [Citation57] studied the mechanistic effects of the initial molar ratios of Fe2+: and Fe2+:OH− in a FeCl2–NaNO3–NaOH aqueous system on the size, morphology and heating rates of the resulting MNPs. 36-nm γ-Fe2O3 were obtained with a Fe2+:
:OH− molar ratio of 3:1:5 and identified as MNPs with the highest heating rates. Furthermore the sequence of adding oxidant (NaNO3) to NaOH and Fe(II) solution had a significant influence on the NP properties ().
Organic phase synthesis of iron oxide cores
Monodisperse iron oxide NPs with very narrow size distributions can essentially be synthesised through the thermal decomposition of organometallic compounds in high boiling organic solvents containing stabilising surfactants. The organometallic precursors are typically iron carbonyls or iron acetylacetonates. Oleic acid, fatty acids, and hexadecylamine are often used as surfactants. In principle, the ratio of the starting reagents including organometallic compounds, surfactant, and solvent are the decisive parameters for the control of the size and morphology of MNPs.
The thermal decomposition of the zerovalent iron in the carbonyl precursor initially leads to formation of the metal, but a two-step procedure can be used to produce the maghemite nanoparticles by subsequent addition of trimethylamine oxide as mild oxidant at elevated temperatures. Hyeon et al. [Citation58] and Park et al. [Citation59] have applied this strategy to synthesise monodisperse γ-Fe2O3 with a size of approximately 13 nm by thermal decomposition of iron pentacarbonyl in a mixture of dioctyl ether and oleic acid. This pioneering thermal decomposition strategy was used by many groups for the tailored design of the particle size for special diagnostic or therapeutic applications, and especially for hyperthermia. The multi-injection seeded-growth approach of Sun and Zeng [60] was used by Levy et al. [61] to prepare monodisperse magnetite NPs by thermal decomposition of iron pentacarbonyl with adjustable sizes from 6 nm to 18 nm for the study of their heating performance. This seeded-growth approach led to the formation of a magnetically frustrated layer in all particles. Thus the heating power of these NPs was much lower than the expected value due to a discrepancy between the real crystal size and the effective magnetic volume. Magnetic disorder was particularly evident for 13–18-nm MNPs, resulting in a drastic loss of their hyperthermia performance. Thus the particular synthesis protocol plays a crucial role for the preparation of MNPs with efficient heating rates [Citation61].
Decomposition of precursors with cationic iron centres leads directly to the oxides. Thus Fe3O4 NPs are obtained if (Fe(acac)3) is decomposed in the presence of 1,2-hexadecanediol, oleylamine, and oleic acid in phenol ether [Citation62]. Hyeon et al. [Citation63] synthesised ferrimagnetic iron oxide nanocubes (FIONs) in the size range from 20 nm to 160 nm with iron(III) acetylacetonate in oleic acid and benzyl ether. Very recently such 30-nm nanocubes were encapsulated in a shell of L-3,4-dihydroxyphenylalanine (DOPA)-conjugated chitosan oligosaccharide [Citation64]. The well dispersed chitosan-FIONs with a hydrodynamic diameter of 158 nm contain multiple 30-nm FIONs that increase the total magnetic moments, which leads to localised accumulation under an applied magnetic field and an extemely high heating rate of 2614 W/g [Citation64]. In a similar approach, magnetite nanocubes having a diameter of 19 nm were obtained, that showed SAR values up to 2452 W/g iron [Citation65].
The large-scale synthesis of such monodisperse nanocrystals from thermal decomposition processes was enabled by the inexpensive formation of the iron oleate precursor from iron chloride and sodium oleate [Citation66]. Further modifications of this approach resulted in larger magnetite NPs having diameters of 12, 13, 14 and 16 nm, which showed the highest SAR value for 16-nm particles. But this value decreased by about 30% in measurements made in simulated biological environments. This decrease was interpreted to arise from blocking of Brownian relaxation contribution. In contrast the 13-nm and 14-nm particles did not show any significant change of SAR even after agglomeration in the same medium, suggesting the interpretation that the dominant loss mechanism is Néel relaxation [Citation67]. Solvents that consist of inert hydrocarbons enabled the thermal decomposition process [Citation66] at higher temperatures. Thus, iron oxide nanocrystals of various sizes and modifications such as polymorphous nanocrystals, spherical nanocrystals and nanowires in the superparamagnetic to ferromagnetic size range of about 20 nm were obtained [Citation68].
Another efficient tool for the synthesis of iron oxide NPs with adjustable sizes is the polyol route. This method is based on the alkaline hydrolysis of iron(II) and iron(III) salts in a stoichiometric mixture of polyols (e.g. diethylene glycol (DEG) and N-methyldiethanolamine (NMDA)). The variation of temperature, nature of precursors, the choice of the solvents and the duration of the reaction influence the size and structure of the resulting MNPs [Citation69]. Hugounenq et al. [Citation69] applied a modified ‘polyol’ protocol and obtained flower-shaped maghemite structures consisting of smaller grains of approximately 11 nm. The heating rate of these nanoflowers is one order of magnitude higher than the SAR reported for conventional 11-nm single-domain maghemite nanoparticles in the same condition of field exposure. Lartigue et al. synthesised multi-core flower-like 30-nm γ-Fe2O3 particles according to the same method, but used a non-stoichiometric DEG/NMDA mixture to obtain multi-core nanoflowers coexisting with single-core particles in a single preparation. This allows the modulation of magnetic interactions in citrate-stabilised suspensions ranging from 10-nm single core to 30-nm multi-core NPs. The close contact between cores ensures a continuity of the crystalline orientation and favours magnetic ordering across the interfaces. Subsequent electrostatic colloidal sorting was used to fractionate the suspensions by size and hence magnetic properties. The resulting 25-nm multi-core NPs consist of 8–10-nm iron oxide cores and show extremely high SAR values of about 2,000 W/g displaying the collective magnetic behaviour of multi-core particles in comparison to analogous singe-core NPs [Citation70] ().
Microemulsion processes
A microemulsion is a thermodynamically stable isotropic dispersion of two immiscible liquids, where the microdomain of either or both liquids is stabilised by an interfacial film of surfactant molecules [Citation71]. In water-in-oil microemulsions, the aqueous phase is dispersed as microdroplets of typically 1–50 nm in diameter, surrounded by a monolayer of surfactant molecules in the continuous oil phase. The size of the reverse micelles is determined by the molar ratio of water to surfactant. Such micelles or emulsion systems were used to precipitate iron oxide inside the aqueous droplets to obtain NPs with a narrow size range. Okoli et al. [Citation72] synthesised magnetic iron oxide nanoparticles from water-in-oil and oil-in-water microemulsions for protein binding and separation. The adsorbed protein still retained its functionality even after binding to the nanoparticles, thus implying the extension of this technique for various biomedical applications.
Microemulsion systems have been successfully used to form iron/iron oxide nanocomposite particles with large specific absorption rates for hyperthermia applications. An iron core with its high saturation magnetisation gives a greater heating effect than iron oxide [Citation73]. Zhang et al. [Citation74] synthesised iron/iron oxide nanoparticles via combination of two water-in-oil microemulsion solutions. Each microemulsion solution contained a surfactant: cetyl trimethyl ammonium bromide (CTAB), a co-surfactant: n-butanol, an oil phase: n-octane, and a water phase of NaBH4 and FeCl3. The formation of the iron oxide shell was achieved by the passivation with trimethylamine N-oxide isopropyl alcohol solution. The CTAB coating on the particles was replaced by a thin hydrophobic hexamethyldisilazane layer to assemble phosphatidylcholine on the nanoparticle surface. The resulting nanocomposite particles have a biocompatible surface and show good stability in both air and aqueous solution. This strategy was enhanced by application of 3-aminopropyltrimethoxysilane for the final coating of the iron oxide shell to introduce functional groups for further conjugation chemistry [Citation75], and by the combination of silane and dextran to provide a biocompatible surface coating [Citation76]. Compared to iron oxide nanoparticles, the nanocomposites show much better heating in an alternating magnetic field [Citation74]. Recently, Basel et al. [Citation77] reported on a similar synthesis of iron/iron oxide nanoparticles in a reverse micelle system (CTAB/n-octane/tert-butyl bromide). The synthesis of iron oxide NPs was performed by adding ammonia to the reverse micelles followed by treatment with sodium borohydride to reduce the outer iron ions. Finally, the particles were coated with 3-(3,4-dihydroxyphenylethylcarbamoyl)propanoic acid tetra-ethylene glycol ester to protect the surface and to decrease the NP toxicity. With these iron/iron oxide NPs it was shown that tumour-homing cells specifically delivering MNPs for AMF therapy can significantly prolong the lives of mice bearing deep and disseminated pancreatic tumours.
Coating and functionalisation of iron oxide NPs
A good colloidal stability and an optimal coating of the MNPs should prevent interactions with the biological surrounding and allow the introduction of functional groups and spacers for the conjugation to specific bioactive molecules, including antibodies, lectins, peptides, hormones, vitamins, nucleotides or drugs.
The charge and the hydrophily/hydrophoby of the particle surfaces have a significant influence on the in vivo particle distribution in each tissue and microscopic compartment. The particle size and surface are essential for the particle association with the biomolecules that are components of biological fluids and lead to a ‘corona’ around the particles by interface organisation of proteins or lipids. Changes in the particle surface are able to entirely influence the nature of the biologically active proteins in the ‘corona’ and thereby also change the cellular uptake and toxicity [Citation78]. Schweiger et al. [Citation79] have compared the quantitative co-localisation of 20-nm iron oxide NPs with a positive and a negative surface charge by real-time quantitative correlation analysis. They found that negatively charged MNPs were detected first in endosomes and later in lysosomes, whereas positively charged particles were exclusively found inside lysosomes. In general, cationic NPs enter cells with a higher efficiency due to the interaction with the negatively charged glycocalix, but can cause a higher toxicity. Nevertheless, negatively charged particles can also be massively incorporated by cells.
Another important parameter in the design of MNPs is the density of the coating layer around the iron oxide cores. Although iron ions are present in the body and essential for life, the potential toxic effects of MNPs result from free iron ions on the particle surface or released iron ions, because of the ability to accept or donate electrons by reduction of Fe3+ or oxidation of Fe2+. These redox reactions may cause an imbalance in body homeostasis and lead to aberrant cellular responses, such as DNA damage, oxidative stress, and inflammatory processes [Citation80]. Furthermore these redox reactions may influence many analytical assays that are based on redox reactions, e.g. the colorimetric bicinchoninic acid (BCA) method for the determination of immobilised proteins or antibodies on the particle surface, that is based on the reduction of copper(II) to copper(I) ions [Citation81]. For a rapid screening of the shielding of the iron oxide cores by the particle cores different assays are available [Citation82] that provide helpful information for the development of a dense coating around the iron oxide cores. Especially for targeted particle applications, the prevention of redox-sensitive interactions with the biological surrounding is essential for a low toxicity and a sufficient circulation time of the NPs in the blood. On the other hand the initiation of reactive oxygen species (ROS) may be advantageous at a direct injection of MNPs into the cancer to make the cancer cells highly vulnerable to subsequent apoptotic magnetic hyperthermia at low temperatures. Therefore, Yoo et al. [Citation83] have designed double-effector MNPs with surface-conjugated gadolinium(III) texapyrin as a producer of ROS to effectively sensitise breast cancer cells before hyperthermia treatment.
The colloidal stability of the iron oxide NPs is essential for an optimal particle coating and functionalisation. Typical monomeric stabilisers, for example carboxylates, phosphates and sulphates, or polymeric stabilisers with terminal carboxylic acid groups such as polyacrylic acid, were used to achieve stable iron oxide colloids. Thereby the stabilisation mechanisms are different for iron oxide NPs from aqueous and organic phase syntheses. The initial colloidal stabilisation is very often the basis for a further coating, for example with silica or polysaccharides. A silica coating of the NPs results in a dense layer around the iron oxide and prevents redox reactions with the surrounding medium. Polysaccharide coatings provide a biocompatible particle surface. The advantages of both coating strategies were successfully combined in the particle surface design for hyperthermia applications.
Stabilisation of iron oxide from wet chemical synthesis
The magnetite resulting from the Massart method is often peptised with tetramethylammonium hydroxide (TMA), which provides a good basis for a final silica coating of the iron oxide [Citation49,Citation84,Citation85]. Rouhana & Schlenoff [Citation86] stabilised TMA peptised Massart Fe3O4 with a zwitterionic siloxane to produce hydrated and electroneutral MNPs for enhanced circulation time in the blood by eliminating the immune recognition and the receptor mediated phagocytosis in the mononuclear phagocyte system. Another stabilisation method for aqueous iron oxide suspensions is the addition of citric acid [Citation50]. Carboxylate groups of citric acid form complexes with iron ions on the particle surface and provide free carboxylic acid groups on the magnetite surface for the charge stabilisation of the colloid [Citation87]. COOH groups of glycine stabilised magnetite NPs and provided amino groups on the particle surface [Citation88]. Block-copolymers having special terminal charges are also a convenient tool to stabilise iron oxide particles to obtain macrophage-evading nanoparticles, with a plasma half-life as long as possible in order to reach the desired target [Citation89]. Thus, positively charged Massart magnetite was stabilised with negatively charged polyacrylic acid (PAA) containing block copolymers (PAA-b-poly(ethylene oxide) and PAA-b-poly(acrylate methoxy poly(ethylene oxide)) to develop highly stable aqueous MNP colloids endowed with protein repellence and thus with stealth [Citation90]. In another approach the magnetite was stabilised with cross-linked PEG-poly(aspartate) block copolymers to provide multifunctional MNPs with a significantly improved biocompatibility, stability and the potential for drug incorporation to combine hyperthermia with MRI and chemotherapy [Citation91].
Stabilisation and phase transfer of iron oxide from organic solvents
The phase transfer of oleic-acid-coated iron oxide NPs from the organic phase into a colloidally stable water phase is essential for the further application of the monodisperse particles that were obtained according to the ‘Hyeon’ strategy [58]. Gonzales and Krishnan et al. [Citation92] transferred the oleic-acid-coated magnetite particles from the organic phase into a colloidal stable water phase by formation of magnetoliposomes, by coating with Pluronic F127 [Citation93,Citation94] or amphiphilic polymers [Citation67]. They studied the influence of particle size and width of size distribution on the heating rates. The SAR decreased with increasing polydispersity of the particle size distribution [Citation94]. Other amphiphilic polymers such as poly(maleic anhydride-alt-1-octadecene)-poly(ethylene glycol) (PMAO-PEG) [Citation67] or poly(maleic anhydride-alt-1-tetradecene) and poly(maleic anhydride-alt-1-octadecene) [Citation61] were tailor-made to improve the long-term colloidal stability of the MNPs. Iron oxide NPs originally coated with oleic acid and oleylamine were formulated in the core of D-α-tocopheryl-co-PEG 1000 succinate micelles using a simple solvent exchange method. These micelles showed increased stability and heating rates, reduced toxic effect and enhanced uptake by cancer cells in comparison to corresponding Pluronic F127 micelles [Citation95].
Coating of iron oxide NPs with polysaccharides
Dextran, starch and chitosan are polysaccharides that are widely used for the coating of iron oxide cores, because of their biocompatibility and affinity to iron oxide surfaces. Especially dextran or carboxydextran are established components of clinically applied MRI contrast agents such as Resovist® and Feridex®.
While dextran and starch-coated NPs have a neutral to slightly negative surface charge in the physiological pH range, a chitosan coating results in more positively charged particles and an improved cell uptake. To achieve a strong binding between the iron oxide surface of FIONs and chitosan, Bae et al. have conjugated DOPA to chitosan and obtained positively charged FIONs under acidic tumour microenvironments, which is advantageous for the prolonged residence of chitosan-FIONs in the tumour tissue. This high tumour affinity is beneficial for repeated tumour irradiation without further MNP injection and leads to successful eradication of cancer cells through caspase-mediated apoptosis [Citation64].
Polysaccharide coatings are a versatile platform to introduce many types of functional groups [Citation43,Citation96]. This is particularly evident for cross-linking with epichlorohydrin followed by introduction of amino groups with ammonia [Citation97] or other bifunctional amines [Citation98], which increases the stability of the polysaccharide coating and provides the link for the attachment of antibodies, peptides or other biomolecules [Citation43].
Silica coating of iron oxide NPs in combination with an outer polysaccharide coating
Silica coating is an efficient method of achieving a dense layer around the iron oxide cores [Citation93] and to introduce functional groups by reaction with corresponding alkoxysilanes. Jordan et al. [Citation2,Citation19] have applied aminosilane-coated iron oxide NPs in clinical trials for hyperthermia treatment of glioblastoma multiforme and prostate cancer. They found an improved tissue distribution of the MNPs after introduction of a neutral or slightly negative outer shell, e.g. a dextran coating around the innermost aminosilane shell [Citation99]. Creixell et al. [Citation85] applied this strategy and conjugated their aminosilane-coated MNPs with carboxymethyl dextran as the basis for further attachment of epidermal growth factor (EGF) to demonstrate the efficient targeting of the EGF receptor. The targeted intracellular hyperthermia led to a dramatic decrease of cell viability of EGF receptor overexpressing cells even without a perceptible temperature rise of the cell medium, which allows the possibility of treating even small tumours and metastatic cancers. Lartigue et al. [Citation100] have prepared magnetite NPs by thermal decomposition in the size range of 4–35 nm and coated it with sugars as recognition vectors for certain lectins. Therefore the sugars were functionalised with bis(trimethylsilyl) phosphonate groups for attachment at the iron oxide surface. The optimum SAR values were obtained for a core size of 16 nm governed by Néel relaxation and of 35 nm for thermally blocked NPs ().
Influence of coating, medium and particle concentration on the SAR of the MNPs
The particle coating as well as the surrounding medium may interact with the surface atoms of the magnetic core and form a magnetically disordered layer, reducing the total amount of the magnetic phase and thus the heating rates of MNPs [Citation101]. Gonzalez-Fernandez et al. [Citation102] have studied the influence of the silica coating of iron oxide NPs on the heating rates of the particles. The heating rate of 30-nm iron oxide particles was reduced by 1% due to a 1-nm silica layer. The heating rates of corresponding 45-nm particles decreased by about 29% due to a 4-nm silica layer. Larumbe et al. [Citation103] also measured a significant decrease of heating rates (26%) from plain magnetite particles to the corresponding silica-coated NPs. Such deviations, enhanced by the silica coating, are associated with the occurrence of surface spin disordered effects. Dennis et al. [Citation104] have compared magnetite NPs with the same core but two different thicknesses of dextran layers, and found that a difference in saturation magnetisation of a factor of 1.5 yields a difference in SAR of a factor of 2.5. The influence of the coating layer on the SAR values underlines the requirement of the exact calculation of the heating rates. Furthermore, the colloidal parameters have a significant influence on the heating rates of MNPs. A remarkable decrease of SAR values was found with increasing particle concentrations [Citation105] and solvent viscosity. This effect was studied on the basis of the magnetic relaxation mechanisms involved [Citation106].
Summary of heating rates of iron oxide NPs
The SAR data of the described MNP types are summarised for the aqueous phase syntheses in and for the organic phase syntheses in . Both tables illustrate the diversity of magnetic field amplitudes, H, and corresponding frequencies, f, that were used for SAR measurements. Very often the original research papers do not delineate a clear relationship of the SAR calculation with the mass of iron, the mass of iron oxide or the mass of the coated particle as well as the medium and particle concentration for SAR measurement. The heating rates of the different MNP types were measured at different AMF parameters with mainly individually designed AFM generators. This demonstrates the strong need for standardised methods and tools to characterise MNPs for hyperthermia applications to enable comparison of the SAR data. The external magnetic field of the used radiation and its frequency have a significant influence on the heat generation. The values of SAR are only comparable if the incident radiation is the same or similar. Gonzales-Weimuller et al. [Citation94] have prepared high-quality magnetite NPs with diameters of 5 nm, 9 nm, 10 nm and 11 nm. By performing calorimetry measurements with these monodisperse particles at a constant frequency of 400 kHz and a constant magnetic field amplitude of 24.5 kA/m, it was demonstrated that the heating rates of superparamagnetic particles are dependent on particle size. A maximum heating rate of 447 W/g was obtained for the 11-nm magnetite NPs ().
Pankhurst et al. [Citation46] have introduced the ‘intrinsic loss power’ (ILP) to make the SAR data of different groups comparable. The ILP is defined as the SAR normalised to H2 and f, assuming a corresponding dependence of SAR on these parameters: ILP = SAR/H2f. This applied proportional dependency of SAR on frequency and squared field strength is limited to the linear response regime and does not fully describe the complex interdependence of particle sizes used, spatial particle distribution, the medium, frequencies, and field strengths as shown by several experimental investigations [Citation56,Citation70,Citation107,Citation108] and was therefore not included in our summary.
Conjugation of iron oxide nanoparticles with target-specific biomolecules
A variety of monoclonal antibodies is available for human therapy [Citation109] providing available ligands for targeted hyperthermia applications with MNPs. Conjugation with monoclonal antibodies, antibody fragments, peptides or other target-specific biomolecules enables the targeted delivery of the particles directly to the cells of interest. After systemic administration the particles may accumulate in sufficiently high concentrations in the local environment of the cancer to provide useful heating at clinically tolerable levels of AMF [Citation47,Citation110,Citation111]. A significant issue with systemic delivery of targeted MNPs for non-invasive hyperthermia is the particle uptake in the liver (Kupffer cells), spleen and bone marrow, thus reducing their specificity and rendering therapy less selective. Recent data presented by Kut et al. highlight the potential of damage to liver and spleen if these high concentrations of particles are inadvertently activated by AMF [Citation112]. Particle uptake by macrophages is strongly dependent on the particle size, their surface charge, and agglomeration tendency, and is presumably mediated by the protein corona that forms from interactions between the nanoparticle surface and blood plasma proteins. Several approaches to minimise the non-specific particle uptake in macrophages were studied [Citation113]. Pretreatment with lovastatin before the MNP injection was found to decrease the non-specific uptake of targeted MNPs in liver and spleen [Citation114]. Another approach that has been proposed is the injection of decoy particles to eliminate plasma opsonins. Simberg et al. [Citation115] found that such a pretreatment with Ni-liposomes led to a five-fold prolongation in particle half-life, but caused a higher toxicity. An interesting variation of this approach is to take advantage of the phagocytic nature of tumour-associated macrophages in order to enhance the local concentration of nanoparticles in the tumour environment [Citation116]. Many of these strategies for tumour-specific nanoparticle delivery are under intense investigation. The interplay between nanoparticles and various components of the innate immune system provides significant potential for delivery and therapy, assuming a sufficient understanding of the associated physiological properties emerges.
Carbodiimide conjugation versus oriented conjugation strategies
A large variety of strategies are known for the covalent binding of the target antibodies or other target molecules on the particle surface [Citation117]. The majority of these strategies requires amino or carboxylic acid groups on the particle surface that can be further modified with hetero-bifunctional cross-linkers for an oriented binding of the antibodies. The antibodies provide amino groups that can be used directly for the conjugation reaction or be further functionalised to obtain, for example, terminal thiol, maleimide or azide groups ( and ).
Figure 1. Overview on typical strategies for antibody conjugation on the surface of MNPs. The precursor particles were obtained by following reactions. (A) Carboxylated MNPs were activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). (B) Aminated MNPs were functionalised with maleimide or PEG-maleimide groups by reaction with sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC) or with succinimidyl-[(N-maleimidopropionamido)-PEG] esters (NHS-PEGn-maleimide) [Citation118]. (C) Aminated MNPs were functionalised with N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP). (D) Aminated MNPs were functionalised with N-succinimidyl iodoacetate (SIA).
![Figure 1. Overview on typical strategies for antibody conjugation on the surface of MNPs. The precursor particles were obtained by following reactions. (A) Carboxylated MNPs were activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). (B) Aminated MNPs were functionalised with maleimide or PEG-maleimide groups by reaction with sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC) or with succinimidyl-[(N-maleimidopropionamido)-PEG] esters (NHS-PEGn-maleimide) [Citation118]. (C) Aminated MNPs were functionalised with N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP). (D) Aminated MNPs were functionalised with N-succinimidyl iodoacetate (SIA).](/cms/asset/980fbf69-c4af-4388-856a-1e2edf3d3448/ihyt_a_835876_f0001_b.jpg)
The activation of carboxylic acid groups on the particle surface with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) provides a semi-stable NHS ester, that reacts with amino groups of the target molecules to form amide bonds between particle surface and antibody (). This method leads to randomly oriented antibodies on the particle surface that often results in a loss of bioactivity of the conjugated antibody. More sophisticated methods use hetero-bifunctional cross-linkers to introduce maleimide, pyridyl disulphide or iodoacetyl groups by reaction of the amino groups on the particle surface with 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid 3-sulpho-N-hydroxysuccinimide ester sodium salt (sulpho-SMCC), N-succinimidyl 3-(2-pyridyldithio)-propionate (SPDP) and N-succinimidyl iodoacetate (SIA), respectively (). These particles can bind target molecules with thiol groups, for example peptides with a terminal cysteine residue or thiolated antibodies. Several approaches have been developed to introduce thiol groups in antibody molecules, e.g. with N-succinimidyl S-acetylthioacetate (SATA) or Traut’s reagent (2-iminothiolane) or to cleave the disulphides between the heavy chains of IgG by treatment with mild reducing agents such as cysteamin [Citation96]. Commercially available heterobifunctional maleimide-PEGn-NHS cross-linkers enable the variation of the spacer length between particle surface and conjugated biomolecule to adjust the flexibility of the target molecules and to reduce the toxicity of the MNPs [Citation118]. A comparison of the antibody binding by EDC/NHS chemistry with maleimide and SPDP-based reactions has clearly demonstrated the advantage of the latter methods. Therefore MNPs were conjugated with a model antibody by the different methods. The bioactivity of the antibody after conjugation was slightly reduced to 88% for the maleimide and SPDP strategy and to 35% for the EDC/NHS chemistry [Citation55]. Similar results were obtained for the binding of 111In-DOTA chimeric L6 antibody (ChL6) to MNPs with PEG-COOH groups on the surface via EDC/NHS chemistry. The in vitro binding of the ChL6-conjugated particles to human breast cancer cells HBT 3477 was decreased to 40–60% in comparison to the free reference antibody [Citation110,Citation119]. Further studies used the corresponding maleimide functionalised particles for binding of di-scFv, a recombinantly generated antibody fragment for imaging and therapy of anti-MUC-1-expressing cancers, and achieved an immunoreactivity of >80% relative to the non-conjugated di-scFv-c [Citation120]. The oriented conjugation of thiolated antibodies to the surface of maleimide functionalised MNPs led to a significant enhancement of the association between target cells and antibody-labelled MNPs in comparison to the naked particles in several in vitro studies. Thus maleimide functionalised MNPs were conjugated with the thiolated ING-1 antibody. Human HT-29 cancer cells show a much higher particle association for ING-1-labelled particles in comparison to plain reference particles as a basis for targeted hyperthermia applications [Citation4]. The same maleimide functionalised MNPs were conjugated in a similar way with Herceptin. Malignant human mammary epithelial cells (SK-BR-3) bound significantly to the Herceptin-labelled particles and could selectively be killed via hyperthermia after AMF treatment [Citation5].
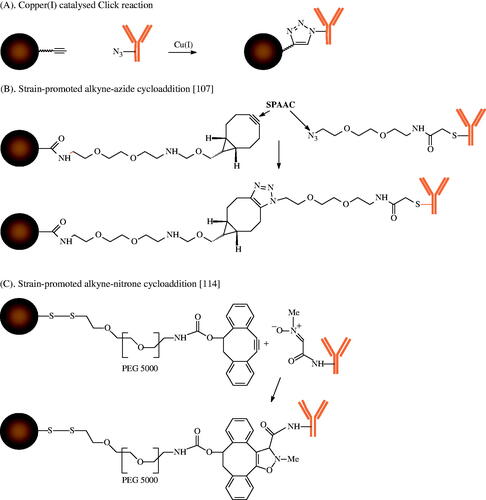
Site-specific conjugation of biomolecules by bioorthogonal reactions
The typical copper(I)-catalysed ‘click’ reaction is a successful method of achieving a strict bioorthogonality of the biomolecules on particle surfaces by reaction of alkyne-derivatised particles and azide functionalised antibodies [Citation121]. The possible interaction of the copper catalyst with the iron oxide of MNPs and the high toxicity of copper ions were a limitation of this method in the past. The seminal work o Bertozzi et al. [Citation122] led to the new strategy of strain promoted alkyne-azide cycloaddition (SPAAC) that does not require metal catalysts. Various compounds with strained alkyne units, for example (BCN) derivatives have recently become commercially available [Citation123] and allow particle functionalisation for reaction with azide functionalised antibodies (). An initial comparison of the biofunctionality of a model antibody that was bound to the surface of MNPs demonstrated the clear advantage of the SPAAC strategy over carbodiimide chemistry, with significant preservation of the biofunctionality of the antibodies by maleimide chemistry [Citation118]. Recent approaches combine the SPAAC strategy with the expressed protein ligation to optimise the antibody density on the particle surface for improved cell binding. Therefore a HER-2 affibody containing a C-terminal thioester reacted with the terminal cysteine of an azido-functionalised peptide for SPAAC with aza-dibenzocyclooctyne-modified MNPs [Citation124]. To increase the reaction rate in comparison to SPAAC, the strain-promoted alkyne-nitrone cycloaddition (SPANC) was developed. Colombo et al. [Citation125] demonstrated the complete conservation of protein effectiveness by binding nitrone functionalised bioengineered scFvs antibodies on the surface of 4-dibenzocyclooctynol-derivatised magnetite NPs via a PEG 5000 spacer. A further important advantage of the bioorthogonal conjugation is the stability of the reaction partners. By contrast, the carbodiimide chemistry is based on the N-hydroxysuccinimide ester as intermediate with a very short stability. The maleimide or SPDP strategies require thiol-functionalised reactants that can be oxidised and inactivated for the conjugation reaction by formation of disulphide bridges. An excellent review on bioorthogonal chemistries for NP conjugation and targeting summarises recent developments, including the strain-promoted inverse-electron-demand Diels-Alder cycloaddition (SPIEDAC) [Citation126].
Conclusion
Many sophisticated synthesis methods for iron oxide based NPs having suitable heating rates for hyperthermia applications are known. The comparison of heating parameters clearly shows the advantages of NPs with multi-crystalline iron oxide cores in comparison to single-core particles. The magnetic cooperative effect of iron oxide cores in specific multi-core NPs and nanocubes led to a significant increase of heating rates and will be a promising field of future development. The detailed analysis of the heating rates of different NP types requires standardisation of AMF parameters and measurement devices as well as the calculation of SAR values in relation to the particle composition, concentration, and the medium to identify the most promising candidates for clinical applications.
The particle stabilisation and coating can be adapted to the special aim of hyperthermia application itself, but can also provide a platform for future multimodal cancer therapy with drug-loaded iron oxide NPs that are conjugated with a site-specific antibody for combination of hyperthermia, chemotherapy, biotherapy and targeting [Citation127].
The clinical success of targeted hyperthermia applications can only be achieved if the MNPs demonstrate high circulation times in the blood and significantly reduced non-specific uptake by non-targeted organs and tissues following systemic delivery and a high specific accumulation in cancer tissue. Therefore, oriented and bioorthogonal conjugation strategies allow the binding of antibodies and other target-selective biomolecules by preserving their biofunctionality. The formation of a protein corona at the particle interaction with the biological surrounding significantly influences the in vivo particle behaviour. Therefore the corona fomation has to be studied for particle targeting applications with the final antibody conjugated MNPs. Successful in vitro and in vivo heating studies with a large variety of iron oxide NPs increase the diversity of future clinical hyperthermia applications and the potential of hyperthermia as powerful sensitiser for radiation and chemotherapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We are grateful to Robert Ivkov for all valuable amendments and critical comments to our manuscript. We thank Silvio Dutz for his assistance in the discussion of the comparability of the heating rates.
References
- Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci 2011;166:8–23
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–74
- Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia 2010;26:790–5
- Hoopes PJ, Tate JA, Ogden JA, Strawbridge R, Fiering SN, Petryk AA, et al. Assessment of intratumor non-antibody directed iron oxide nanoparticle hyperthermia cancer therapy and antibody directed IONP uptake in murine and human cells. Proc SPIE 2009;7181:71810P-1
- Zhang J, Dewilde AH, Chinn P, Foreman AR, Barry S, Kanne D, et al. Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cancer cells in vitro via hyperthermia. Int J Hyperthermia 2011;27:682–97
- Marcos-Campos I, Asin L, Torres TE, Marquina C, Tres A, Ibarra MR, et al. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology 2011;22:205101
- Asin L, Ibarra MR, Tres A, Goya GF. Controlled cell death by magnetic hyperthermia: Effects of exposure time, field amplitude, and nanoparticle concentration. Pharm Res 2012;29:1319–27
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103:317–24
- Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes JP. Magnetic nanoparticle hyperthermia in cancer treatment. Nano LIFE 2010;1:17–32
- Lehmann J, Natarajan A, DeNardo GL, Ivkov R, Foreman AR, Catapano C, et al. Nanoparticle thermotherapy and external beam radiation therapy for human prostate cancer cells. Cancer Biother Radiopharm 2008;23:265–71
- Tietze R, Lyer S, Dürr S, Alexiou C. Nanoparticles for cancer therapy using magnetic forces. Nanomedicine 2012;7:447–57
- Ren Y, Zhang H, Chen B, Cheng J, Cai X, Liu R, et al. Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int J Nanomedicine 2012;7:2261–9
- Kim M-H, Yamayoshi I, Mathew S, Lin H, Nayfach J, Simon SI. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann Biomed Eng 2013;41:598–609
- Thomas LA, Dekker L, Kallumadil M, Southern P, Wilson M, Nair SP, et al. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J Mater Chem 2009;19:6529–35
- Franke K, Kettering M, Lange K, Kaiser WA, Hilger I. The exposure of cancer cells to hyperthermia, iron oxide nanoparticles, and mitomycin C influences membrane multidrug resistance protein expression levels. Int J Nanomedicine 2013;8:351–63
- Grazu V, Silber A, Moros M, Asin L, Torres T, Marquina C, et al. Application of magnetically induced hyperthermia in the model protozoan Crithidia fasciculata as a potential therapy against parasitic infections. Int J Nanomedicine 2012;7:5351–60
- Kopp AF, Laniado M, Dammann F, Stern W, Grönewäller E, Balzer T, et al. MR imaging of the liver with Resovist: Safety, efficacy, and pharmacodynamic properties. Contrast Media 1997;204:749–56
- Wang Y-XJ. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg 2011;1:35–40
- van Landeghem FKH, Maier-Hauff K, Jordan A, Hoffmann K-T, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30:52–7
- Tartaj P, Morales MdP, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 2003;36:182–97
- Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 2008;108:2064–110
- Laurent S, Boutry S, Mahieu I, Elst VL, Muller RN. Iron oxide based MR contrast agents: From chemistry to cell labeling. Curr Med Chem 2009;16:4712–27
- Pankhurst QA, Thanh NKT, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2009;42:224001
- Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev 2011;63:789–808
- Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007;2:23–39
- Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res Lett 2008;3:397–415
- Lodhia J, Mandarano G, Ferris N, EU P, Cowell S. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed Imaging Interv J 2009;6:1–12
- Takafuji M, Shundo A, Ihara H. Organic layered magnetic nanoparticles. In: Nalwa HS, ed. Magnetic Nanostructures. Stevenson Ranch, CA: American Scientific Publishers, 2009, pp. 603–21
- Safarik I, Horska K, Safarikova M. Magnetic nanoparticles for biomedicine. In: Prokop A, ed. Intracellular Delivery: Fundamentals and Applications. New York, NY: Springer, 2011, pp. 363–72
- Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J Mater Chem 2009;19:6274–93
- Chen B, Wu W, Wang X. Magnetic iron oxide nanoparticles for tumor-targeted therapy. Curr Cancer Drug Targets 2011;11:184–9
- Hergt R, Andrä W, d’Ambly CG, Hilger I, Kaiser WA, Richter U, et al. Physical limitations of hyperthermia using magnetite fine particles. IEEE Trans Magn 1998;34:3745–54
- Jordan A, Scholz R, Wust P, Fähling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mat 1999;201:413–19
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mat 2002;252:370–4
- Kawashita M, Tanaka M, Kokubo T, Inoue Y, Yao T, Hamada S, et al. Preparation of ferrimagnetic magnetite microspheres for in situ hyperthermic treatment of cancer. Biomaterials 2005;26:2231–8
- Verges MA, Costo R, Roca AG, Marco JF, Goya GF, Serna CJ, et al. Uniform and water stable magnetite nanoparticles with diameters around the monodomain- multidomain limit. J Phys D Appl Phys 2008;41:134003
- Hergt R, Dutz S, Müller R, Zeisberger M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J Phys Condens Matter 2006;18:S2919–34
- Molday RS. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Meth 1982;52:353–67
- Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, et al. Superparamagnetic iron oxide; clinical application as a contrast agent for MR imaging of the liver. Radiology 1988;168:297–301
- Weissleder R, Bogdanov A, Neuwelt EA, Papisov M. Long-circulating iron oxides for MR imaging. Adv Drug Deliv Rev 1995;16:321–34
- Ferguson RM, Minard KR, Khandhar AP, Krishnan KM. Optimizing magnetite nanoparticles for mass sensitivity in magnetic particle imaging. Med Phys 2011;38:1619--26
- Eberbeck D, Dennis CL, Huls NF, Krycka KL, Grüttner C, Westphal F. Multicore magnetic nanoparticles for magnetic particle imaging. IEEE Trans Magn 2013;49:269–74
- Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics and therapy. Acc Chem Res 2011;44:842–52
- Rimkus G, Grüttner C, Bremer-Streck S, Herrmann K-H, Krumbein I, Reichenbach JR, et al. mVCAM-1 specific iron oxide nanoparticles based probes for multimodal imaging purposes. Biomed Tech 2012;57:77–80
- Ling Y, Pong T, Vassiliou CC, Huang PL, Cima MJ. Implantable magnetic relaxation sensors measure cumulative exposure to cardiac biomarkers. Nature Biotechnol 2011;29:273–7
- Kallumadil M, Tada M, Nakagawa T, Abe M, Southern P, Pankhurst QA. Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mat 2009;321:1509–13
- DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Grüttner C, et al. Thermal dosimetry predictive of efficacy of 111InChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. J Nucl Med 2007;48:437–44
- Reimers GW, Khalafalla SE, inventors. Production of magnetic fluids by peptization techniques. USA patent US 3,843,540, 1974
- Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn 1981;17:1247–8
- Fortin J-P, Wilhelm C, Servais J, Menager C, Bacri JC, Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 2007;129:2628–35
- Lefebure S, Dubois E, Cabuil V, Neveu S, Massart R. Monodisperse magnetic nanoparticles: Preparation and dispersion in water and oils. J Mater Res 1998;13:2975–81
- Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, et al. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int J Nanomed 2011;6:373–80
- Dutz S, Clement JH, Eberbeck D, Gelbrich T, Hergt R, Müller R, et al. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J Magn Magn Mat 2009;321:1501–4
- Cheraghipour E, Javadpour S, Mehdizadeh AR. Citrate capped superparamagnetic iron oxide nanoparticles used for hyperthermia therapy. J Biomed Sci Eng 2012;5:715–19
- Grüttner C, Müller K, Teller J, Westphal F, Foreman AR, Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J Magn Magn Mat 2007;311:181–6
- Bordelon DE, Cornejo C, Grüttner C, Westphal F, DeWeese TL, Ivkov R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl Phys 2011;109:124904
- Li Z, Kawashita M, Araki N, Mitsumori M, Hiraoka M, Doi M. Preparation of magnetic iron oxide nanoparticles for hyperthermia of cancer in a FeCl2-NaNO3-NaOH aqueous system. J Biomater Appl 2011;25:643–61
- Hyeon T, Lee SS, Park J, Chung Y, Bin Na H. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc 2001;123:12798–801
- Park J, Lee E, Hwang N-M, Kang MK, Kim SC, Hwang Y, et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew Chem Int Ed 2005;44:2872–7
- Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc 2002;124:8204–5
- Levy M, Quarta A, Espinosa A, Figuerola A, Wilhelm C, Garcia-Hernandez M, et al. Correlating magneto-structural properties to hyperthermia performance of highly monodisperse iron oxide nanoparticles prepared by a seeded-growth route. Chem Mater 2011;23:4170–80
- Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, et al. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J Am Chem Soc 2004;126:273–9
- Kim D, Lee N, Park M, Kim BH, An K, Hyeon T. Synthesis of uniform ferrimagnetic magnetite nanocubes. J Am Chem Soc 2009;131:454–5
- Bae KH, Park M, Do MJ, Lee N, Ryu JH, Kim GW, et al. Chitosan oligosaccharide-stabilized ferrimagnetic iron oxide nanocubes for magnetically modulated cancer hyperthermia. ACS Nano 2012;6:5266–73
- Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, et al. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012;6:3080–91
- Park J, An K, Hwang Y, Park J-G, Noh H-J, Kim J-Y, et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater 2004;3:891–5
- Khandhar AP, Ferguson RM, Krishnan KM. Monodispersed magnetite nanoparticles optimized for magnetic fluid hyperthermia: Implications in biological systems. J Appl Phys 2011;109:7B310--7B3103
- Armijo LM, Brandt YI, Mathew D, Yadav S, Maestas S, Rivera A, et al. Iron oxide nanocrystals for magnetic hyperthermia applications. Nanomaterials 2012;2:134–46
- Hugounenq P, Levy M, Alloyeau D, Lartigue L, Dubois E, Cabuil V, et al. Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J Phys Chem C 2012;116:15702–12
- Lartigue L, Hugounenq P, Alloyeou D, Clarke SP, Levy M, Bacri JC, et al. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012;6:10935–49
- Langevin D. Micelles and microemulsions. Annu Rev Phys Chem 1992;43:341–69
- Okoli C, Sanchez-Dominguez M, Boutonnet M, Järås S, Civera C, Solans C, et al. Comparison and functionalization study of microemulsion-prepared magnetic iron oxide nanoparticles. Langmuir 2012;28:8479–85
- Zeng Q, Baker I, Loudis JA, Liao Y, Hoopes PJ, Weaver JB. Fe/Fe oxide nanocomposite particles with large specific absorption rate for hyperthermia. Appl Phys Lett 2007;90:233112
- Zhang G, Liao Y, Baker I. Surface engineering of core/shell iron/iron oxide nanoparticles from microemulsions for hyperthermia. Mater Sci Eng C Mater Biol Appl 2010;30:92–7
- Kekalo K, Koo K, Zeitchick E, Baker I. Microemulsion synthesis of iron core/iron oxide shell magnetic nanoparticles and their physicochemical properties. MRS Proc 2012;1416
- Cassim SM, Giustini AJ, Baker I, Hoopes JP. Development of novel magnetic nanoparticles for hyperthermia cancer therapy. Proc SPIE, Energy-based Treatment of Tissue and Assessment VI 2011;7901:700115
- Basel MT, Balivada S, Wang H, Shrestha TB, Seo GM, Pyle M, et al. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int J Nanomed 2012;7:297–306
- Laurent S, Burtea C, Thirifays C, Rezaee F, Mahmoudi M. Significance of cell ‘observer’ and protein source in nanobiosciences. J Colloid Interface Sci 2013;392:431–45
- Schweiger C, Hartmann R, Zhang F, Parak WJ, Kissel TH, Rivera Gil P. Quantification of the internalization patterns of superparamagnetic iron oxide nanoparticles with opposite charge. J Nanobiotechnol 2012;10(28):1–11
- Häfeli UO, Riffle JS, Harris-Shkhawat L, Carmichael-Baranauskas A, Mark F, Daily JP, et al. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm 2009;6:1417–28
- Rimkus G, Bremer-Streck S, Grüttner C, Kaiser WA, Hilger I. Can we accurately quantify nanoparticle associated proteins when constructing high-affinity MRI molecular imaging probes. Contrast Media Mol Imaging 2011;6:119–25
- Grüttner C, Müller K, Teller J. A rapid assay to measure the shielding of iron oxide cores by the particle shell. IEEE Trans Magn 2013;49:177–81
- Yoo D, Jeong H, Preihs C, Choi J-S, Shin T-H, Sessler JL, et al. Double-effector nanoparticles: A synergistic approach to apoptotic hyperthermia. Angew Chem Int Ed 2012;51:12482–5
- Philipse AP, van Bruggen MPB, Pathmamanoharan C. Magnetic silica dispersions: Preparation and stability of surface-modified silica particles with a magnetic core. Langmuir 1994;10:92–9
- Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011;5:7124–9
- Rouhana LL, Schlenoff JB. Aggregation resistant zwitterated superparamagnetic nanoparticles. J Nanopart Res 2012;14:835
- Nigam S, Barick KC, Bahadur D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J Magn Magn Mat 2011;323:237–43
- Barick KC, Hassan PA. Glycine passivated Fe3O4 nanoparticles for thermal therapy. J Colloid Interface Sci 2012;369:96–102
- Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem 2004;14:2161–75
- Aqil A, Vasseur S, Duguet E, Passirani C, Benoit JP, Roch A, et al. PEO coated magnetic nanoparticles for biomedical application. Eur Polymer J 2008;44:3191–9
- Dan M, Scott DF, Hardy PA, Wydra RJ, Hilt JZ, Yokel RA, et al. Block copolymer cross-linked nanoassemblies improve particle stability and biocompatibility of superparamagnetic iron oxide nanoparticles. Pharm Res 2013;30:552–61
- Gonzales M, Krishnan KM. Synthesis of magnetoliposomes with monodisperse iron oxide nanocrystal cores for hyperthermia. J Magn Magn Mat 2005;293:265–70
- Gonzales M, Krishnan KM. Phase transfer of highly monodisperse iron oxide nanocrystals with pluronic F127 for biomedical applications. J Magn Magn Mat 2007;311:59–62
- Gonzales-Weimuller M, Zeisberger M, Krishnan KM. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J Magn Magn Mat 2009;321:1947–50
- Chandrasekharan P, Maity D, Yong CX, Chuang K-H, Ding J, Feng S-S. Vitamin E (D-alpha-tocopheryl-co-poly(ethylene glycol) 1000 succinate) micelles-superparamagnetic iron oxide nanoparticles for enhanced thermotherapy and MRI. Biomaterials 2011;32:5663–72
- Hermanson GT. Bioconjugate Techniques. San Diego, CA: Academic Press, 2008
- Josephson L, Tung C-H, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconj Chem 1999;10:186–91
- Chen T-J, Cheng T-H, Chen C-Y, Hsu SCN, Cheng T-L, Liu G-C, et al. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem 2009;14:253–60
- Lesniak C, Schiestel T, Schmidt H, Jordan A, inventors. Nanoscale particles having an iron oxide-containing core enveloped by at least two shells. USA Patent US 2003/0180370 A1, 2003
- Lartigue L, Innocenti C, Kalaivani T, Awwad A, del Mar Sanchez Duque M, Guari Y, et al. Water-dispersible sugar-coated iron oxide nanoparticles. An evaluation of their relaxometric and magnetic hyperthermia properties. J Am Chem Soc 2011;133:10459–72
- Yuan Y, Rende D, Altan CL, Bucak S, Ozisik R, Borca-Tasciuc D-A. Effect of surface modification on magnetization of iron oxide nanoparticle colloids. Langmuir 2012;28:13051–9
- Gonzalez-Fernandez MA, Torres TE, Andres-Verges M, Costo R, de la Presa P, Serna CJ, et al. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J Solid State Chem 2009;182:2779–84
- Larumbe S, Gomez-Polo C, Perez-Landazabal JI, Pastor JM. Effect of a SiO2 coating on the magnetic properties of Fe3O4 nanoparticles. J Phys Condens Matter 2012;24:266007
- Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Hoopes PJ, et al. The influence of magnetic and physiological behaviour on the effectiveness of iron oxide nanoparticles for hyperthermia. J Phys D Appl Phys 2008;41:134020
- Linh PH, Thach PV, Tuan NA, Thuan NC, Manh DH, Phuc NX, et al. Magnetic fluid based on Fe3O4 nanoparticles: Preparation and hyperthermia application. J Phys Conf Ser 2009;187:012069
- Pinero-Redondo Y, Banobre-Lopez M, Pardinas-Blanco I, Goya GF, Lopez-Quintela MA, Rivas J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res Lett 2011;6:1–7
- Eggeman AS, Majetich SA, Farrell D, Pankhurst QA. Size and concentration effects on high frequency hysteresis of iron oxide nanoparticles. IEEE Trans Magn 2007;43:2451–3
- Müller R, Dutz S, Neeb A, Cato ACB, Zeisberger M. Magnetic heating effect of nanoparticles with different sizes and size distributions. J Magn Magn Mat 2013;328:80–5
- Arruebo M, Valladares M, Gonalez- Fernandez A. Antibody-conjugated nanoparticles for biomedical applications. J Nanomaterials 2009;2009:439389
- DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Grüttner C, et al. Development of tumor targeting bioprobes (111In-Chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clin Cancer Res 2005;11:S7087–92
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnology J 2011;6:1342–7
- Kut C, Zhang Y, Hedayati M, Zhou H, Cornejo C, Bordelon DE, et al. Preliminary study of injury from heating systemically delivered, nontargeted dextran-superparamagnetic iron oxide nanoparticles in mice. Nanomedicine 2012;7:1697–711
- Peng X-H, Qian X, Mao H, Wang AY, Chen ZG, Nie S, et al. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine 2008;3:311–21
- Rogers WJ, Basu P. Factors regulating macrophage endocytosis of nanoparticles: Implications for targeted magnetic resonance plaque imaging. Artherosclerosis 2005;178:67–73
- Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci USA 2007;104:932–6
- Toraya-Brown S, Sheen MR, Baird JR, Barry S, Demidenko E, Turk MJ, et al. Phagocytes mediate targeting of iron oxide nanoparticles to tumors for cancer therapy. Intregr Biol 2013;5:159–71
- Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 2009;62:284–304
- Grüttner C, Müller K, Teller J. Comparison of strain-promoted alkyne-azide cycloaddition with established methods for conjugation of biomolecules to magnetic nanoparticles. IEEE Trans Magn 2013;49:172–6
- Natarajan A, Grüttner C, Ivkov R, DeNardo GL, Mirick G, Yuan A, et al. NanoFerrite particle based radioimmunonanoparticles and in vivo pharmacokinetics. Bioconj Chem 2008;19:1211–18
- Natarajan A, Xiong C-Y, Grüttner C, DeNardo GL, DeNardo SJ. Development of multivalent radioimmunonanoparticles for cancer imaging and therapy. Cancer Biother Radiopharm 2008;23:82–91
- Lin P-C, Ueng S-H, Yu S-C, Jan M-D, Adak AK, Yu C-C, et al. Surface modification of magnetic nanoparticle via Cu(I)-catalyzed alkyne-azide [2 + 3] cycloaddition. Org Lett 2007;9:2131–4
- Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 2008;320:664–7
- van Hest JCM, van Delft FL. Protein modification by strain-promoted alkyne-azide cycloaddition. Chembiochem 2011;12:1309–12
- Elias DR, Poloukhtine A, Popik V, Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013;9:194–201
- Colombo M, Sommaruga S, Mazzucchelli S, Polito L, Verderio P, Galeffi P, et al. Site-specific conjugation of ScFvs antibodies to nanoparticles by bioorthogonal strain-promoted alkyne-nitrone cycloaddition. Angew Chem Int Ed 2012;51:496–9
- Rahim MK, Kota R, Lee S, Haun JB. Bioorthogonal chemistries for nanomaterial conjugation and targeting. Nanotechnol Rev 2013;2:215–27
- Mi Y, Liu X, Zhao J, Ding J, Feng S-S. Multimodality treatment of cancer with herceptin conjugated, thermomagnetic iron oxides and docetaxel loaded nanoparticles of biodegradable polymers. Biomaterials 2012;33:7519–29