Abstract
Though the concepts of magnetic fluid hyperthermia (MFH) were originally proposed over 50 years ago, the technique has yet to be successfully translated into routine clinical application. Significant challenges must be addressed if the field is to progress and realise its potential as an option for treatment of diseases such as cancer. These challenges include determining the optimum fields and frequencies that maximise the effectiveness of MFH without significant detrimental off-target effects on healthy tissue, achieving sufficient concentrations of magnetic nanoparticles (MNPs) within the target tumour, and developing a better mechanistic understanding of MNP-mediated energy deposition and its effects on cells and tissue. On the other hand, emerging experimental evidence indicates that local thermal effects indeed occur in the vicinity of energy-dissipating MNPs. These findings point to the opportunity of engineering MNPs for the selective destruction of cells and/or intracellular structures without the need for a macroscopic tissue temperature rise, in what we here call magnetically mediated energy delivery (MagMED).
Introduction
Magnetic nanoparticles (MNPs) are typically single or multiple inorganic crystals of a magnetic material coated with or embedded within a biocompatible polymer, or a gold or silica shell enabling functionalisation. MNPs are versatile tools, enabling a wide variety of biomedical applications. For most biomedical applications, MNPs are made from ferrimagnetic iron oxides, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3). These iron oxides are particularly useful in biomedical applications as they respond to external magnetic fields by mechanical motion (linear or rotational, depending on particle size and domain state) or dissipation of thermal energy [Citation1,Citation2].
An advantage of MNPs over other inorganic nanoparticles proposed for biomedical applications is the extensive knowledge base regarding their effects in vitro and in vivo, which appears to indicate that iron oxide-based MNPs are to a great extent biocompatible. The first clinical application of MNPs approved by the US Food and Drugs Administration (FDA) was the use of magnetic iron oxide nanoparticles as negative contrast agents in magnetic resonance imaging (MRI) to improve tumour detection in the liver [Citation3,Citation4]. Another clinical application involves magnetic iron oxide nanoparticles for the treatment of anaemia due to iron deficiency [Citation5]. Currently, MNPs are being investigated for use in biomedical applications such as magnetically assisted drug delivery [Citation6,Citation7], magneto-mechanical actuation of cell surface receptors [Citation8,Citation9], magnetic gene transfection [Citation10,Citation11] and magnetic fluid hyperthermia (MFH), the subject of this commentary.
Local energy delivery is crucial for clinical application of hyperthermia in cancer treatment
Hyperthermia, the elevation of body temperature to 40–44 °C [Citation12], is of clinical interest in cancer therapy. The rationale behind the use of hyperthermia in combination with chemotherapy and/or radiation therapy for cancer treatment relies on the fact that tumours are heterogeneous tissues with areas of necrosis, low pH, and low oxygen [Citation12]. Cells in these areas are often in cell cycle arrest (G0 phase) and therefore most resistant to growth prohibiting (cytostatic) drugs [Citation13]. It remains unclear whether these cells are susceptible to heat damage; however, heat can stimulate vascularisation and increase oxygenation of the tissue, thereby improving the concentration and effectiveness of cytostatic and cytotoxic drugs and radiosensitivity [Citation13]. In other parts of the tumour, cells undergoing DNA replication (S phase of the cell cycle) are resistant to ionising radiation, but are susceptible to heat [Citation14]. These findings have been confirmed in a number of clinical studies [Citation15,Citation16] which indicate that elevation of the temperature within the tumour has a cytotoxic effect on radio-resistant cells when heated to temperatures above 42 °C.
There are a variety of techniques [Citation17,Citation18] to increase the temperature within the human body. Hyperthermia for the treatment of cancer can be divided into whole body hyperthermia, partial or regional hyperthermia, and local hyperthermia. Whole body hyperthermia is typically proposed for metastatic cancer, and aims to increase the whole body temperature to 41.8 °C using thermal chambers or hot water blankets [Citation12]. Reported side effects include transient nausea, vomiting and diarrhoea. Partial or regional hyperthermia, being considered to treat deep-seated or locally advanced cancer, is often achieved through isolated perfusion [Citation17] or the use of external applicators or antennas emitting microwaves [Citation18]. Side effects include blisters and superficial burns, discomfort and pain. Local hyperthermia is confined to areas smaller than limbs or organs. It induces the least severe side effects and is therefore the most frequently studied kind of hyperthermia [Citation18]. Local hyperthermia can be achieved through a variety of approaches, including radiofrequency ablation, focused ultrasound, or laser ablation and, more recently, MFH. Dissipation of magnetic field energy in the form of heat by MNPs is an attractive approach to achieving local hyperthermia because MNPs can be engineered to accumulate in cancer tissues and modified to specifically target cancer cells.
MFH has recently been approved in Europe for treatment of glioblastoma multiforme in a procedure that requires direct delivery of MNPs to the cancer site. Further clinical trials are currently being conducted by MagForce (Berlin, Germany) to study the application of MFH for treating prostate and pancreatic cancer. Although this approval and these clinical trials are an important step in translating MFH to the clinic, further work is needed to achieve the full potential of this promising cancer nanotechnology.
MNPs have been engineered for high energy dissipation rates
The approach we currently call ‘magnetic fluid hyperthermia’ was originally proposed in 1957 by Gilchrist et al. [Citation19] to destroy cancer in lymph nodes by injecting them with ‘particulate matter’ of maghemite and applying an alternating magnetic field to induce ‘selective inductive heating’. When single domain MNPs are subjected to an alternating magnetic field they dissipate heat through relaxation losses. The mechanism of energy dissipation depends on the size and magnetic material, and can either be through Néel relaxation, the rapid internal re-orientation of the particle’s magnetic moment with the applied field, or Brownian relaxation, frictional heating caused by the physical rotation of the magnetic particle. Rosensweig [Citation20] developed a simple model for the volumetric energy dissipation rate (P), show in Equation 1.
where μ0 is the permeability of free space (4π × 10−7 T m A−1), χ0 is the initial (low field) susceptibility of the particles, H is the magnetic field amplitude, f is the magnetic field frequency in Hz, and τ is the effective relaxation time. This equation illustrates that the heating potential and mechanism for energy deposition of MNPs in an alternating magnetic field depends on the conditions of the applied alternating magnetic field (i.e. its amplitude and frequency), and the properties and concentration (by way of the initial susceptibility) of the particles in the tissue [Citation20]. In terms of dependence on field parameters, it would appear that increasing the field amplitude and frequency leads to an increase in energy dissipation rate; however, as will be discussed below, this cannot be done indiscriminately as alternating magnetic fields can lead to undesirable non-specific eddy heating in tissues, affecting patient comfort and leading to side effects such as pain and blisters. On the other hand, the dependence of the energy dissipation rate on the properties of the particles has motivated substantial recent research on optimising the energy dissipation rate by selection of the magnetic core material [Citation21] and control of particle core size [Citation22,Citation23], both of which affect the initial susceptibility and relaxation time, and by developing core-shell [Citation24] and aggregate structures [Citation25] with tuned interactions leading to effective relaxation times that are close to the inverse of the applied alternating magnetic field frequency.
Typically, the heating potential of MNPs is reported in terms of the so-called specific absorption rate (SAR). To determine this parameter a suspension of MNPs of known concentration is subjected to an alternating magnetic field of known amplitude and frequency. The initial increase in temperature is recorded and the SAR calculated according to Equation 2
where c is the heat capacity of the suspension (typically assumed to be the heat capacity of the suspension medium for low concentrations of MNPs), msample is the mass of the sample, miron oxide is the mass of iron oxide in the sample, and
is the initial slope of the time-dependent heating curve [Citation26]. The volumetric energy dissipation rate P and SAR are related by Equation 3
where
is the density of iron oxide,
is the volume fraction of iron oxide, and
is the mass of iron oxide per unit volume. It is important to note that for the above equation to yield an accurate estimate of the heating capacity of the nanoparticles the suspension must be in thermal equilibrium with its surroundings at the start of the experiment. Otherwise heat flow to/from the environment can confound the measurement. Furthermore, care must be taken to ensure that the sample is subjected to a uniform magnetic field (typically achieved by using a sample that fits within the small central region of the coil used to generate the field) and to ensure that the sample heats uniformly. These and other experimental considerations are discussed in the literature [Citation27,Citation28].
The growing interest in MFH and the availability of commercial devices that can generate the required alternating magnetic fields has led to a large body of research aiming to characterise and optimise the SAR of MNPs, taking advantage of the significant advances made in the synthesis of MNPs with controlled size and shape [Citation29–32]. Engineering nanoparticles to have higher SAR values is important because higher SAR values would require smaller amounts of MNPs to be targeted to the tumour. Unfortunately, because there are no standards for the alternating magnetic field amplitude and frequency, each research group characterises their particles under different field conditions. As is evident in Equation 1, the energy dissipation rate is a sensitive function of the amplitude and frequency of the alternating magnetic field, as such SAR measurements will significantly vary if made under different field conditions. As a consequence, SAR values obtained by different research groups even for the same nanoparticle suspension will differ, as systems with different magnetic field strengths and frequencies are used. To address this issue, Kallumadil et al. [Citation33,Citation34] introduced the concept of intrinsic loss power (ILP), where SAR is normalised against the frequency and magnetic field strength, facilitating direct comparison of the heating potential of MNPs.
In recent years, a major focus in the MFH community has been on producing MNPs with exceptional SAR values, through exploration of alternative magnetic materials such as cobalt or cobalt alloys, or by tuning the size and/or structure of the particles [Citation32], which could potentially result in greater rates of energy dissipation. The most promising candidates so far, with regard to SAR, are CoFe2O4@MnFe2O4 core@shell nanoparticles synthesised by Lee et al. [Citation24], demonstrating SAR values of up to 4,000 W/g at 500 kHz and 37.3 kA/m (ILP = 5.75). However, the in vivo toxicity can be expected to be a problem due to the use of cobalt. On the other hand, the best heating by purely iron oxide nanoparticles currently in the literature appears to be iron oxide nanocubes generating exceptional SAR values of up to 2,452 W/g at 520 kHz and 29 kA/m (ILP = 5.61) [Citation35].
Additional research is needed to elucidate the maximum allowable magnetic field amplitude and frequency for magnetic field hyperthermia
As discussed above, according to Rosensweig’s model [Citation20] the energy dissipation rate rises with an increase in magnetic field strength and frequency, so one might think that heating potential or SAR could be simply improved by increasing the magnetic field amplitude. However, this approach is limited by the possibility of non-specific heating due to eddy currents in tissue, which can result in damaging levels of heat in healthy tissue. According to Atkinson [Citation36], the volumetric power dissipation due to eddy current heating (Peddy) is strongly dependent on the diameter of the object subjected to the AMF, as described by Equation 4
where σt is the electrical conductivity of the object, μ0 is the permeability of free space, H0 and f are the magnetic field amplitude and frequency, and r is the radius of the object. The generation of eddy currents by MNPs themselves is negligible because of their small size; however, eddy heating in the body under the alternating magnetic field conditions used in MFH could be significant. Eddy heating and tissue power absorption is quite a complex function of field and tissue parameters. For example, the electrical conductivity varies with tissue and may also vary with the frequency of the applied magnetic field.
In order to limit off-target, detrimental effects on healthy tissue associated with exposure to electromagnetic radiation, guidelines and limits for the exposure to alternating magnetic fields have been proposed by the International Commission on Non-Ionizing Radiation Protection (ICNIRP). These guidelines apply for both occupational and public exposure to electromagnetic fields and were developed on the basis of laboratory studies of cellular, tissue, and animal systems exposed to electromagnetic fields in the frequency range of 100 kHz–300 GHz and epidemiological studies on cancer risk [Citation37]. To provide protection against known adverse effects and to obtain a body temperature increase less than 1 °C, the ICNIRP has set a whole body average tissue SAR of 0.4 W/kg for an occupational exposure of 30 min, intended to provide a large margin of safety for other limiting conditions such as high temperature, high physical activity, or humidity [Citation37]. However, we note that these values are ten times lower than the SAR limits for MRI equipment specified in the FDA guidelines [Citation38], which are intended to leave a wide margin of safety for frequencies of 100 kHz–300 GHz.
In the field of MFH many authors have cited a product of magnetic field frequency and amplitude of 4.85 × 108 A/m s as the limit for applied AMFs in humans. This number was derived apparently from the work of Atkinson et al. [Citation36], who studied the effect of alternating magnetic field amplitude on patient comfort by subjecting patients’ extremities with a radius of 15 cm to an alternating magnetic field generated using a single turn coil at a frequency of 13.56 MHz and various magnetic field amplitudes. They found that amplitudes above 35.8 A/m led to discomfort. Later, Oleson et al. [Citation39] reported non-specific heating and discomfort through skin blistering in some patients subjected to an alternating magnetic field of 13.56 MHz frequency and unreported amplitude. Blistering was attributed to eddy heating.
Because the effects of exposure to alternating magnetic fields on tissues have been poorly studied, it would seem practical to extrapolate the observations of Atkinson et al. [Citation36] and Oleson et al. [Citation39] to obtain guidelines as to the maximum frequency and amplitude that can be applied in MFH. Hence, the adoption of the product of magnetic field amplitude and frequency (in units of Hz) of 4.85 × 108 A/m.s as the upper limit by the MFH research community. However, such extrapolation must be done with caution and in consideration of the other parameters that affect eddy heating in tissues, namely, the maximum diameter possible for an eddy current through the affected tissue and the electrical conductivity of the affected tissue. In terms of the effect of diameter it is evident that smaller tissue regions could tolerate larger field-frequency products and that this effect would scale quadratically, making it significant only when comparing whole body application of the AMF to regional/local application. On the other hand, the electrical conductivity of tissue can vary significantly with the frequency of the applied alternating magnetic field [Citation40]. This is specially the case for skin, which the observations of Oleson et al. [Citation39] suggest is the principal source of discomfort, damage (blistering), and pain. The conductivity of dry skin decreases from 0.238 S/m at 13.56 MHz to 1.06 × 10−3 S/m at 200 kHz [Citation41]. Hence, to result in the same rate of non-specific heating in the skin, the field amplitude-frequency product would need to be 7.3 × 109 A/(m s) in order to result in pain and damage in the skin. Alternatively, we can translate the ICNIRP guidelines and FDA guidelines for MRI into estimates of maximum allowable magnetic field amplitude and frequency product. In the case of the ICNIRP guidelines the maximum magnetic field amplitude and frequency product to avoid damage to skin would be 8.1 × 107 A/(m s) at 13.56 MHz and 1.2 × 109 A/(m s) at 200 kHz. On the other hand, considering the FDA guidelines for MRI, the corresponding maximum magnetic field amplitude and frequency product to avoid damage to skin would be 2.55 × 108 A/(m s) at 13.56 MHz and 3.83 × 109 A/(m s) at 200 kHz. It is perhaps a coincidence that the estimated maximum magnetic field amplitude and frequency products are similar for the FDA MRI guidelines and extrapolation of the results of Atkinson et al. [Citation36] when considering the variation of skin electrical conductivity with magnetic field frequency. Obviously, these calculations are only estimates, but they serve to illustrate the need for more detailed study of the effects of alternating magnetic fields on normal tissues under conditions typical of MFH in order to adequately determine the limits to which the applied alternating magnetic field can be optimised.
MNP delivery is a crucial challenge for the clinical application of MFH
For MFH to successfully eradicate cancer one must be able to locally dissipate sufficient heat to overcome losses due to conduction to surrounding tissue and due to blood perfusion. This can be achieved through a combination of optimising the energy dissipation properties of the particles, operating at the highest allowable alternating magnetic field conditions, and maximising the amount of particles that can be deposited to the targeted tissue by passive and active targeting. The field has made major advances in terms of optimising the energy dissipation properties of MNPs under typical MFH conditions; however, it is increasingly clear that there is only so much more improvement possible and it is likely that the highest possible SAR are in the range of 1–10 kW/g. In terms of allowable magnetic field conditions, the discussion above suggests that it may be possible to apply magnetic field amplitudes that are higher than previously thought; however, additional research is needed to determine exactly what conditions are allowable. Furthermore, it would seem evident from the discussion above that no more than an order-of-magnitude increase in the product of magnetic field amplitude and frequency would be possible. Therefore, for MFH to be successful it is critical to design MNPs that can accumulate in cancer tissues at concentrations that are sufficient, for a given particle SAR and magnetic field condition, to achieve hyperthermia.
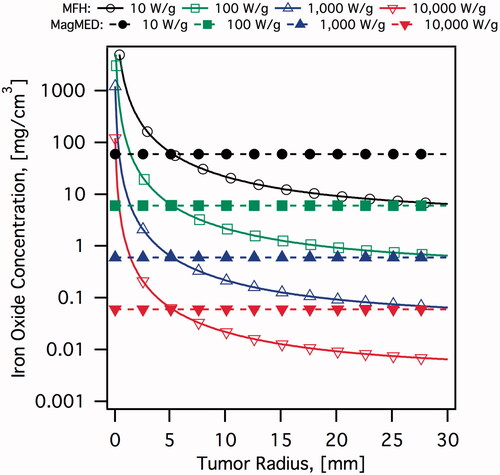
The concentration of particles needed to achieve hyperthermia in a tumour mass can be estimated using an admittedly simplistic model of heat transfer in tissues. One can consider a spherical tumour region, of radius R, with a uniform distribution of MNPs that dissipate heat at a volumetric energy dissipation rate P. The tumour and tissue can be modelled as an effective continuum using Penne’s bio-heat equation [Citation42,Citation43]. The solution is straightforward and here we only consider the temperature at the interface between the tumour and healthy tissue as the figure of merit, as the temperature within the tumour will necessarily be higher than this value. The resulting steady-state temperature at the interface between the tumour and healthy tissue, , is given by Equation 5
where
is the arterial temperature of the blood perfusing through the tissue, k is the tissue thermal conductivity,
is the blood perfusion rate through the tissue,
is the blood density, and
is the specific heat capacity of blood. Equation 5 can be combined with Equation 3 to estimate the iron oxide concentration
needed to achieve a desired interface temperature for a tumour of a given radius when the particles have a given SAR. illustrates this calculation for an interface temperature of 45 °C and a range of SAR values. Other parameters are
,
,
,
, and
[Citation44]. Clearly as the tumour becomes smaller the concentration of particles needed to raise the temperature to the hyperthermia range rises rapidly. As an illustrative example, for particles with SAR of the order of 1,000 W/g (representative of the best SAR values currently reported) and a tumour diameter of 5 mm a concentration of approximately 650 μg/cm3 would be required.
Figure 1. Estimated iron oxide concentrations required to eradicate a spherical tumour as a function of tumour radius. It is assumed that magnetic nanoparticles are uniformly distributed throughout the tumour volume. MFH calculations assume a temperature of 45 °C at the interface between tumour and healthy tissue. MagMED calculations assume a thermal dose of 18 μJ/cell required, over 2 h of AMF application, and a cell density of 2 × 108 cells/cm3.
One can consider two general approaches to deliver MNPs to cancer tissues: (1) direct local delivery, and (2) systemic delivery. The first is perhaps the most straightforward, and therefore is the approach used in most in vivo MFH studies [Citation45,Citation46] and in recent clinical trials treating glioblastoma, prostate and pancreatic cancer [Citation47–50]. Direct local delivery of the MNPs is possible when the location and extent of a tumour is known and is easily accessible for delivery, typically through an injection. This is obviously the case in animal models with subcutaneous tumours [Citation51], but it is hardly the case in some forms of cancer, especially when the disease has advanced to the metastatic state. These potential clinical limitations of direct local delivery motivate the interest in systemic delivery. Systemic delivery consists of designing MNPs that can be injected into the blood stream and once there circulate long enough to deposit in highly vascularised cancer tissue. Alternatively, nanoparticles could be injected into arteries supplying blood to a targeted organ, enhancing particle deposition locally [Citation52]. For this approach to be successful the MNPs must be designed to be large enough to avoid renal clearance (>5.5 nm [Citation53]), small enough to avoid the reticuloendothelial system (<200 nm [Citation26]) and to permeate the so-called ‘leaky vasculature’ of a cancer tumour (through the so-called ‘enhanced permeation and retention’ (EPR) effect [Citation54–61]). Furthermore, the particles should have adequate surface chemistry to avoid adsorption of plasma proteins that can affect the in vivo transport and fate of the nanoparticles. Because the hydrodynamic size of the nanoparticles is so important to ensure long blood circulation time and transport through the tumour vasculature, it is imperative to develop MNP platforms that resist aggregation in the complex biological milieu, characterised by high ionic strength, presence of species with high affinity for the iron oxide surface, and large concentrations of biomacromolecules such as proteins. This fact has motivated research on routes to modify the MNP surface that leads to colloidal stability under conditions mimicking the biological environment [Citation62,Citation63]. Finally, the use of so-called active targeting agents, such as biomolecules (e.g. folic acid [Citation64–69]), antibodies [Citation70–72], antibody fragments [Citation73,Citation74], receptor ligands [Citation75,Citation76], and more recently aptamers [Citation77–80], could prove beneficial to enhance deposition of MNPs in the intended tissue by promoting uptake of the particles by targeted cells, thereby maintaining a concentration gradient between the particles in the blood stream and particles in the extracellular space of the tumour. Additionally, besides the possibility of greater MNP deposition in cancer tissues, active targeting could lead to control over the intracellular localisation of MNPs, which could prove advantageous in enhancing the therapeutic effects of thermal energy delivered by the MNPs. This is discussed further below.
The concepts of local and systemic delivery, passive targeting through the EPR, and active targeting using surface bound ligands have been proposed and discussed since the inception of the field of MFH, although thus far there has been very limited success, particularly with respect to systemic passive (i.e. solely through the EPR) and active delivery (i.e. using active targeting agents). As noted above, many of the in vivo studies of MFH relied on direct local delivery of the nanoparticles [Citation46,Citation50], often to subcutaneous tumour xenografts [Citation51]. Systemic delivery of MNPs to cancer tumours and tissue has been studied in the context of using MNPs as negative MRI contrast agents [Citation4,Citation81]; however, the amount of particles that need to be deposited for that application is much lower than that needed to achieve hyperthermia. There have also been reports of systemically delivered antibody-targeted magnetic iron oxide nanoparticles for MRI contrast imaging of different kinds of cancers [Citation82–84]. Unfortunately, although these studies are important, they provide limited insight into designing MNPs for systemic delivery and use in MFH. Thus, the state of the field is that, although there have been studies of the in vivo efficacy of MFH using systemic delivery [Citation85], substantial additional work is needed to fully understand the phenomena governing MNP transport and deposition in tumours (particularly small metastatic masses), so that we can design future generations of MNPs that can be delivered at high enough concentrations to achieve hyperthermia in the cancer tissue.
Local thermal effects due to energy-dissipating MNPs could circumvent some of the challenges of MFH
The application of MNPs for hyperthermia is often predicated on the potential advantages of nanoscale energy delivery, whether to achieve greater control over temperature distribution in a tumour, or due to the hope that thermal energy delivered by internalised nanoparticles could be more effective at destroying cancer cells. For example, Gordon et al. [Citation86] suggested that internalised MNPs could be more effective at killing cancer cells because the cell membrane would act as an insulator enhancing the hyperthermic effect. With the advent of nanotechnology and the possibility of targeting MNPs to specific structures within the cell, the idea of selective destruction of these intracellular targets has become more attractive. In fact, if local heating effects could be realised one could think of killing cancer cells without the need for a macroscopic temperature increase. Because this would no longer require the elevation of tissue temperature to the hyperthermia range the term magnetic fluid hyperthermia would no longer be applicable. Here we introduce the term ‘magnetically mediated energy delivery’ (MagMED) to denote situations wherein magnetic field energy is locally transformed into other forms (e.g. heat or rotational/translational work) in order to control the fate of cells or intracellular components, without the need to achieve a macroscopic tissue temperature rise. (The term ‘magnetically mediated energy delivery’ arose from conversations between Carlos Rinaldi, University of Florida, and O. Thompson Mefford, Clemson University.)
Although promising, the idea that local heating effects due to MNPs in alternating magnetic fields can be exploited to kill cancer cells is not without controversy. For example, theoretical analysis by Rabin [Citation87] indicates that nanoscale heating should have negligible effect on cells, as the rate of energy removal due to conduction to the surroundings far exceeds the rate of energy dissipation possible with MNPs. As such, the temperature on the surface of a MNP is expected to be negligibly higher than the temperature of the surrounding bulk fluid. Similarly, according to Rabin [Citation87], the temperature of a cell full of MNPs would be practically the same as that of the surrounding tissue. Finally, Rabin’s calculations [Citation87] suggest that tumours have to be at least 1.1 mm in diameter in order to achieve temperatures in the hyperthermia range, posing major limitations for using MFH to eradicate small tumours and metastatic cells. Later Keblinsky et al. [Citation88] made similar theoretical calculations, reaching similar conclusions.
The theoretical arguments of Rabin [Citation87] and Keblinsky et al. [Citation88] stand in stark contrast with a growing body of experimental evidence in support of the existence and biological relevance of local thermal effects in the vicinity of MNPs that are dissipating heat due to the action of an alternating magnetic field. For example, Huang et al. [Citation89] used targeted MNPs in combination with an alternating magnetic field to thermally activate calcium channels without evidence of a rise in tissue temperature. Perhaps more importantly, in their study Huang et al. [Citation89] monitored the surface and bulk environment through thermally responsive fluorophores, one grafted to the nanoparticle surface and another free in solution. They observed that immediately upon application of the AMF the particle-bound fluorophore reported a change in fluorescence intensity, which they interpreted to correspond to a rise (as great as 20 °C) in nanoparticle surface temperature. This happened even though the fluorophore that was free in solution did not report a change in temperature of the bulk fluid. Similar observations were made by Polo-Corrales and Rinaldi [Citation90], who used iron oxide nanoparticles coated with the fluorescent thermoresponsive polymer consisting of poly(n-isopropylacrylamide) co-polymerised with a fluorescent benzofurazan monomer. Under external heating the fluorescence of the polymerbound particles was constant up to the lower critical solution temperature (LCST) of approximately 35 °C, as the intensity of the fluorescence of the benzofurazan dye rapidly increased with decreasing solvent polarity (i.e. as its environment shifted from hydrophillic to hydrophobic) [Citation91]. On the other hand, when heating was achieved through application of an alternating magnetic field and energy dissipation from within the MNPs, the fluorescence intensity was observed to increase immediately upon application of the AMF, even though the bulk temperature was about 12 °C below the LCST. Polo-Corrales and Rinaldi [Citation90] interpreted this as evidence of substantial surface heating of the MNPs upon application of an AMF.
Indirect evidence of the practical relevance of local thermal effects in the vicinity of MNPs in alternating magnetic fields can be found in the work of Amstad et al. [Citation92], who studied triggered release of cargo from magnetoliposomes wherein the MNPs were engineered to adhere to the lipid bilayer. AMF treatment of the magnetoliposomes resulted in increased permeability even though the bath temperature had not reached the melting temperature of the liposomes. Because the MNPs were directly associated with the liposome’s lipid bilayer this observation suggests that local heating effects due to energy dissipating MNPs can affect (and perhaps damage) structures such as lipid bilayers.
Rodriguez-Luccioni et al. [Citation93] approached the topic of local thermal effects due to energy dissipation by MNPs differently, by comparing cell viability after hyperthermia mediated through nanoparticles or through the use of a hot water bath. Comparisons were made between cell cultures subjected to similar time–temperature profiles, such that the thermal dose and cumulative equivalent minutes [Citation94] were the same. While no differences in cell viability were observed between hot water bath hyperthermia (HWH) with and without nanoparticles, comparison of HWH and MFH showed that MFH resulted in a greater decrease in cell viability and activation of apoptotic pathways. Along these lines, Lee et al. [Citation95] observed that MFH increased the effectiveness of cisplatin treatment, even at cisplatin concentrations a magnitude below the half maximal inhibitory concentration (IC50). They observed that MFH was significantly more effective than HWH in enhancing the effectiveness of cisplatin, regardless of the order in which thermal treatment and cisplatin treatment were applied. Berrios-Alvarez et al. [Citation96] explored membrane permeabilisation as a potential mechanism for the enhanced activity of cisplatin in combination with MFH or HWH. Cisplatin is actively transported into the cell through the cell copper transporter (CTR1) and therefore can be hindered by using extracellular copper. The experiments of Berrios-Alvarez et al. [Citation96] demonstrated that both forms of hyperthermia resulted in increased uptake of cisplatin; however, this effect was greater for MFH treatment. Furthermore, they found that MFH resulted in a significant increase in membrane fluidity and permeability, an effect not observed with HWH. These findings seem to agree with the arguments of Lepock [Citation97], who hypothesised that hyperthermia could cause irreversible lipid and protein denaturation and result in increased membrane fluidity and permeability.
Although the above studies hint at the possibility of using local thermal effects due to MNPs in alternating magnetic fields, it is not clear if these effects can be harnessed to destroy cancer cells without the need for a macroscopic tissue temperature rise to the hyperthermia range. Creixell et al. [Citation75] addressed this possibility using MNPs, as they hypothesised that active targeting would be crucial to achieve the close contact required for local thermal effects to seriously damage cellular structures. To this end they conjugated the peptide epidermal growth factor (EGF) to carboxymethyl-dextran-coated MNPs, obtaining particles that selectively target the epidermal growth factor receptor (EGFR). Their experiments demonstrated greater uptake of EGF-conjugated MNPs over non-conjugated MNPs in breast cancer cells that over-express the EGFR, whereas there was no difference between targeted and non-targeted nanoparticle uptake in cells that did not over-express the EGFR. More importantly, Creixell et al. [Citation75] demonstrated that significant (up to 99.9%) reductions in cell viability were possible using only the MNPs taken up by the cells and without the need for a macroscopic temperature rise (that is, the temperature of their cell cultures remained at 37 °C throughout their experiments). This reduction in cell viability was not observed with non-targeted MNPs or in cells that did not over-express the EGFR. Furthermore, the observed reduction in cell viability was shown to vary with the amplitude of the applied AMF, and hence was dependent on the rate of energy dissipation by the nanoparticles. Although these results are promising, Creixell et al. [Citation75] were unable to provide a mechanistic explanation to their observations. They did, however, note that according to confocal microscopy EGF-MNP conjugates localised to the cell membrane and lysosomes, suggesting that damage to these cell structures could be responsible for the observed reductions in cell viability. This potential mechanism was further investigated by Domenech et al. [Citation98], who demonstrated that EGFR-targeted MNPs accumulate in the lysosomes of cells that over-express the EGFR and lead to disruption of these intracellular structures upon application of an AMF. In cancer cells they also observed that disruption of lysosomes by internalised EGFR-targeted MNPs upon application of an AMF resulted in a significant reduction in cell viability and an increase in reactive oxygen species generation, even though the sample temperature remained at 37 °C throughout the experiment.
The observations of Creixell et al. [Citation75] and Domenech et al. [Citation98] are significant because they suggest that MNPs can be engineered to achieve significant reductions in cancer cell viability without the need for a macroscopic tissue temperature rise to the hyperthermia range and using only the particles that are internalised into the cells. This is an example of MagMED. In this case it is no longer necessary to achieve concentrations as high as in MFH, and in fact it would be expected that the concentration required would be independent of the size of the cancer mass being treated. We illustrate this possibility in where we have estimated the concentration of particles needed to destroy cancer cells on the basis of the thermal doses used by Creixell et al. [Citation75] and representative SAR values. These estimates indicate that MagMED using EGF-conjugated MNPs that target the EGFR could be more effective than MFH at treating small cancer tumours. Clearly, the results of Creixell et al. [Citation75] and Domenech et al. [Citation98] are most promising for the treatment of small tumours, such as in early stage and metastatic disease.
The above discussion illustrates that there is a growing body of experimental evidence supporting the existence of local heating effects in the vicinity of MNPs in alternating magnetic fields, and that these local heating effects can be biologically relevant. These observations appear to contradict theoretical calculations and further work is needed to reconcile theory with experimental evidence. However, if we accept the existence of such nanoscale thermal effects and consider that MNPs can be engineered to target specific cellular structures, it becomes evident that there is a need to understand the biological mechanisms by which MagMED using MNPs can lead to cell death. Unfortunately there is a dearth of studies on the mechanisms by which thermal energy delivered by MNPs leads to cell death. Most past studies have focused solely on quantifying reductions in cell viability in vitro, using metabolic assays or clonogenic survival, without investigating the mechanisms involved. It is suggested that future studies investigate biological responses within the cell by taking advantage of the wide variety of cell death assays used in drug discovery [Citation99].
Concluding remarks
The application of thermal energy delivered by magnetic particulates has been under development for almost six decades and yet the field is far from achieving its full clinical potential. An important first milestone was reached with the clinical approval of MFH for glioblastoma multiforme in Europe. In the past two decades attention has focused on using MNPs to raise the temperature of tumour tissue to the hyperthermia range, in what is commonly referred to as magnetic fluid hyperthermia. Recent research has focused on optimising the energy dissipation properties of a variety of compositions and morphologies of MNPs, with current specific absorption rates in the order of 2 kW/g for biocompatible iron oxide-based nanoparticles. Progress in this area is also beginning to suggest that it is unlikely that order of magnitude improvements in SAR will be possible. As such, the research community needs to shift its attention to address the remaining challenges to the clinical application of MFH, namely (1) quantifying the maximum allowable magnetic field conditions for MFH, and (2) engineering MNPs to achieve substantial accumulation in cancer tumours after systemic delivery. Unfortunately, heat transfer arguments suggest that MFH will be intrinsically ineffective in treating small tumours in early stage and metastatic disease if a macroscopic temperature rise to the hyperthermia range is the only mechanism by which energy dissipation by MNPs in alternating magnetic fields leads to cancer cells. However, a growing body of experimental evidence supports the notion that MNPs can be engineered to cause local thermal effects in their vicinity, which can lead to disruption of cellular structures and cell death without the need for a macroscopic temperature rise. Additional work is needed to elucidate the mechanisms by which this form of MagMED can be exploited to treat cancer, among a variety of other potential applications.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2003;36:167–81
- Pankhurst QA, Thanh NKT, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2009;42:224001
- Marincek B. Diagnostic improvement in MRI of gynecological neoplasms. J Belg Radiol 1996;79:13–17
- Reimer P, Balzer T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur Radiol 2003;13:1266–76
- Auerbach M. Ferumoxytol as a new, safer, easier-to-administer intravenous iron: Yes or no? Am J Kidney Dis 2008;52:826–9
- Owen J, Pankhurst Q, Stride E. Magnetic targeting and ultrasound mediated drug delivery: Benefits, limitations and combination. Int J Hyperthermia 2012;28:362–73
- Sarwar A, Lee R, Depireux DA, Shapiro B. Magnetic injection of nanoparticles into rat inner ears at a human head working distance. IEEE Trans Magn 2013;49:440–52
- Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, Ingber DE. Nanomagnetic actuation of receptor-mediated signal transduction. Nat Nanotechnol 2008;3:36–40
- Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nat Nanotechnol 2008;3:139–43
- Plank C, Scherer F, Schillinger U, Bergemann C, Anton M. Magnetofection: Enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J Liposome Res 2003;13:29–32
- Schillinger U, Rutz M, Fischer C, Jahnke AJ, Walsch F, Ferizi M, et al. Novel immunostimulatory therapy by adjuvant magnetofection prolongs relapse-free survival of fibrosarcoma bearing cats: A veterinary clinical study. Hum Gene Ther 2010;21:1210–11
- Otte J. Hyperthermia in cancer therapy. Eur J Pediatr 1988;147:560–9
- van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int J Hyperthermia 2007;23:315–23
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103:317–24
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Chichel A, Skowronek J, Kubaszewska M, Kanikowski M. Hyperthermia – Description of a method and a review of clinical applications. Rep Pract Oncol Radiother 2007;12:267–75
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg 1957;146:596–606
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002;252:370–74
- Liu TY, Hu SH, Liu DM, Chen SY, Chen IW. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today 2009;4:52–65
- Gonzales-Weimuller M, Zeisberger M, Krishnan KM. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J Magn Magn Mater 2009;321:1947–50
- Suto M, Hirota Y, Mamiya H, Fujita A, Kasuya R, Tohji K, Jeyadevan B. Heat dissipation mechanism of magnetite nanoparticles in magnetic fluid hyperthermia. J Magn Magn Mater 2009;321:1493–6
- Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol 2011;6:418–22
- Lartigue L, Hugounenq P, Alloyeau D, Clarke SP, Levy M, Bacri JC, et al. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012;6:10935–49
- Krishnan KM. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans Magn 2010;46:2523–58
- Natividad E, Castro M, Mediano A. Adiabatic magnetothermia makes possible the study of the temperature dependence of the heat dissipated by magnetic nanoparticles under alternating magnetic fields. Appl Phys Lett 2011;98:243119
- Huang S, Wang SY, Gupta A, Borca-Tasciuc DA, Salon SJ. On the measurement technique for specific absorption rate of nanoparticles in an alternating electromagnetic field. Meas Sci Technol 2012;23:035701
- Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine 2010;5:1401–14
- Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Account Chem Res 2011;44:875–82
- Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Account Chem Res 2011;44:853–62
- Ling D, Hyeon T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2012;9:1450–66
- Kallumadil M, Tada M, Nakagawa T, Abe M, Southern P, Pankhurst QA. Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mater 2009;321:1509–13
- Kallumadil M, Tada M, Nakagawa T, Abe M, Southern P, Pankhurst QA. Corrigendum to: “Suitability of commercial colloids for magnetic hyperthermia” (vol 321, pg 1509, 2009). J Magn Magn Mater 2009;321:3650–1
- Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, et al. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012;6:3080–91
- Atkinson WJ, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng 1984;31:70–5
- ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Available from http://www.icnirp.de/documents/emfgdl.pdf (accessed 1 May 2013)
- FDA. A primer on medical device interactions with magnetic resonance imaging systems. Available from http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm107721.htm (accessed 1 May 2013)
- Oleson JR. A review of magnetic induction methods for hyperthermia treatment of cancer. IEEE Trans Biomed Eng 1984;31:91–7
- Stuchly MA. Health effects of exposure to electromagnetic fields. Aspen, CO: Aerospace Applications Conference; 1995. pp 351–68
- Carrara N. An internet resource for the calculation of the dielectric properties of body tissues in the frequency range of 10 Hz–100 GHz. Available from http://niremf.ifac.cnr.it/tissprop/ (accessed 28 May 2013)
- Bagaria HG, Johnson DT. Transient solution to the bioheat equation and optimization for magnetic fluid hyperthermia treatment. Int J Hyperthermia 2005;21:57–75
- Giordano MA, Gutierrez G, Rinaldi C. Fundamental solutions to the bioheat equation and their application to magnetic fluid hyperthermia. Int J Hyperthermia 2010;26:475–84
- Diller K, Valvano J, Pearce J. Bioheat transfer. In: Kreith F, Timmerhaus K, Lior N, Shaw H, Shah RK, Bell KJ, editors. The CRC Handbook of Thermal Engineering. Boca Raton, FL: CRC Press; 2000. pp 114–87
- Giustini AJ, Ivkov R, Hoopes PJ. Magnetic nanoparticle biodistribution following intratumoral administration. Nanotechnology 2011;22:345101
- Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, et al. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia 1997;13:587–605
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103:317–24
- van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009;30:52–7
- Johannsen M, Gneueckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, et al. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 2007;52:1653–62
- Johannsen M, Jordan A, Scholz R, Koch M, Lein M, Deger S, et al. Evaluation of magnetic fluid hyperthermia in a standard rat model of prostate cancer. J Endourol 2004;18:495–500
- Jordan A, Scholz R, Maier-Hauff K, van Landeghem FKH, Waldoefner N, Teichgraeber U, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol 2006;78:7–14
- Moroz P, Jones SK, Winter J, Gray BN. Targeting liver tumors with hyperthermia: Ferromagnetic embolization in a rabbit liver tumor model. J Surg Oncol 2001;78:22–9
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165–70
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000;65:271–84
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001;41:189–207
- Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 2011;63:136–51
- Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release 2012;164:108–14
- Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J Control Release 2012;161:175–87
- Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release 2012;164:138–44
- Taurin S, Nehoff H, Greish K. Anticancer nanomedicine and tumor vascular permeability; Where is the missing link? J Control Release 2012;164:265–75
- Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 2013;65:71–9
- WC Miles, Goff JD, Huffstetler PP, Reinholz CM, Pothayee N, Caba BL, et al. Synthesis and colloidal properties of polyether-magnetite complexes in water and phosphate buffered saline. Langmuir 2009;25:803–13
- Creixell M, Herrera AP, Latorre-Esteves M, Ayala V, Torres-Lugo M, Rinaldi C. The effect of grafting method on the colloidal stability and in vitro cytotoxicity of carboxymethyl dextran coated magnetic nanoparticles. J Mater Chem 2010;20:8539
- Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate–dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. MAGMA 2001;12:104–113
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995–4021
- Sonvico F, Mornet S, Vasseur S, Dubernet C, Jaillard D, Degrouard J, et al. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjugate Chem 2005;16:1181–8
- Sun C, Sze R, Zhang M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res Part A 2006;78A:550–7
- Yoo M-K, Park I-K, Lim H-T, Lee S-J, Jiang H-L, Kim Y-K, et al. Folate-PEG-superparamagnetic iron oxide nanoparticles for lung cancer imaging. Acta Biomater 2012;8:3005–13
- Mahajan S, Koul V, Choudhary V, Shishodia G, Bharti AC. Preparation and in vitroevaluation of folate-receptor-targeted SPION–polymer micelle hybrids for MRI contrast enhancement in cancer imaging. Nanotechnology 2012;24:015603
- Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA. Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn Reson Med 2003;49:1006–13
- Grüttner C, Müller K, Teller J, Westphal F, Foreman A, Ivkov R. Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy. J Magn Magn Mater 2007;311:181–6
- Xiong F, Zhu Z.-Y, Xiong C, Hua X-Q, Shan X-H, Zhang Y, Gu N. Preparation, characterization of 2-deoxy-D-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pharm Res 2011;29:1087–97
- Le B, Shinkai M, Kitade T, Honda H, Yoshida J, Wakabayashi T, Kobayashi T. Preparation of tumor-specific magnetoliposomes and their application for hyperthermia. J Chem Eng Jap 2001;34:66–72
- Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small 2008;5:235–43
- Creixell M, Bohórquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011;5:7124–9
- Creixell M, Herrera AP, Ayala V, Latorre-Esteves M, Pérez-Torres M, Torres-Lugo M, Rinaldi C. Preparation of epidermal growth factor (EGF) conjugated iron oxide nanoparticles and their internalization into colon cancer cells. J Magn Magn Mater 2010;322:2244–50
- Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, et al. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. Chem Med Chem 2008;3:1311–15
- Yu MK, Kim D, Lee I-H, So J-S, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small 2011;7:2241–9
- Bamrungsap S, Shukoor MI, Chen T, Sefah K, Tan W. Detection of lysozyme magnetic relaxation switches based on aptamer-functionalized superparamagnetic nanoparticles. Anal Chem 2011;83:7795–9
- Bamrungsap S, Chen T, Shukoor MI, Chen Z, Sefah K, Chen Y, Tan W. Pattern recognition of cancer cells using aptamer-conjugated magnetic nanoparticles. ACS Nano 2012;6:3974–81
- Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomedicine 2004;17:484–99
- Yang L, Mao H, Cao ZH, Wang YA, Peng XH, Wang XX, et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009;136:1514–25
- Hadjipanayis CG, Machaidze R, Kaluzova M, Wang LY, Schuette AJ, Chen HW, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 2010;70:6303–12
- Chen TJ, Cheng TH, Chen CY, Hsu SCN, Cheng TL, Liu GC, Wang YM. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem 2009;14:253–60
- Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, DeNardo GL. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res 2005;11:S7093–103
- Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med Hypotheses 1979;5:83–102
- Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int J Hyperthermia 2002;18:194–202
- Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. J Applied Phys 2006;100:054305
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol 2010;5:602–6
- Polo-Corrales L, Rinaldi C. Monitoring iron oxide nanoparticle surface temperature in an alternating magnetic field using thermoresponsive fluorescent polymers. J Applied Phys 2012;111:07B334
- Uchiyama S, Matsumura Y, de Silva AP, Iwai K. Fluorescent molecular thermometers based on polymers showing temperature-induced phase transitions and labeled with polarity-responsive benzofurazans. Anal Chem 2003;75:5926–35
- Amstad E, Kohlbrecher J, Müller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett 2011;11:1664–70
- Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, Torres-Lugo M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int J Nanomedicine 2011;6:373–80
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009;25:3–20
- Lee JS, Rodriguez-Luccioni HL, Méndez J, Sood AK, Lpez-Berestein G, Rinaldi C, Torres-Lugo M. Hyperthermia induced by magnetic nanoparticles improves the effectiveness of the anticancer drug cis-diamminedichloroplatinum. J Nanosci Nanotechnol 2011;11:4153–7
- Alvarez-Berrios MP, Castillo A, Mendez J, Soto O, Rinaldi C, Torres-Lugo M. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int J Nanomedicine 2013;8:1003–13
- Lepock JR. Involvement of membranes in cellular responses to hyperthermia. Radiat Res 1982;92:433–8
- Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano 2013;7:5091–101
- Kepp O, Galluzzi L, Lipinski M, Yuan JY, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov 2011;10:221–37
