Abstract
The development of phased array transducers and their integration with magnetic resonance (MR) guidance and thermal monitoring has established transcranial MR-guided focused ultrasound (tcMRgFUS) as an attractive non-invasive modality for neurosurgical interventions. The presence of the skull, however, compromises the efficiency of transcranial FUS (tcFUS) therapy, as its heterogeneous nature and acoustic characteristics induce significant phase aberrations and energy attenuation, especially at the higher acoustic frequencies employed in tcFUS thermal therapy. These aberrations may distort and shift the acoustic focus as well as induce heating at the patient’s scalp and skull bone. Phased array transducers feature hundreds of elements that can be driven individually, each with its own phase and amplitude. This feature allows for compensation of skull-induced aberrations by calculation and application of appropriate phase and amplitude corrections. In this paper, we illustrate the importance of precise refocusing and provide a comprehensive review of the wide variety of numerical and experimental techniques that have been used to estimate these corrections.
Introduction
Since the earliest reports on the use of high intensity focused ultrasound (HIFU) to create lesions deep inside animal brains by Lynn et al. in 1942 [Citation1,Citation2], this promising technology has evolved and matured significantly. Groundbreaking research by Fry et al. in the 1950s was the first demonstration of focused ultrasound (FUS) used for brain tumour surgery and functional neurosurgery in animals and humans [Citation3–6], despite the necessity for craniotomy and the absence of suitable modalities for intervention monitoring. Since then, this technology has seen major advances and increased acceptance by the scientific community.
The most notable technological breakthrough in the field of transcranial FUS (tcFUS) surgery in the past 20 years, has been the development in the 1990s of phased array transducers that are manufactured to be magnetic resonance (MR) compatible, i.e. non-magnetic and shielded from radiofrequency (RF) radiation. Combined with the utilisation of the temperature-dependent proton resonance frequency (PRF) shift, which allows for MR thermometry (MRT), these advances have yielded MR-guided FUS (MRgFUS) technology, which enables non-invasive, image-guided, and temperature-monitored transcranial MRgFUS (tcMRgFUS) interventions [Citation7–11].
The history of FUS neurosurgical interventions is briefly summarised in . A comprehensive review of the physics, the underlying mechanisms and the historical clinical applications associated with FUS and MRgFUS are beyond the scope of this article; however, the reader is directed to a number of recent review articles on the topic [Citation12–18], as well as some books on US [Citation19–21] and ultrasonic surgical interventions [Citation22,Citation23].
Figure 1. A historical timeline of FUS neurosurgical intervention [Citation76].
![Figure 1. A historical timeline of FUS neurosurgical intervention [Citation76].](/cms/asset/d1ccb8e8-7863-48a5-859b-6d89b513446c/ihyt_a_861519_f0001_b.jpg)
Experimental prototypes and commercial systems
Technological advances have led to the development and evaluation of several tcFUS systems, and a multitude of small, multi-element arrays have been reported and used in in vitro and ex vivo transcranial sonication experiments [Citation10,Citation11,Citation24–28]. Those systems, however, were not manufactured for MR compatibility, a characteristic required for clinical in vivo applications [Citation29], and therefore are not considered here.
tcFUS systems are typically hemispherically shaped ultrasonic arrays that feature from several hundred to over one thousand elements, each of which is driven by an independent RF signal with computer-controlled amplitude and phase. Clement et al. [Citation30,Citation31] presented a 500-element hemispherical array with a diameter of 30 cm, operating at 700–800 kHz. Chauvet et al. [Citation32] introduced a high power 512-element system operating at 1 MHz, the highest frequency utilised for transcranial brain treatment. These prototypes have been employed in a wide range of experimental tcFUS studies, many of which are reviewed in this paper.
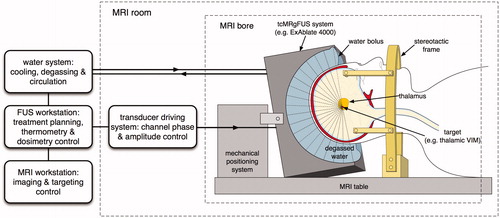
Currently, ExAblate® 4000 (InSightec, Haifa, Israel), the only Conformité Européenne (CE)-certified tcFUS system in clinical use, is under evaluation for clinical safety and efficacy in functional neurosurgery and brain tumour ablation (see and ). The ExAblate® 4000 applicator consists of a 30-cm diameter hemispherical phased array transducer with 1024 elements operating at either 230 or 650 kHz. This device is driven by a 1024-channel RF-amplifier, which allows phase and amplitude control of each transducer element in the phased array.
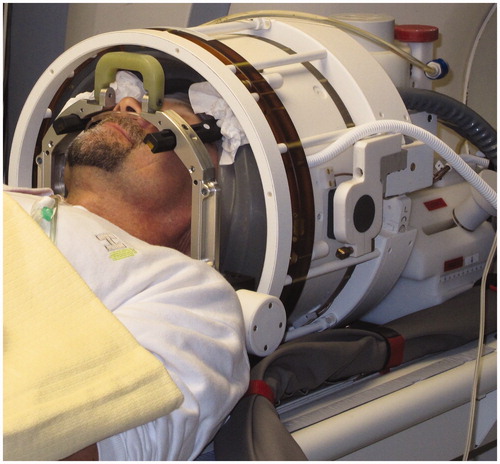
Figure 3. Schematic diagram of a tcFUS treatment with a phased array transducer like the ExAblate® 4000. The patient’s head is fully shaved, fixed to the table with a stereotactic frame and positioned in the helmet-like cavity of the transducer, which is filled with circulating, degassed water for scalp cooling and held back with a flexible membrane seal. The entire system is MR-compatible and integrated into a standard MR system.
Current state of clinical tcFUS brain interventions
tcFUS has been increasingly applied in the field of brain tumour surgery and functional neurosurgery over the past few years with encouraging results. Medical centres around the world are exploring this technology and setting up clinical trials for the treatment of neuropathic pain, essential tremor, movement disorders, centrally located brain tumours, and Parkinson’s disease [Citation18]. Jolesz et al. [Citation33] treated three patients with glioblastomas using multiple ultrasound exposures with real-time MRT feedback to ensure accurate targeting. These treatments demonstrated technical feasibility, but were not therapeutically successful as they could not induce ablation of the tumour tissue. Martin et al. [Citation34] were the first to use tcFUS to treat chronic neuropathic pain in nine patients by means of selective medial thalamotomy. tcFUS thalamotomy is being performed by several groups to treat patients with essential tremor: Elias et al. in the USA [Citation35] recently completed a phase I clinical trial, Jeanmonod and co-workers in Switzerland [Citation36] (no treatment results published so far), and Lozano and co-workers in Canada [Citation37]. In Korea, Chang and co-workers use tcFUS to treat patients with obsessive–compulsive disorder (OCD).
Reported clinical complications and limitations of tcFUS
Despite the benefits of tcFUS, complications have been reported in human trials. In the case of a patient treated for a thalamic glioma, the tumour was ablated but the patient died from delayed intraventricular haemorrhage on the contralateral side five days after the treatment [Citation16,Citation22]. An in-depth investigation of the incident by InSightec and the Food and Drug Administration (FDA), however, could establish neither a cause nor a causal relation between the treatment and this event. In a publication by Jeanmonod et al. [Citation38], on the clinical and neurophysiological results over one year after the neuropathic pain treatments carried out by the Martin et al. group [Citation34], a complication in one of the patients was reported. In the case of that patient an 8–10-mm bleed and ischaemic changes were detected immediately after the treatment. These were causal to motor symptoms of right-side motor hemineglect with dysmetria of the arm and leg, and dysarthria. As reported, the symptoms had entirely disappeared one year later, but the event was causally related to the treatment during an extended investigation. However, no cause for this incident could be established.
Even though tcFUS shows potential for the non-invasive treatment of malignancies and areas deep inside the brain, the technology exhibits certain physical limitations. Ultrasound cannot penetrate air-filled viscera, causing the high energy sound waves to be reflected at tissue-air interfaces, possibly producing unwanted high temperature increases in those locations. Similar, though not as pronounced, effects are observed at bone–tissue interfaces due to the large acoustic impedance mismatch between bone and soft tissue [Citation12,Citation16]. Moreover, the intracranial treatment envelope is limited due to physical restrictions imposed by the presence of the skull, making it impossible to target areas adjacent to the skull surface [Citation18]. Furthermore, treatment times, due to the time consuming MR imaging procedures, typically require many hours of patient immobility. Lastly, the thermal ablation of target volumes larger than a few millimeters requires multiple sonications, each of which must be followed by a cooling period of several minutes due to the limited cooling capacity of the brain and the cerebrospinal fluid (CSF) [Citation17,Citation18,Citation34].
Overview of compensation techniques for skull-induced aberrations
The reported complications and inherent limitations of tcFUS indicate that precise targeting and effective focusing in the treatment region are crucial. Also, it would be desirable to predict, and subsequently avoid, secondary effects of the procedure, such as standing pressure waves – especially in the case of low frequency ultrasound or the long sonications used in tcFUS thermal therapy – and generation of secondary hot spots at bone–tissue and air–tissue interfaces. Currently, however, this is possible only by numerically modelling the entire procedure to estimate 3D pressure and temperature distributions.
The foremost barrier when treating the brain with tcFUS is the presence of the skull. When compared to soft tissues, bone exhibits twice the speed of sound and density, and at least an order of magnitude higher attenuation [Citation39], absorbing a large percentage of the acoustic energy, especially at higher frequencies (e.g. 650–1000 kHz) [Citation21,Citation40]. Combined with the complex heterogeneous nature of the skull, which is multi-layered, liquid-filled and porous, the presence of the skull causes distortion of the acoustic focus, focal shift, and significant decrease of the thermal gain – i.e. the ratio of energy deposition at the focus to the energy deposition on the scalp and skull-bone – thus compromising the treatment (see ).
Figure 4. Simulated acoustic pressure distributions from an idealised model of the ExAblate® 4000 system applicator in the presence of a human head model segmented from MR data [Citation77,Citation78] on planes through the location of the geometric focus. (Left) Pressure distribution in the presence of skull-induced aberrations, including shifting and distortion of the focal spot, significant energy deposition on the skull bone and scalp, as well as the potential generation of secondary foci and standing waves. (Right) Pressure distribution after application of a ‘virtual source’ phase-correction approach.
![Figure 4. Simulated acoustic pressure distributions from an idealised model of the ExAblate® 4000 system applicator in the presence of a human head model segmented from MR data [Citation77,Citation78] on planes through the location of the geometric focus. (Left) Pressure distribution in the presence of skull-induced aberrations, including shifting and distortion of the focal spot, significant energy deposition on the skull bone and scalp, as well as the potential generation of secondary foci and standing waves. (Right) Pressure distribution after application of a ‘virtual source’ phase-correction approach.](/cms/asset/be121916-3ac6-4cdf-894c-12257ffc070a/ihyt_a_861519_f0004_b.jpg)
The solution to the above problem is to use large, multi-element – several hundred to over a thousand – transducer arrays, where each element is driven with an optimised phase and amplitude. The large transducer surface permits the acoustic energy to be distributed on the skull surface, thus diminishing the local deposition on the scalp and bone. In addition, the ability to drive the transducer elements individually with appropriately corrected phases and amplitudes allows compensation of focal distortion effects.
Significant effort has been made by many research teams to calculate and/or measure these phase, and, optionally, amplitude corrections by various approaches. One non-craniotomy approach used to overcome the limitations imposed by the skull involves the use of low frequency ultrasound in the order of 250 kHz, where the acoustic wavelength in bone (∼12 mm) is comparable to the skull thickness, which reduces the impact of the aberrations.
The success of this approach has been demonstrated [Citation41]; however, it is known that lowering the ultrasonic frequency exhibits certain drawbacks. The larger acoustic wavelength results in an increased focal size that causes larger lesions. The cavitation threshold, which is proportional to frequency, consequently decreases, thus increasing the risk of unwanted cavitation [Citation42,Citation43]. Finally, since the attenuation coefficient in soft tissue is lower, energy deposition is reduced with a consequent need for increased acoustic intensities to create lesions in the targeted area, which again increases the risk of cavitation. Because of these issues, the clinical use of tcFUS for thermal ablation is performed at acoustic frequencies in the order of 600–1000 MHz. At these frequencies, however, skull-induced aberrations are significant, making precise aberration corrections and focusing mandatory. An overview of techniques tailored to compensate for these skull-induced aberrations and improve transcranial focusing at higher frequencies is presented in this paper.
Time reversal techniques
The phase conjugate mirror (PCM) approach for phase aberration correction, which involves insonation of an acoustically reflective target and the use of the transducer elements as receivers to measure relative time delays in the different elements, was first proposed for transcranial ultrasonic imaging by Phillips et al. [Citation44]. After inversion, the appropriate phase shifts are computed to achieve in-phase superposition [Citation45]. The time reversal approach was initially presented as a wave mirroring method by Fink et al. [Citation46]. Instead of merely detecting and inverting the phases of reflected waves, the entire temporal pressure waveform is stored, reversed, and re-emitted to focus on a reflective target (convergent waves) that behaves as a source when insonated (divergent waves), providing both phase and amplitude corrections. The main advantage of the time reversal over the PCM approach is that it can be applied to broadband acoustic signals and is not limited to harmonic waves. This method was first applied to lithotripsy, where such reflective sources (e.g. kidney stones) occur naturally in the body [Citation46]. Later, for application of this approach to brain therapy involving transcranial sonication, it was proposed that the lack of such a reflector in the brain could be compensated for by implanting an artificial source or sensor in the targeted area, e.g. a monoelement transducer or a hydrophone in the tumour [Citation47,Citation48]. This method saw further improvement (e.g. amplitude compensation [Citation8]) in the following years, although a detailed historical overview is outside the scope of this article. Fink et al. [Citation48] published a review of time reversal techniques and its applications in acoustics in general, ranging from imaging to lithotripsy and brain therapy, and another brief review of the time reversal approaches can be found in Vignon et al. [Citation49]. Three types of approaches employing time reversal are presented below.
Implanted hydrophone
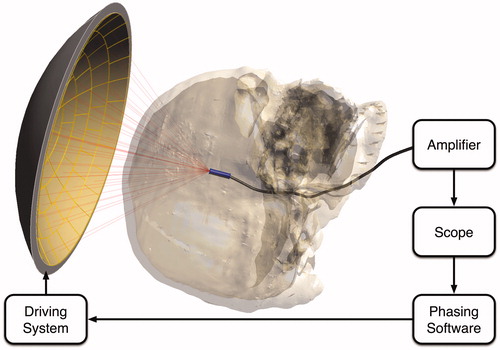
Hynynen et al. [Citation9] presented an ‘implanted’ hydrophone approach, where in ex vivo skull studies, a hydrophone was placed at the location of the desired focus. After placement, every element of the transducer array was powered separately, and this procedure was followed sequentially for all involved elements. With the hydrophone, the phase shifts induced by the presence of the skull were measured, inverted and applied to all elements in the array to correct for the phase shifts and make the waves converge at the focal spot in phase, resulting in maximum deposited acoustic energy through constructive interference. A schematic diagram of this approach is shown in .
Figure 5. Schematic diagram of the implanted hydrophone correction technique, which is considered the ‘gold standard’ in experimental studies. A hydrophone implanted at the intended target location records the complex pressure for each element of a phased array transducer activated in a sequential manner. The recorded phases are processed and inverted before being applied back to the transducer elements to correct for the skull aberrations and refocus at the target location.
While this approach is believed to provide the best possible aberration correction – and therefore maximum energy deposition compared to any other focusing approach – it exhibits two substantial limitations. First, the approach is invasive, requiring the insertion of a receiver hydrophone in the brain when applied in vivo. Second, to produce a new focal point in another location, e.g. when overlapping multiple smaller lesions for the treatment of a large tumour volume, the receiver would need to be moved and the procedure repeated, which would increase both the treatment time and the risk of complications.
In a later study, Clement and Hynynen [Citation24] from the same group, expanded upon this method to overcome the latter of the two restrictions by introducing a beam-steering approach. The method still depends on the use of a small catheter-inserted hydrophone at the location of the intended focus and measurement of the phase shifts for all array elements, as described above. However, when the initial pressure measurements are performed at locations remote from the focus, in the absence of the ex vivo skull, it was possible to analytically calculate new phase corrections for those remote locations and reconstruct the focus after the skull was re-introduced. Thus, this study demonstrated the ability to steer the focus without repositioning the implanted hydrophone within the skull. This approach produced clinically viable acoustic intensity values and temperature increases within a 25-mm radius of the catheter location as determined by a ∼50% drop in acoustic intensity.
It should be mentioned that the ‘refocusing’ strategy in Clement and Hynynen [Citation24] was previously introduced by Seip et al. [Citation50], where similar dynamic refocusing approaches and resulting ranges were reported, but only in soft tissue. Clement and Hynynen [Citation24] verified this approach in the presence of an ex vivo skull, and in vivo in Hynynen et al. [Citation51]. This hydrophone approach is still considered the ‘gold standard’ in experimental studies. It has also been used in recent ex vivo studies, e.g. Song et al. [Citation52], as well as others. The study by Pernot et al. [Citation26] demonstrated in vivo the ability to produce thermal lesions deep inside the brains of 10 sheep with implanted hydrophones by means of time reversal techniques.
Acoustic stars
In an attempt to compensate for the absence of passive or active sources in the brain with a non-invasive technique, Pernot et al. [Citation53] suggested the use of two ultrasonic arrays: first, a high power array to generate a short, intense ultrasonic pulse focused in one area of the brain to create a cavitation bubble, the collapse of which generates a shock wave that is detected by a second ultrasonic imaging array. With this application of a time reversal approach, the authors reported successful refocusing in an in vitro study involving an artificial rubber aberrator placed in front of a tissue phantom, and claimed that, as this approach generated only a single cavitation bubble, it should be possible to prevent the occurrence of any tissue damage.
This method was improved and applied to focusing in a tissue-mimicking phantom contained in an ex vivo half skull in a later study [Citation28]. Instead of two transducer arrays, a single cylindrical array was employed to induce cavitation, record the generated shock wave, and focus on the target through time reversal. The presence of the skull, however, caused strong wave aberrations and did not initially allow the pressure amplitudes to rise high enough and induce cavitation at the target. To compensate, initial aberration corrections were computed through a 3D finite-difference time-domain (FDTD) simulation based on CT image data (first presented in Aubry et al. [Citation11]) with a ‘virtual’ point source at the location of the desired focus as described below. This initial focusing allowed for the induction of cavitation and application of the focusing approach presented in the first study, whereby 97% of the implanted hydrophone-based reference pressure amplitude was restored at the focal location.
To avoid the high pressures required to induce cavitation, a technique was developed [Citation54] that involves injection of liquid droplets into the target volume that are consequently vaporised with a high frequency, high power ultrasonic array to create cavitation bubbles before time reversal-based aberration correction is performed as described in Pernot et al. [Citation53].
Virtual source
The recent increase in computational power has allowed for efficient simulations of acoustic wave propagation, which has yielded a ‘virtual’ version of the time reversal approach, first applied to transcranial sonication by Marquet et al. [Citation27]. In this study, CT image data from an ex vivo primate and human skulls were segmented and used as input in a 3D FDTD code to solve a linear acoustic wave equation. In a simulation of this set-up with a ‘virtual’ point source placed at the desired focus location, the temporal pressure waveforms at the level of the transducer elements were recorded and transmitted experimentally with a 300-element spherical array transducer system after time reversal: precise focusing, with a positioning error of 0.7 mm at 90% of the peak pressure amplitude recorded with an implanted hydrophone, was reported.
A similar approach was recently reported involving the sonication of an ex vivo skull with the ExAblate® 4000 system operating at 230 kHz [Citation52]. In this study, linear acoustic equations for propagation in soft tissues and elastic wave equations for propagation within the bone were used in an attempt to investigate standing wave effects during transcranial sonication; a point source was used at each of four desired focal locations to record the waves at the transducer element level, with only the phase of the imprinting waves inverted and without amplitude correction.
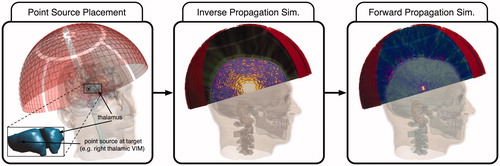
Many recent studies have employed this approach to correct for skull aberrations, e.g. the numerical studies on tcFUS by Pinton et al., in which a 3D FDTD linear acoustic solver was used to explore cavitation effects [Citation55], and where a non-linear 3D FDTD solver was utilised to investigate the impact of acoustic non-linearity [Citation56]. Alternative numerical approaches have recently been investigated, and have demonstrated a significant reduction in computational time. These include the demonstration of the use of a hybrid finite-different and phase-projection algorithm [Citation57], and one where a k-space numerical propagation model was used instead [Citation58]. In another recent study [Citation59] Leduc et al. employed the ‘virtual source’ approach with a 3D FDTD linear acoustic solver, not only to achieve refocusing at the intended target location, but also to eliminate secondary foci by iteratively placing additional point sources at those locations and inducing destructive interference in subsequent simulations. A schematic diagram of the ‘virtual source’ approach is shown in .
Figure 6. Schematic diagram showing the concept of the ‘virtual source’ correction technique. A point source is placed at the intended target location, here, in the right thalamic ventral intermediate nucleus (VIM). The elements of the phased array transducer are used as receivers, and an ‘inverse propagation’ simulation allows the elements to record the pressure waves as either the entire waveform or the complex pressure phasors. These recorded pressure waves are inverted – either reversed in time for waveforms, or conjugated for complex pressure phasors – and a forward propagation simulation or experiment yields refocusing at the intended target.
CT-based techniques
Analytical techniques
Clement and Hynynen [Citation60] employed computed tomography (CT) scans of ex vivo human skulls segmented to either a single bone layer or three distinct outer cortical, central trabecular, and inner cortical layers. The segmented models were assumed to exhibit either homogeneous speed of sound across a layer or an averaged ‘effective’ speed of sound based on CT-derived density variation information. Phase corrections – analytically calculated based on the thickness of the different skull layers, the wave frequency, and the speed of sound, either homogeneous or effective, in bone – were applied to each of the four skull segmentation types in 10 ex vivo skulls, and hydrophone-based measurements at the focal location yielded 63% and 76% (with and without density variation corrections) of the focal peak of the non-aberrated case in the absence of the skull.
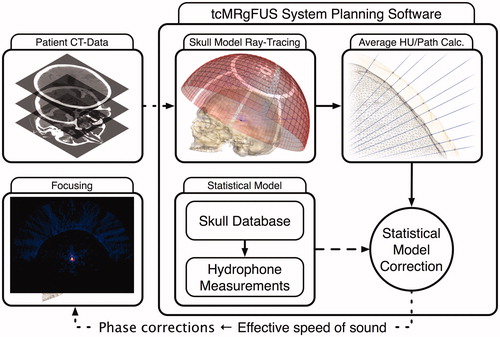
A slightly modified version of this approach is commonly employed today by modern tcFUS systems. CT scans and hydrophone measurements of multiple ex vivo skulls were used to derive a statistical model, which establishes a linear relation between an ‘effective’ speed of sound in bone and the Hounsfield units (HU). For every patient, a new CT scan is performed and the image data is used as input for the tcFUS system planning software. The skull is segmented as a single bone layer, and the software performs ray-tracing analysis on the ray cast from the element centre towards the intended target for each of the array elements to calculate the average HU along each ray’s path. The data is entered into the statistical model, from which an effective speed of sound is obtained and used to analytically calculate the phase correction for each element. This procedure is graphically depicted in .
Figure 7. Schematic diagram of a CT-based phase correction approach concept commonly employed in modern tcFUS systems. The patient’s CT scan is entered into the system’s planning software, which segments the skull as a single layer of bone and performs a ray-tracing analysis to calculate the average HU values along the path of the ray for each array element. These HU values are entered into an existing statistical model, based on measurements of ex vivo skulls, to yield an effective speed of sound, which can be used to calculate phase corrections and achieve re-focusing.
Simulation techniques
Clement and Hynynen [Citation25] used CT images of the skull to derive thickness, density, and geometry information, which was entered into a planar propagation numerical model. The model involves splitting the transducer into groups of elements, the acoustic fields of which are projected onto a plane directly above the skull, and an approach similar to the FFT-based angular spectrum method (ASM) employed in the FOCUS software [Citation61] is used to propagate those fields through the focus. The quality of this focusing approach was evaluated by comparing the acoustic focus created by the simulation-based corrections against an ideal case of hydrophone-based corrections, where the phase-shift of each transducer segment is measured at the intended focal location and applied to achieve focusing. The algorithm was applied to 10 ex vivo skulls to yield an average peak value of 45% compared to the ideal case. In a later study [Citation62], the same authors used the above approach with deliberately induced shear waves at the skull to enhance their propagation algorithm with longitudinal–shear wave conversion at the skull, to evaluate whether the transmission of focused ultrasound beams purely as shear waves is possible and advantageous. Results showed that the peak amplitude due to shear wave propagation was 35−55% smaller than the peak achieved with longitudinal waves. However, they reported that, due to the similar shear wave speed in water, soft tissues, and bone, these waves suffer less distortion. Thus, this shear transmission method could be used as an additional delivery strategy, especially for focusing at high incident angles, e.g. when focusing close to the skull surface where shear waves are prominent. The approach presented in Clement and Hynynen [Citation25], when applied to sonication of rabbit thigh muscle and brain tissue with an ex vivo human skull between the tissue and a 500-element hemispherical transducer, yielded consistent results [Citation51].
MR-ARFI techniques
MR acoustic radiation force imaging (MR-ARFI) [Citation63] is a recently developed technique that utilises MRI through motion-sensitive encoding MR gradients, similar to those used in MR elastography [Citation64,Citation65], to measure the micron-scale static tissue displacement induced by FUS waves as phase shifts in the resulting MR image [Citation66,Citation67]. Since this tissue displacement is proportional to the local acoustic intensity, i.e. the square of the acoustic pressure, MR-ARFI allows non-invasive pressure measurements anywhere in the insonated anatomy.
Larrat et al. [Citation68] proposed a method called ‘energy-based adaptive focusing’ in which MR-ARFI measurements are used to estimate the local tissue displacement induced by the acoustic radiation force of the beams at the location of the desired focus. With one of the transducer elements as a reference, four such acquisitions are performed for each of the other elements of the transducer to estimate the phase shift between that element and the chosen focal spot. Those calculated phase shifts are then inverted and applied to the transducer elements to achieve a strong focus. This approach was successfully applied on a simple phantom set-up [Citation68], in which a five-fold increase in intensity was reported. In a previous study [Citation67], the same research group applied this theoretical model to speckle-tracking obtained from pulse-echo ultrasound sequences to define tissue displacement in simplified phantoms containing reflective targets/scatterers. However, despite the success of this technique in a proof-of-concept experiment, the authors reported that ultrasound imaging cannot be accurately used in the presence of a medium which causes strong aberrations, e.g. the skull, which prompted the use of MR-ARFI [Citation68]. The same group recently published a study [Citation69] where this MR-ARFI approach, applied to a 512-element transcranial sonication system with human cadaver heads, resulted in a factor of 2.2 higher intensity at the focal spot compared to the non-phase-corrected sonication, and a factor of 1.5 compared to the focusing pattern achieved through CT-based aberration corrections. However, this case required over 10,000 sonications and MR-ARFI acquisitions, which amount to ∼2 h of measurements, to define the phase corrections for a single focal spot. This implies that the method, although promising, may not yet be appropriate for the clinical environment, especially for treatment of large volumes with multiple overlapping focal spots.
In an attempt to decrease the number of MR-ARFI acquisitions, Hertzberg et al. [Citation70] employed a similar approach: instead of calculating the phase correction for each element in reference to another, they grouped the transducer array elements and assigned a total of 26 random phases to each group. Subsequently, they performed MR-ARFI acquisitions for sonications with different phase shifts for each group to identify the optimal phases; i.e. the phases that produced the maximal displacement at the focal location. This approach was applied in vivo with the ExAblate® 4000 system to sonicate a porcine brain exposed by craniotomy, with a human skull between the animal tissue and the transducer to mimic human treatments. The maximum tissue displacements induced at the intended focus by the employed method were compared to those found with CT-based and hydrophone correction approaches and against the displacement in the absence of aberrations. The presented MR-ARFI approach yielded a 27% maximum displacement compared to the non-aberrated case, better than the 8–12% obtained with CT-image-based corrections, and was deemed comparable to the hydrophone approach, which yielded a 40% displacement.
Grissom et al. [Citation71] and Vyas et al. [Citation72] proposed the use of MR-ARFI measurements to improve the numerically calculated phase corrections obtained through unfocused simulations, i.e. random phases that disregard actual patient anatomy, using the Field II [Citation73] and the hybrid angular spectrum methods [Citation74], respectively. The proposed method allowed for successful refocusing when applied to a simple phantom set-up with a significantly smaller number of MR-ARFI acquisitions (4 and 1 respectively) when compared to the ‘energy-based adaptive focusing’ approach presented above.
The study by Kaye et al. [Citation66] is built on the method presented in Larrat et al. [Citation68], which requires four MR-ARFI acquisitions for each transducer element to measure the acoustic intensity at given locations in the brain. However, an alternative ultrasonic wave encoding, based on Zernike polynomials instead of Hadamard matrices, allowed 90% of the non-aberrated focal intensity to be achieved with less than 20% of the MR-ARFI acquisitions. The same study proposed the utilisation of a phase correction ‘database’ based on CT datasets of other patients, similar to the approach presented in Clement and Hynynen [Citation60], to extract an initial approximate phase correction set, which is then improved based on the MR-ARFI measurements.
Critical assessment
The techniques presented above range from purely analytical or numerical calculations of the required aberration corrections to entirely experimental approaches, with a varying degree of invasiveness, usability, and success. A qualitative comparison of the approaches presented can be seen in .
Table 1. A qualitative comparison of the presented approaches.
Highly effective time reversal techniques, like the implanted hydrophone and the ‘acoustic stars’, undoubtedly provide the highest refocusing quality achievable. In the case of the former, the only viable solution that would allow the clinical use of this technique would involve a catheter-inserted microscopic hydrophone that can be introduced in the vicinity of the intended focal region through the cerebral vascular network. However, this approach would be invasive and could even result in undesirable tissue damage, i.e. brain haemorrhage. Moreover, even when complemented with semi-analytical steering capabilities, as proposed in Clement and Hynynen [Citation24], the resulting steering range is limited to a couple of centimetres around the implanted hydrophone, after which the focusing quality deteriorates with distance. The ‘acoustic stars’ technique is a non-invasive alternative, and could theoretically be employed to achieve refocusing anywhere in the insonated soft tissue of the anatomy. However, as it depends on the administration of sonications exhibiting HIFU level pressure amplitudes, and acoustically induced microbubble cavitation, this approach may entail safety risks. It has been theorised that by focusing acoustic energy only in the intended target location, and with sonications gradually increasing in power, the technique should avoid uncontrolled cavitation outside that region. However, there exists no indication as to whether it may induce any of the tissue damage or disruption effects that have been associated with acoustic cavitation [Citation43,Citation75]. The aforementioned concerns and the absence of literature on the in vivo application of these techniques on humans suggest that these approaches are unlikely to be utilised in the clinical environment. Nonetheless, they remain an invaluable tool for in vitro and ex vivo experiments, feasibility studies, and the development of tcFUS systems.
Approaches based entirely on analytical calculations or simulations to derive phase and, optionally, amplitude corrections (see ‘Virtual source’ and ‘CT-based techniques’ above) are entirely non-invasive, do not require the presence of the patient, and are already being employed on modern tcFUS systems. However, despite their speed and ease of use, the purely analytical methods are inherently limited as they do not account for the entire range of wave propagation phenomena, unlike full-wave simulation-based techniques. Thus, these methods are not entirely effective for estimating the corrections required, resulting in a limited treatment envelope and focusing quality. Moreover, the existing statistical models utilised for these analytical approaches are based on measurements of a limited number of adult ex vivo skulls, which limits the applicability of the models, e.g. excluding paediatric patients.
Simulation-based techniques have shown great promise and will undoubtedly replace their analytical counterparts, since they allow modelling of the complex patient anatomy, most notably the skull, in far more detail than analytical approaches, potentially even in a patient-specific manner. Full-wave simulations can also account for most of the physical phenomena, e.g. reflections, refractions and attenuations, that occur during wave-propagation, while some models can even account for non-linear wave propagation, cavitation and shear waves. Simulation-based techniques are inherently amenable to permitting treatment planning and optimisation, e.g. through multiple simulations or even genetic optimisation techniques. They offer the possibility to predict and avoid secondary effects of the treatment, e.g. skull heating, the formation of secondary foci and unwanted cavitation. The complexity of acoustic wave propagation, however, in highly complex, heterogeneous anatomical structures, is a computationally intensive problem that requires huge amounts of computational resources to allow such simulations to run in viable time frames. The required resources and computational time increase even further at higher acoustic frequencies (e.g. 1 MHz), or when accounting for effects such as non-linearity and shear waves. The choice of the simulation technique typically involves a trade-off between physical accuracy, simulation time and computational resources, and requires validation of the chosen method. But the employment of high-end hardware and state-of-the-art parallelisation techniques is a promising track, and such simulations are now becoming feasible; multiple optimisation simulations that can be run overnight will soon be achievable with affordable hardware.
The MR-ARFI techniques also show promise and could prove invaluable for estimating required corrections. These techniques are entirely non-invasive and have demonstrated the ability to produce high quality focusing. In addition, they exhibit the unique characteristic of directly monitoring the quantity of interest, i.e. acoustic pressure, and offer the possibility of closed-loop control of transcranial sonication. Nonetheless, MR-ARFI techniques are still in an experimental stage and, in their current form, require hours of measurement in the presence of the patient and the careful management of the transducer element grouping and activation. Despite this, the promise of MR-ARFI aberration correction approaches captures the interest of many researchers, and may well prove to be a valuable clinical solution if technical advances are made that help overcome the aforementioned challenges.
Conclusions and outlook
Despite the inherent limitations of therapeutic ultrasound, and, in the case of tcFUS, the major problems caused by skull-induced aberrations, this modality has shown great promise in a number of neurosurgical interventions, including cancer treatment, motor disorders such as essential tremor and Parkinson’s disease, and improved drug delivery for treatment of conditions such as Alzheimer’s disease and multiple sclerosis. Consequently, medical centres throughout the world are establishing clinical trials to explore the capabilities of this technology.
Many different approaches to compensate for skull-induced aberrations have been reported over the years. Each has its own set of advantages and limitations which are discussed in this article. As the aberrations constitute the main obstacle to the efficacious employment of this technology, significant progress has been noted towards resolving this issue over the past decade, but further research is clearly required to establish an effective, efficient, and non-invasive procedure for tcFUS to be accepted by the clinical community.
In view of their non-invasive nature, flexibility, and the wealth of information offered by 3D simulations, their use will likely become increasingly common and they will be available for online treatment delivery adaptation and correction, e.g. by leveraging pre-computed solutions.
Declaration of interest
This work was financed by the Swiss National Centre of Competence in Research on Computer Aided and Image Guided Medical Interventions (NCCR COME). The authors alone are responsible for the content and writing of the paper.
References
- Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942;26:179–93
- Lynn JG, Zwemer RL, Chick AJ. The biological application of focused ultrasonic waves. Science 1942;96(2483):119–20
- Fry WJ, Fry F, Barnard J, Krumins R, Brennan J. Ultrasonic lesions in mammalian central nervous system. Science 1955;122(3168):517–18
- Fry WJ, Barnard JW, Fry FJ, Brennan JF. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med Rehabil 1955;34:413–23
- Fry WJ. Intense ultrasound in investigations of the central nervous system. Adv Biol Med Phys 1958;6:281–348
- Fry WJ, Fry F. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960;3:166–81
- Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr 1992;16:956–65
- Tanter M, Thomas JL, Fink M. Focusing and steering through absorbing and aberrating layers: Application to ultrasonic propagation through the skull. J Acoust Soc Am 1998;103:2403–10
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24:275–83
- Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol 2003;48:2577--89
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am 2003;113:84–93
- Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: Current potential and oncologic applications. Am J Roentgenol 2008;190:191–9
- Jagannathan J, Sanghvi NT, Crum LA, Yen CP, Medel R, Dumont AS, et al. High-intensity focused ultrasound surgery of the brain: Part 1 – A historical perspective with modern applications. Neurosurgery 2009;64:201–11
- Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med 2009;60:417–30
- Medel R, Monteith SJ, Elias WJ, Eames M, Snell J, Sheehan JP, et al. Magnetic resonance–guided focused ultrasound surgery: Part 2: A review of current and future applications. Neurosurgery 2012;71:755–63
- Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics 2010;50:221–9
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005;5:321–7
- Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery: A review. J Neurosurg 2013;118:215–21
- Cobbold R. Foundations of Biomedical Ultrasound. New York: Oxford University Press, 2006
- Azhari H. Basics of Biomedical Ultrasound for Engineers. Hoboken, NJ: Wiley, 2010
- Szabó TL. Diagnostic Ultrasound Imaging: Inside Out. Waltham, MA: Elsevier Academic Press, 2004
- Martin E, Jolesz FA. Noninvasive treatment for brain tumors: Magnetic resonance-guided focused ultrasound surgery. In: Hayat MA, ed. Tumors of the Central Nervous System. Volume 3. Part I. Dordrecht, Netherlands: Springer, 2011, pp. 227–36
- Jolesz FA, Hynynen KH. MRI-Guided Focused Ultrasound Surgery. Abingdon, UK: Taylor & Francis, 2007
- Clement GT, Hynynen K. Micro-receiver guided transcranial beam steering. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:447–53
- Clement G, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol 2002;47:1219–36
- Pernot M, Aubry JF, Tanter M, Boch AL, Marquet F, Kujas M, et al. In vivo transcranial brain surgery with an ultrasonic time reversal mirror. J Neurosurg 2007;106:1061–6
- Marquet F, Pernot M, Aubry J, Montaldo G, Marsac L, Tanter M, et al. Non-invasive transcranial ultrasound therapy based on a 3D CT scan: Protocol validation and in vitro results. Phys Med Biol 2009;54:2597--613
- Gâteau J, Marsac L, Pernot M, Aubry JF, Tanter M, Fink M. Transcranial ultrasonic therapy based on time reversal of acoustically induced cavitation bubble signature. IEEE Trans Biomed Eng 2010;57:134–44
- Marquet F, Boch AL, Pernot M, Montaldo G, Seilhean D, Fink M, et al. Non-invasive ultrasonic surgery of the brain in non-human primates. J Acoust Soc Am 2013;134:1632–9
- Clement G, Sun J, Giesecke T, Hynynen K. A hemisphere array for non-invasive ultrasound brain therapy and surgery. Phys Med Biol 2000;45:3707–19
- Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med 2005;24:1117–25
- Chauvet D, Marsac L, Pernot M, Boch AL, Guillevin R, Salameh N, et al. Targeting accuracy of transcranial magnetic resonance-guided high-intensity focused ultrasound brain therapy: A fresh cadaver model: Laboratory investigation. J Neurosurg 2013;118:1046–52
- McDannold N, Clement G, Black P, Jolesz F, Hynynen K. Transcranial MRI-guided focused ultrasound surgery of brain tumors: Initial findings in three patients. Neurosurgery 2010;66:323–32
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol 2009;66:858–61
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640–8
- Moser D, Zadicario E, Schiff G, Jeanmonod D. Measurement of targeting accuracy in focused ultrasound functional neurosurgery: Technical note. Neurosurg Focus 2012;32:E2--E7
- Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. Lancet Neurol 2013;12:462–8
- Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus 2012;32:E1--E11
- Pinton G, Aubry JF, Bossy E, Muller M, Pernot M, Tanter M. Attenuation, scattering, and absorption of ultrasound in the skull bone. Med Phys 2012;39:299--307
- Pichardo S, Sin VW, Hynynen K. Multi-frequency characterization of the speed of sound and attenuation coefficient for longitudinal transmission of freshly excised human skulls. Phys Med Biol 2011;56:219--50
- Yin X, Hynynen K. A numerical study of transcranial focused ultrasound beam propagation at low frequency. Phys Med Biol 2005;50:1821–36
- Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng 2004;6:229–48
- Humphrey VF. Ultrasound and matter-physical interactions. Prog Biophys Mol Biol 2007;93:195–211
- Phillips D, Smith S, Von Ramm O, Thurstone F. Sampled aperture techniques applied to B-mode echoencephalography. Acoust Hologr 1975;6:103–20
- Flax S, O’Donnell M. Phase-aberration correction using signals from point reflectors and diffuse scatterers: Basic principles. IEEE Trans Ultrason Ferroelectr Freq Control 1988;35:758–67
- Fink M. Time reversal of ultrasonic fields. I. Basic principles. IEEE Trans Ultrason Ferroelectr Freq Control 1992;39:555–66
- Thomas JL, Fink MA. Ultrasonic beam focusing through tissue inhomogeneities with a time reversal mirror: Application to transskull therapy. IEEE Trans Ultrason Ferroelectr Freq Control 1996;43:1122–9
- Fink M, Montaldo G, Tanter M. Time-reversal acoustics in biomedical engineering. Annu Rev Biomed Eng 2003;5:465–97
- Vignon F, Aubry J, Tanter M, Margoum A, Fink M. Adaptive focusing for transcranial ultrasound imaging using dual arrays. J Acoust Soc Am 2006;120:2737–45
- Seip R, VanBaren P, Ebbini ES. Dynamic focusing in ultrasound hyperthermia treatments using implantable hydrophone arrays. IEEE Trans Ultrason Ferroelectr Freq Control 1994;41:706–13
- Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med 2004;52:100–07
- Song J, Pulkkinen A, Huang Y, Hynynen K. Investigation of standing-wave formation in a human skull for a clinical prototype of a large-aperture, transcranial MR-guided focused ultrasound (MRgFUS) phased array: An experimental and simulation study. IEEE Trans Biomed Eng 2012;59:435–44
- Pernot M, Montaldo G, Tanter M, Fink M. ‘Ultrasonic stars’ for time-reversal focusing using induced cavitation bubbles. Appl Phys Lett 2006;88:034102–3
- Haworth KJ, Fowlkes JB, Carson PL, Kripfgans OD. Towards aberration correction of transcranial ultrasound using acoustic droplet vaporization. Ultrasound Med Biol 2008;34:435–45
- Pinton G, Aubry JF, Fink M, Tanter M. Numerical prediction of frequency dependent 3D maps of mechanical index thresholds in ultrasonic brain therapy. Med Phys 2012;39:455–67
- Pinton G, Aubry JF, Fink M, Tanter M. Effects of nonlinear ultrasound propagation on high intensity brain therapy. Med Phys 2011;38:1207-16
- Pinton GF, Aubry JF, Tanter M. Direct phase projection and transcranial focusing of ultrasound for brain therapy. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:1149–59
- Jing Y, Meral FC, Clement GT. Time-reversal transcranial ultrasound beam focusing using a k-space method. Phys Med Biol 2012;57:901–17
- Leduc N, Okita K, Sugiyama K, Takagi S, Matsumoto Y. Focus control in HIFU therapy assisted by time-reversal simulation with an iterative procedure for hot spot elimination. J Biomech Sci Eng 2012;7:43–56
- Clement G, Hynynen K. Correlation of ultrasound phase with physical skull properties. Ultrasound Med Biol 2002;28:617–24
- Zeng X, McGough RJ. Evaluation of the angular spectrum approach for simulations of near-field pressures. J Acoust Soc Am 2008;123:68–76
- Clement G, White P, Hynynen K. Enhanced ultrasound transmission through the human skull using shear mode conversion. J Acoust Soc Am 2004;115:1356--64
- McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys 2008;35:3748–58
- Souchon R, Salomir R, Beuf O, Milot L, Grenier D, Lyonnet D, et al. Transient MR elastography (t-MRE) using ultrasound radiation force: Theory, safety, and initial experiments in vitro. Magn Reson Med 2008;60:871–81
- Plewes D, Betty I, Urchuk S, Soutar I. Visualizing tissue compliance with MR imaging. J Magn Reson Imaging 1995;5:733–8
- Kaye EA, Hertzberg Y, Marx M, Werner B, Navon G, Levoy M, et al. Application of Zernike polynomials towards accelerated adaptive focusing of transcranial high intensity focused ultrasound. Med Phys 2012;39:6254–63
- Herbert E, Pernot M, Montaldo G, Fink M, Tanter M. Energy-based adaptive focusing of waves: Application to noninvasive aberration correction of ultrasonic wavefields. IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:2388–99
- Larrat B, Pernot M, Montaldo G, Fink M, Tanter M. MR-guided adaptive focusing of ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57:1734–47
- Marsac L, Chauvet D, Larrat B, Pernot M, Robert B, Fink M, et al. MR-guided adaptive focusing of therapeutic ultrasound beams in the human head. Med Phys 2012;39:1141--9
- Hertzberg Y, Volovick A, Zur Y, Medan Y, Vitek S, Navon G. Ultrasound focusing using magnetic resonance acoustic radiation force imaging: Application to ultrasound transcranial therapy. Med Phys 2010;37:2934–42
- Grissom WA, Kaye E, Pauly KB, Zur Y, Yeo D, Medan Y, et al. Rapid HIFU autofocusing using the entire MR-ARFI image. AIP Conf Proc 2012;1503:162–7
- Vyas U, Kaye E, Pauly KB. Transcranial phase aberration correction using beam simulations and MR-ARFI. AIP Conf Proc 2012;1503:185–90
- Jensen JA, Svendsen NB. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans Ultrason Ferroelectr Freq Control 1992;39:262–7
- Vyas U, Christensen D. Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:1093–100
- Leighton TG. What is ultrasound? Prog Biophys Mol Biol 2007;93:3–83
- Martin E, Werner B. Focused ultrasound surgery of the brain. Curr Radiol Rep 2013;1:126–35
- Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, et al. The Virtual Family – Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol 2010;55:N23--N38
- ZMT. iSEG internet site. http://www.zurichmedtech.com/products/sim4life/iseg/iseg/ [last accessed 27 Nov 2013]