Abstract
Purpose: The aim of this review is to evaluate the value of ultrasound (US)-guided high intensity focused ultrasound (HIFU) ablation in the treatment of primary malignant tumours of the bony pelvis. Materials and methods: Eleven patients with primary malignant tumours of the bony pelvis received US-guided HIFU ablation. The maximum tumour size ranged from 5.6 to 25.0 cm (median 10.5 cm). Treatment was curative in four patients and palliative in seven patients. During follow-up, the effectiveness of HIFU ablation was assessed by contrast-enhanced magnetic resonance (MR). Results: Significant coagulative necrosis was obtained in all patients after scheduled HIFU ablations; the volume ablation ratio was 86.7% ± 12.5% (range 65–100%). Complete tumour necrosis was achieved in all patients receiving curative HIFU ablation. No major complications were encountered. No patients died of local tumour progression during follow-up. Conclusions: US-guided HIFU ablation may be a safe and effective minimally invasive technique for the local treatment of primary malignant tumours of the bony pelvis.
Introduction
Approximately 10–15% of all primary malignant bone tumours are located in the pelvis, including a variety of pathological types, such as osteosarcoma, chondrosarcoma, giant cell tumour and Ewing’s sarcoma [Citation1,Citation2]. These tumours are often large and present late. Because of the complexity of pelvic anatomy and the extent of tumour growth, management of primary malignant tumours of the bony pelvis remains one of the greatest challenges in orthopaedic oncology [Citation3].
Hemipelvectomy has been the accepted surgical treatment of primary malignant tumours of the bony pelvis for many years. However, it was associated with a significant percentage of complications and a devastating functional and psychological outcome [Citation1–6]. Local recurrence after surgery was not rare, and further resection may be extremely difficult. Therefore, a safe and effective minimally invasive treatment would be valuable to control local tumour progression in these tumours.
High intensity focused ultrasound (HIFU) ablation is a new minimally invasive technique for the treatment of solid tumours [Citation7]. Under imaging guidance, coagulative necrosis can be induced at depth in the focal area without damaging the overlying tissue. In the past decade it has been successfully used for the treatment of a wide variety of tumours [Citation8–14], yielding effective local tumour control. However, to our knowledge, no study has focused on HIFU ablation of malignant tumours originating from the bony pelvis. Thus, the purpose of this study was to assess ultrasound (US)-guided HIFU ablation in the treatment of primary malignant tumours of the bony pelvis.
Materials and methods
Patients
From February 2002 to January 2011, eleven patients with pathologically proven primary malignant tumours of the bony pelvis were treated by US-guided HIFU ablation in our department. The malignancy was established by previous surgery and/or US-guided core-needle biopsy before treatment. This study was approved by the ethics committee of our hospital. All patients signed informed consent at enrolment.
Multidisciplinary discussions were held before treatment. The tumours were considered unresectable in seven patients and resectable in four patients by experienced surgeons. All patients with resectable tumours refused surgery. HIFU was chosen as the preferred method for local treatment of the bony pelvic tumours by consensus. The inclusion criteria for HIFU ablation were as follows: (1) the patient either refused to undergo surgery or was not a candidate for surgery, (2) no other standard forms of local treatment remained, (3) no critical structures such as bowel were in the planned acoustic pathway. The exclusion criteria were (1) invasion of tumour into the skin, (2) extensive scar in the planned pathway of HIFU, (3) thickened skin due to previous radiation therapy. Extensive scars and thickened skin in the planned pathway of HIFU was considered a contraindication because they could absorb too much energy during HIFU exposure which may result in severe skin burns. Neither metastasis nor chemotherapy was considered an exclusion criterion. Patients with tumours susceptible to chemotherapy received two to four cycles of neoadjuvant chemotherapy before HIFU ablation.
There were five male patients and six female patients. The age of the patients ranged from 12 to 70 years (median 19 years). Of these, eight patients had tumours involving the ilium, one patient had a tumour involving the ischium, two patients had tumours involving both the ilium and ischium (). Five patients had received previous surgical resection, eight patients had received chemotherapy before HIFU ablation. The maximum size of the tumour ranged from 5.6 to 25.0 cm (median 10.5 cm). Before treatment, all patients had mild to moderate pain in the affected region, necessitating oral analgesics in six patients.
Table I. Patient and tumour characteristics.
Equipment
A HIFU system (model JC; Chongqing Haifu Technology, Chongqing, China) was used in this study. It has three major parts: a therapeutic transducer located in a degassed water tank, a 3.5–5.0 MHz convex US imaging probe situated in the centre of the therapeutic transducer, and a computer-controlled system for firing and controlling the movement of the therapeutic transducer. The therapeutic transducer had a diameter of 20 cm and a focal length of 150 mm operating at a frequency of 0.9 MHz. The focal region is 9.8 mm along the beam axis and 1.3 mm in the transverse direction.
HIFU ablation procedures
HIFU ablation was performed under general anaesthesia by an experienced physician (W.W.). Patients were carefully positioned – either prone, supine, or on the side according to the tumour location – so that the skin overlying the tumour could be easily placed in contact with the membrane enclosing the degassed water. When ablating the pelvic portion of the tumour, degassed water balloons were placed on the skin to push the bowel away from the acoustic pathway. The locations of the tumour and surrounding tissue were identified on US, guided by previously obtained computerised tomography (CT and magnetic resonance (MR) images. The targeted region was divided into sections along the transverse axis of the diseased bone by real-time US, with a 5–10 mm separation between the sections to ensure reliable coverage of the planned ablation region. Four patients were treated with a curative aim; seven patients were treated with a palliative aim because of large tumour size or proximity to critical structures. For curative treatment, both the tumour and 2 cm of adjacent normal tissue were ablated. For palliative treatment, the target region did not extend beyond the tumour margin. HIFU energy with a power output of 70–150 W was intermittently exposed. A power output of 70 W was used first, if no echogenic changes occurred on US, the power output was gradually increased at an increment of 20 W until the echogenic changes occurred inside the tumour. Each energy exposure lasted for 1–2 seconds separated by 2–3 seconds. The ablation was repeated section by section, from the deep to shallow regions until the planned target regions were ablated. For large tumours, HIFU ablation was repeated in several sessions to minimise collateral thermal damage to the overlying tissue in the acoustic pathway.
Post-treatment follow-up
After HIFU ablation, patients were observed for 2–3 h for possible complications. They were discharged within 3 days after treatment if no major complications were encountered. After scheduled HIFU ablations, patients entered the follow-up protocol which consisted of contrast-enhanced MR at 1, 3, and 6 months and every 6–7 months thereafter. If recurrent tumour was found or the residual tumour enlarged significantly during follow-up, further HIFU ablation was planned if the patient still met the inclusion criteria.
Results
US-guided HIFU ablation was performed uneventfully in all patients. Each ablation session lasted for 1–4 h. Immediately after treatment, the skin overlying the tumour became slightly swollen in all patients; small blisters were seen in two patients requiring no medication. Mild to moderate pain was encountered in all patients after treatment, requiring oral analgesics in three patients. The pain settled within three days after the treatment. During follow-up, the pain in the affected region was significantly alleviated in all patients after HIFU ablation; analgesic medication was discontinued in all patients after scheduled HIFU ablation sessions. No major complications such as severe skin burn, infection or nerve damage were encountered in this study.
After HIFU treatment, a non-perfused ablation region with clear margins was observed in all patients on contrast-enhanced MR. The ablation region enveloped the entire tumour in all patients receiving curative treatment (). In patients receiving palliative treatment, a significant part of the tumour was successfully ablated after scheduled HIFU ablations (). The volume ablation ratio measured by three-dimensional contrast-enhanced MR was 86.7 ± 12.5% (range 65–100%) after scheduled HIFU ablations.
Figure 1. Transverse MR in a 19-year-old girl with Ewing’s sarcoma in the left ilium who received curative HIFU ablation. (A) The tumour margin (arrow) was shown on T2-weighted MR before HIFU ablation. (B) The tumour (arrow) showed rich enhancement on T1-weighted contrast-enhanced MR before HIFU ablation. (C) One month after HIFU ablation, the ablation area (arrow) enveloped the entire tumour, which showed no enhancement on T1-weighted contrast-enhanced MR. (D) One year after HIFU ablation no enhancement was found in the ablation area (arrow) on T1-weighted contrast-enhanced MR.
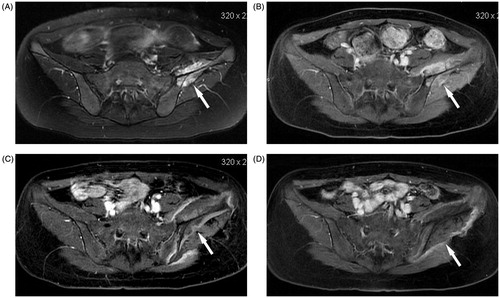
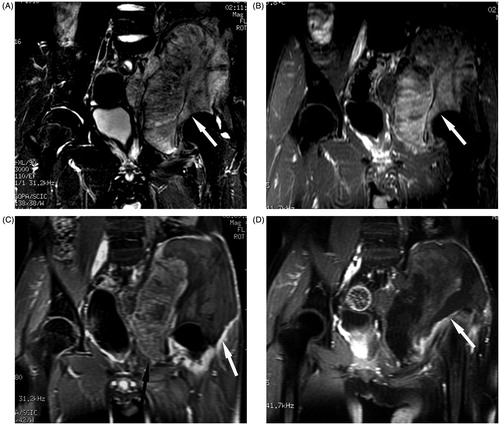
Figure 2. Coronal MR in a 15-year-old boy with osteosarcoma in the left ilium who received palliative HIFU ablation. (A) T2-weighted MR of the tumour (arrow) before HIFU ablation. (B) The tumour showed evident enhancement on T1-weighted contrast-enhanced MR before HIFU ablation. (C) After one HIFU ablation session, the ablation area showed no enhancement (white arrow), the residual tumour (black arrow) still had enhancement. (D) After the second HIFU ablation session, almost the entire tumour showed no enhancement (arrow).
Patients with tumours sensitive to chemotherapy also received three to four cycles of chemotherapy after treatment. The patients were followed up until December 2012. In a median follow-up of 22 months (range 11–154 months), seven patients receiving palliative ablation died of metastatic disease. Enlargement of residual tumour was observed in all patients receiving palliative HIFU ablations 6–24 months after study entry. Four patients who received palliative HIFU treatment had additional HIFU ablations. The remaining four patients receiving curative ablation are still alive. Local recurrence was observed in one patient receiving curative HIFU ablation, which was retreated by additional HIFU ablation; no local recurrence was observed thereafter.
Discussion
Extensive en bloc tumour resections had been considered the most important curative treatment for primary malignant tumours of the bony pelvis. However, surgery is highly demanding because of the irregular and complex shape of the bony pelvis, numerous muscle attachments, and the proximity of major blood vessels, nerves and visceral organs [Citation1–6]. The surgery was associated with a high rate of morbidity and long hospital stay. The quality of life was significantly impaired even after limb-salvaging resections. Therefore, new techniques need to be developed for the treatment of primary malignant tumours of the bony pelvis.
As a minimally invasive technique, HIFU ablation requires no incision or insertion of therapeutic applicators. It can induce large-volume tumour necrosis without damaging the overlying normal tissue. The treatment is repeatable if necessary. These properties make HIFU ablation a good candidate for treating solid tumours with high surgical risks [Citation7–10].
Because ultrasound beams are strongly reflected and attenuated by bone, it was initially believed that HIFU could not penetrate bone and would not be suitable for treating bone malignancy. However, as the bone cortex has a high acoustic absorption and low thermal conductivity, it is possible to use a relatively low level of HIFU energy to achieve localised coagulative necrosis of bone malignancy without damaging adjacent tissue [Citation11–13].
In this study, HIFU ablation appeared to be safe and effective for the treatment of primary malignant tumours of the bony pelvis. Significant coagulative necrosis was obtained after scheduled ablations in all patients. No major complications were encountered. Complete tumour necrosis was achieved in four patients with a relatively small tumour size, suggesting HIFU ablation may be a curative treatment in selected patients. Significant tumour necrosis was obtained in all patients with large inoperable tumours, the local tumour progression could be effectively controlled by repeated HIFU ablation, which suggested that HIFU ablation may be used as a palliative limb-salvaging treatment for inoperable primary malignant tumours of the bony pelvis. As HIFU ablation could be repeated, it was advantageous for the treatment of recurrent tumours.
This study was limited by its retrospective nature, the small number of subjects, and limited subtypes of tumours. A cost–benefit analysis was not performed. Further prospective studies are warranted to enrol more patients with pelvic tumours in different anatomical locations for better assessment of the value of HIFU ablation in the treatment of primary malignant tumour of the bony pelvis. Additional studies to compare HIFU with other available techniques such as limb-salvaging surgery would be important for rational selection of treatment in individual patients.
Conclusion
In conclusion, US-guided HIFU ablation may be a safe and effective minimally invasive technique for the local treatment of primary malignant tumours of the bony pelvis.
Declaration of interest
The authors of this article have no potential conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Aboulafia AJ, Malawer MM. Surgical management of pelvic and extremity sarcoma. Cancer 1993;71:3358–66
- Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after excision of malignant tumors. J Bone Joint Surg 1997;79:773–9
- Mavrogenis AF, Soultanis K, Patapis P, Guerra G, Fabbri N, Ruggieri P, et al. Pelvic resections. Orthopedics 2012;35:e232–43
- Dominkus M, Darwish E, Funovics P. Reconstruction of the pelvis after resection of malignant bone tumours in children and adolescents. Recent Results Cancer Res 2009;179:85–111
- Hillmann A, Hoffmann C, Gosheger G, Rödl R, Winkelmann W, Ozaki T. Tumors of the pelvis: Complications after reconstruction. Arch Orthop Trauma Surg 2003;123:340–4
- Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop Scand 1996;67:333–8
- Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: Current potential and oncologic applications. Am J Roentgenol 2008;190:191–9
- Chapman A, Harr G. Thermal ablation of uterine fibroids using MR-guided focused ultrasound – A truly non-invasive treatment modality. Eur Radiol 2007;17:2505–11
- Kennedy JE, Wu F, ter Harr GR, Gleeson FV, Phillips RR, Middleton MR. High-intensity focused ultrasound for the treatment of liver tumors. Ultrasonics 2004;42:931–5
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer. Radiology 2005;236:1034–40
- Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: Effective treatment with high-intensity focused ultrasound ablation. Radiology 2010;255:967–78
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging – Guided focused ultrasound. Radiology 2008;249:355–63
- Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010;116:3934–42
- Wang Y, Wang W, Tang J. Ultrasound-guided high-intensity focused ultrasound treatment for extra-abdominal desmoid tumours: Preliminary results. Int J Hyperthemia 2011;27:648–53
