Abstract
Focused ultrasound surgery (FUS) is a non-invasive method for tissue ablation that has the potential for complete and controlled local tumour destruction with minimal side effects. The treatment of abdominal organs such as the liver, however, requires particular technological support in order to enable a safe, efficient and effective treatment. As FUS is applied from outside the patient’s body, suitable imaging methods, such as magnetic resonance imaging or diagnostic ultrasound, are needed to guide and track the procedure. To facilitate an efficient FUS procedure in the liver, the organ motion during breathing and the partial occlusion by the rib cage need to be taken into account in real time, demanding a continuous patient-specific adaptation of the treatment configuration. Modelling the patient’s respiratory motion and combining this with tracking data improves the accuracy of motion predictions. Modelling and simulation of the FUS effects within the body allows the use of treatment planning and has the potential to be used within therapy to increase knowledge about the patient status. This article describes integrated model-based software for patient-specific modelling and prediction for FUS treatments of moving abdominal organs.
Introduction
The combination of high-intensity focused ultrasound (FUS) with imaging techniques such as magnetic resonance imaging (MRI) and diagnostic ultrasound (US) has created new therapeutic access to a multitude of pathological conditions since the 1990s [Citation1]. The therapeutic potential of FUS lies in the fact that it makes it possible to deposit very localised, high energy doses in the depths of the human body without harm to adjacent structures. The combination with imaging allows for planning, control and direct monitoring of the treatment progress. Applications range from the thermal treatment of benign and malignant lesions to targeted drug delivery up to the treatment of thromboses (sonothrombolysis), neurological disorders and much more [Citation2]. Great challenges for the focusing of the ultrasound waves are posed by partial or full occlusion of the target site by bones or air-filled structures as well as target motion [Citation3–5].
It has been shown in the past that for treatment techniques such as radiation therapy the use of mathematical modelling, simulation and optimisation is indispensable for thorough planning and a good quality of treatment [Citation6]. Also, for the treatment of lesions with thermal ablation, such as radiofrequency current (RFA) and microwaves (MWA), it has been hypothesised that mathematical modelling, simulation and optimisation can lead to superior quality of treatment [Citation7–9]. In these cases the mathematical models represent biophysical processes that take place during the respective treatments and they assist in predicting the outcome of the therapy, thus supporting the awareness for risks and problems that might arise.
Similarly, to exploit the full potential of extracorporeal FUS to safely and precisely destroy tissue in the depths of a moving organ requires sophisticated software and hardware. In abdominal FUS the combination of different imaging modalities and computer support for the simulation and optimisation in the planning and conducting of the FUS therapy is being investigated. Here, again, mathematical modelling and numerical simulation can be used to predict the consequences of treatment decisions and the FUS therapy can be optimised before performing the treatment on the patient [Citation10]. To allow for safe and efficient treatment in abdominal organs without the need for apnea, both planning and execution have to be performed specifically according to the patient’s individual anatomy during breathing-related motion. Corresponding mathematical models and simulations support the monitoring of the organ motion and thus are components that enable a treatment of the moving organ with focal tracking.
This article gives an overview of recent progress that the authors have made in the integration of models and simulations for enabling FUS interventions for abdominal organs. The paper thereby focuses mainly on support for MR-guided FUS (MRgFUS), however, the authors assume that much of the technology presented here can be applied to ultrasound-guided FUS (USgFUS) as well.
Review of related work
FUS in abdominal organs
Most abdominal FUS applications for the treatment of liver, pancreas or kidney tumours to date have been performed under US-guidance. Despite the technical challenges of abdominal FUS stated above, most studies report that FUS is feasible and safe [Citation11–16]. In general, high ablation efficiency and minor side effects (necrosis of the ribs, localised vertebral body necrosis, biliary obstruction, symptomatic pleural effusions, fistula formation) are described [Citation15–17]. To date, thousands of abdominal USgFUS treatments have been performed, especially in Asia during recent decades. An initial clinical study under more controlled conditions was conducted by Illing et al. [Citation13]. The first MR-guided liver ablation was performed by Okada et al. in 2006 [Citation18]. Recently, Anzidei et al. [Citation19,Citation20] reported the successful treatment of selected patients with pancreatic adenocarcinoma and patients with a single liver tumour using a breath-holding technique under general anaesthesia.
Transcostal delivery
To compensate for the difficulties in delivering ultrasound energy through the rib cage, multi-element phased array transducers and adaptive focusing techniques have been developed. The approaches range from geometrical ray-tracing with deactivation of elements to methods including scattering and diffraction mechanisms [Citation21–25]. As an alternative to adaptive beam forming to protect ribs from overheating, an acoustic shielding was proposed to block energy deposition [Citation26].
Respiratory motion
Breath-holding or gating techniques can be applied to overcome the motion of abdominal organs due to breathing [Citation18,Citation19]. These techniques extend the treatment time due to limited duration and repetition frequency of the anaesthesia and the reduction of active FUS exposure time. A more efficient approach is to follow the moving target with the ultrasound focus enabling a continuous and localised energy delivery. In this case, multi-element phased array transducers with real-time electronic beam steering can be used [Citation27]. To track the motion of the organ, several US and MR-based methods have been developed. In Pernot et al. [Citation27] a 3D motion tracking was achieved using a US method based on 1D cross-correlation of a backscatter signal and was successfully used to compensate liver motion in pigs [Citation28]. Auboiroux et al. [Citation29] used US images for 2D motion tracking based on an optical flow method. An example of MR-based real-time 3D tracking with subsequent FUS motion compensation is given by Ries et al. [Citation30].
Therapy monitoring
Thermometry methods are utilised to directly monitor the temperature within the patient during the treatment. Both ultrasound and MR imaging can in principle be used for thermometry [Citation31,Citation32]. While MR thermometry is currently a major component of many clinical FUS systems, US thermometry is not yet applied in the clinic. To compensate for motion artefacts in MR thermometry of moving targets, real-time motion monitoring is necessary [Citation29,Citation30]. Approaches include self-reference or reference-less MR thermometry methods [Citation33–35], hybrid, and also model-based approaches [Citation32]. Furthermore, monitoring FUS therapy is possible using acoustic radiation force imaging (MR-ARFI) and also the change of elasticity of the tissue during ablation may potentially be assessed using both ultrasound imaging [Citation36] and MRI [Citation37].
Increasing ablation efficiency
Volumetric ablation techniques – in contrast to single-focus sonications – can be used to heat larger volumes by continuously moving the focus along trajectories. Thermometry feedback can be used to control energy delivery [Citation38]. Furthermore, inducing non-linear effects or controlled bubble clouds (cavitation/tissue boiling) make it possible to enhance focal energy deposition [Citation39].
Clinical perspective
Patient population
To date, the primary use cases for FUS treatments of liver tumours are hepatocellular carcinoma (HCC) and liver metastases. For patients with early stage HCC, with a preserved liver function, the first line treatment option is surgical resection [Citation40,Citation41]. Local ablation is considered the standard of care for patients with early tumours not suitable for surgery and the local ablation techniques currently most widely used clinically are RFA and MWA. Considering reported data in literature, RFA/MWA provides 5-year survival rates of 40–70% and even beyond in highly selected candidates [Citation40]. As the therapeutic effect of RFA/MWA and of MRgFUS is based on local thermal ablation, it is possible to consider these treatments suitable for the same groups of patients. Potential candidates for local ablation of HCC may be divided into four general categories: (1) patients who are poor surgical candidates due to inadequate liver function, from underlying cirrhosis or prior hepatectomy, or due to co-morbid conditions, (2) patients who are ineligible for surgical resection due to the anatomical distribution of the tumours, (3) patients who are surgical candidates but for whom the test-of-time approach is favoured to limit unnecessary repeated hepatectomies in case of the occurrence of new tumours, and (4) patients who undergo ablation to control tumour as a bridge to liver transplantation.
Clinical workflow
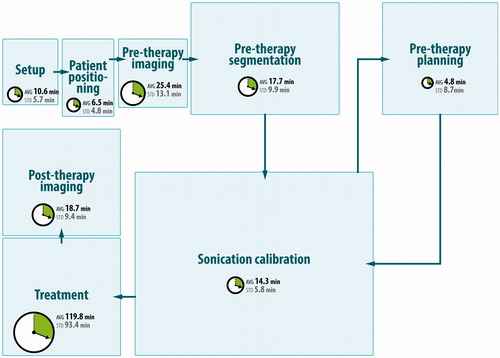
To study potential application and workflow scenarios for the models and software planning, we have analysed the clinical workflow for a total of 15 MRgFUS treatments of uterine fibroids in clinics in Dachau, Germany and Rome, Italy. The treatments were conducted with the ExAblate 2100 system (InSightec, Haifa, Israel). The mean total treatment time was 213.5 min (SD 107.22) for a mean of 56.8 (SD 41.4) sonications per treatment. These studies were the basis for the identification of a detailed workflow of current clinical practice in MRgFUS. Detailed descriptions of the workflow and the analysis can be found in Fernandez-Guttierez [Citation42]. shows a high level visualisation of the workflow. The workflow consists of eight main phases. In the ‘set-up’ and ‘patient positioning’ phases, the patient is placed in the MRI and the FUS transducer and MRI coils are set in place. Afterwards, planning scans are acquired in the ‘pre-therapy imaging’ phase and if necessary the patient is re-positioned. In the ‘pre-therapy segmentation’ phase important structures are defined such as the target region, transducer, skin line, gall bladder, kidneys and potentially tracking fiducials. The following ‘pre-therapy planning’ phase allows the definition of sonication targets. Next, the ‘sonication calibration’ phase consists of low-power sonications that are performed to verify the focal spot location, and then the actual ‘treatment’ phase allows the performance of the planned sonications until the target volume is ablated. Finally, the ‘post-therapy imaging’ phase consists of perfusion scans to verify treatment success.
Figure 1. High-level visualisation of the current workflow of MRgFUS for uterine fibroids with timings for each workflow step. The mean total treatment time is 213.5 min (SD 107.22) for a mean of 56.8 (SD 41.4) sonications per treatment.
Pre-procedural and follow-up MR examinations were performed using the following protocol: multiplanar T2-weighted turbo spin echo (TSE) sequences, axial fat-suppressed T1-weighted sequences and 3D or multiplanar post-contrast fat-suppressed T1-weighted sequences. MR protocol during the FUS procedure includes multiplanar T2-weighted TSE sequences and real-time thermometry EPI sequences acquired during sonications. After FUS treatment completion, multiplanar post-contrast fat-suppressed T1-weighted sequences were acquired.
Descriptive statistics on the gathered data showed that most of the treatment time was spent on the actual sonication of the target region (119.8 min of 213.5 min mean total treatment time). The results further showed that most delays were caused by organ motion – the repetition of parts of the workflow made necessary due to organ motion consumed up to 30% of the total procedure time, even for this well-established treatment of the uterus, which has a low level of motion. Based on this analysis, a workflow simulation model was developed and implemented within the process simulation software Delmia Quest (Dassault Systèmes, Paris, France). The model was validated and verified against the observation data. The simulations showed that MRgFUS procedure times increase exponentially with the number of organ motion events during the treatment. Further simulation studies were conducted to predict the effects on procedure times caused by introducing automated segmentation, execution of the treatment plan, and motion tracking and compensation. Even when treating static organs, these results showed a 24% procedure time reduction. For details we refer the reader to Fernandez-Guttierez [Citation42].
Based on these findings, we have developed a future workflow for MRgFUS for moving abdominal organs, which features automation of most of the currently manual tasks and decisions (with user supervisory control at all times for safety). This envisioned workflow was the basis for the development of software that integrates mathematical models and simulations as well as image processing and user interaction.
Patient-specific modelling and simulation of FUS in moving organs
For safe, effective and efficient FUS of abdominal organs, modelling and simulation can be regarded as enabling technology. A patient-specific simulation of organ motion and ultrasound propagation helps in planning the treatment by predicting the potential outcome. This makes it possible to perform a risk analysis and to determine therapy plans that lead to successful treatment.
As the motion of the target directly influences treatment decisions, it is beneficial to account for respiratory motion already during the treatment planning. For the execution of the treatment, actual motion observations are required, whereby the motions of all relevant structures are of importance, although these might not be fully observable. Here, models can be used to supply the missing motion information by interpolating between and extrapolating from the observations [Citation43,Citation44].
Modelling and simulating the distribution of temperature in the patient’s body in a similar approach makes it possible to push the limits of imaging [Citation45]. Temperature measurement and model predictions can be combined to improve the measurement accuracy or increase imaging resolution.
For clinical applicability, the numerical simulation of the models must have a high simulation performance with respect to computing time. The motion model must be able to predict both spatially and temporally with real-time performance and must be able to simulate patient-specific normal respiratory motion when no tracking is available during treatment planning.
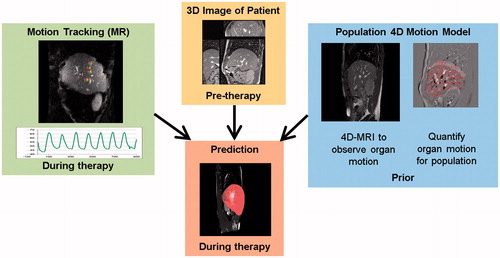
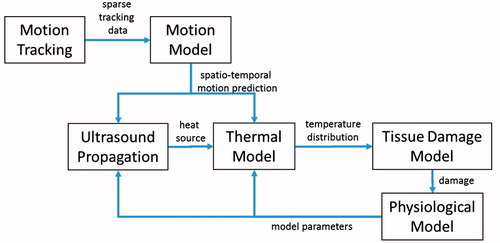
Our system comprises a predictive model for FUS in the abdomen during respiratory motion. The main components of this model are: (1) a respiratory organ motion model of the patient-specific deformation of the relevant anatomical structures during breathing, (2) an ultrasound propagation model describing the ultrasound energy distribution within the patient, and (3) a thermal and physiological model capturing the tissue’s response and the effects on the therapy. These model components are integrated into software which orchestrates the interplay between the models and supplies them with model parameters that are extracted from patient-specific imaging data. shows a flow chart of the interactions and data flows between the different models as an overview.
Figure 2. Overview of the interactions and data flows between the different models. Motion tracking data is fed into the motion model. Spatio-temporal motion predictions are recurrently fetched from the motion model and are used as a basis for the ultrasound propagation model and the thermal model. The ultrasound model computes the local heat source that is fed into the thermal model. Successively, the temperature distribution in the patient deforming during respiration is computed by the thermal model which constitutes the basis for the computation of a damage map by the tissue damage model. The physiological model adjusts based on the tissue damage the parameter values representing the patient status.
Related work
Respiratory motion models have been mainly developed for lungs and liver [Citation43,Citation44]. These models relate partial motion observations to organ displacements by either fitting predefined functions or by learning their statistical relationship.
For the simulation of FUS, an ultrasound propagation model needs to be combined with a thermal model and a tissue damage model. Several numerical models for ultrasound simulation are described in the literature, most prominently the linear angular spectrum approach [Citation46,Citation47] (for reasons of efficiency) and the Khokhlov-Zabolotskaya-Kuznetsov (KZK) equation [Citation48] as an extension of Burgers’ equation to account for non-linear effects. Commercial general purpose simulation packages can be utilised for the solution of the models (e.g. [Citation49]). While allowing for flexible modelling choices, the tools do not reveal sufficient performance in our case. Furthermore, freely available FUS simulation packages (HIFU simulator [Citation50], k-wave [Citation51]) have been developed. While some work already focuses on achieving interactive simulation performance [Citation52] for use in treatment planning, numerical FUS simulation under motion has not been addressed much. The diffusion of heat and the cooling by vascular structures in thermal ablation, however, have been widely studied in the context of interstitial laser ablation, RFA, MWA and related techniques [Citation53,Citation54]. The modelling of ablation during motion of the target and the domain has not been considered in the literature. Methods available for the numerical discretisation of the underlying partial differential equations include finite difference, finite volume and finite element methods [Citation55]. Time-stepping methods are available for the solution of the resulting ordinal differential equations in the time domain after spatial discretisation ranging from explicit methods such as forward Euler to implicit methods such as backward Euler, Crank-Nicolson and leapfrog stepping [Citation55]. While explicit schemes such as forward Euler introduce potential numerical instabilities (dependent on time-step), they allow for good parallelisation of the solution.
Respiratory organ motion model
We developed 4D motion models for the abdomen that predict the motion of the liver as a whole from partial motion observations [Citation56] as illustrated in . Firstly, the irregularity of free breathing and the long-term motion effects (drift) were captured by 4D MRI acquisitions based on 2D navigators and retrospective sorting [Citation57]. Generic motion models, which capture the correlation between the displacements of liver locations, were then created from the motion fields determined by intra-subject image registration of the 4D MRIs. A combined generic motion model is then constructed based on semi-automatically defined inter-subject correspondences. The generic motion model can then be individualised by spatially mapping it to the patient using a breath-hold 3D image acquired in therapy position. During the live-use scenario, the individualised motion model predicts the motion of unobserved regions from partial motion observations given by US or MR tracking. Temporal prediction is achieved by extrapolating the partial motion observations [Citation58]. The effectiveness of the 4D motion models was evaluated with respect to the 4D MRI motion data in leave-one-out tests and recently also after integration with MR tracking. The leave-one-out tests (12 subjects, 40–75 min of observations) showed that when accurately tracking three 3D liver locations (on diaphragm, entrance of portal vein, central liver vessel), a mean ± SD spatial prediction accuracy of 1.0 ± 0.7 mm can be achieved for the right liver lobe [Citation56]. Finally, the more realistic in vivo validation experiment (10 subjects, 4.5 min of observations) included 2D MR tracking of liver vessels and temporal prediction for a latency of 72 ms, and resulted in a mean spatio-temporal prediction accuracy of 1.8 mm and a 95% quantile of 4.3 mm in comparison to manually selected liver vessel landmarks. This performance is similar to state-of-the-art results achieved by motion predictions based on 2D US tracking [Citation59]. After this successful spatio-temporal prediction of the right liver lobe, we will focus on also predicting the motion of the main risk structures, which are the ribs enclosing the liver. Substantial progress has already been made on the challenging task of detecting and registering ribs in MR images [Citation60].
Ultrasound propagation model
Our ultrasound model is based on the hybrid angular spectrum approach [Citation47], which permits a fast simulation of ultrasound propagation through heterogeneous media (sound velocity and acoustic absorption). The propagation of the acoustic field from the transducer through the 3D volume is constituted by two sequential steps: given all hardware parameters and the transducer geometry, it first calculates the initial acoustic field plane of the volume and then simulates the propagation of this pressure distribution through the volume. The calculation of the frequency domain representation of the initial acoustic field plane, the transducer plane, is done analytically for flat phased array transducers comprised of arbitrarily distributed square elements. The analytical solution (super-position of sync functions related to the directivity function of the transducer’s rectangle elements) gives good results at any sampling rate and thereby allows the reduction of the size of the simulation grid and the calculation burden. The angular spectrum method [Citation46] can then be used to simulate the propagation of the pressure distribution from the initial plane through a homogeneous medium. The propagation is performed slice-wise in the frequency domain. Medium heterogeneities can then be corrected for in the spatial domain [Citation47]. We developed a novel technique for the linear propagation filter that handles the spatial heterogeneity in both sound velocity and acoustic attenuation. The novel propagator takes into account both acoustic properties and the local beam direction when calculating the beam propagation at each step. The simulation method was validated numerically against analytical simulations in layered media.
Non-linear modelling
Our simulation of the non-linear propagation of the acoustic field uses the phenomenological method of operator splitting proposed by Zemp [Citation61]. This operator-splitting technique is valid for small propagation steps, where the effects of diffraction, attenuation and non-linearity may be considered to be independent. While diffraction is accounted for by the linear heterogeneous medium acoustic propagation model, the non-linear effects are added to the result in a separate sub-step. The non-linear effects are calculated in the frequency domain via a Fourier series solution to Burgers’ equation (which includes attenuation) as described by Zemp [Citation61].
Transcostal focusing
In the treatment execution scenario, several focus updates per second are required. In order to fulfil this real-time requirement, a fast ray-tracing beam forming was implemented.
Thermal and physiological model
The physiological model describes the interplay between the FUS treatment and the patient’s organ physiology. In our research, the main treatment effect modelled is thermal ablation of tissue while neglecting non-thermal effects.
Bioheat transfer under motion and deformation of the domain
We use a modification of the classical Pennes bioheat equation [Citation62] to describe the spatio-temporal distribution of temperature in the moving and deforming tissue subject to blood perfusion. Motion and deformation of the organs is introduced into the equation by letting the spatial coordinates be time dependent. To discretise the problem we use a Lagrangian approach – describing the problem from a location within the object, i.e. an anatomical location, and letting the physical quantity move with the object. Since in the Lagrangian approach we can choose the grid freely, we have been able to implement the simulation very efficiently using finite differences on a regular hexahedral grid using graphical processing units for fast computations (GPGPU). To allow for fast motion interpolations and computations of the necessary Jacobian of deformation, the motion predictions from the motion model are bound to a tetrahedral mesh. The simulation is performed with a fine temporal resolution (0.1-s time steps) to resolve the continuous respiratory motion.
Vascular structures
Especially for treatments in the liver with its complex vascular system, it is essential to take perfusion-induced cooling effects into account. To this end we extract a geometrical model of vascular structures from anatomical images (MR scans or CT scans) [Citation63]. To model convective heat transfer, the vessels are treated in the simulation as Dirichlet nodes fixed to body temperature. Thereby, tissue in the vicinity of the vessel is cooled down. Temperature within the vessel is not modelled because flow ratios inside the liver are high enough to prevent a considerable temperature rise of the blood. However, the model takes into account the fact that small vessels may coagulate and thus stop their cooling influence as described in Kröger et al. [Citation8].
Tissue damage
Based on the temperature distribution over time, an Arrhenius-type tissue-damage model predicts the degree of tissue damage in the entire domain [Citation8,Citation64]. The physiological model adjusts the ultrasound absorption, the speed of sound, and the local tissue perfusion rate based on a dependency on the grade of tissue damage. This leads to a reduction of perfusion rate and increased absorption and speed of sound in ablated tissue. The actual parameters of these dependencies are currently under investigation.
Ultrasound pre-computations for interactive simulation
Our GPGPU ultrasound simulation needs a computing time of a few seconds to generate a single 3D pressure field of sufficient accuracy [Citation52]. When accounting for motion of the domain, however, several ultrasound simulations are needed for each second of treatment time. For FUS simulations with interactive response times, as required for treatment planning during motion, even the angular spectrum approach implemented on GPGPU is currently not fast enough. To remedy this, we developed an approximation method based on pre-computations of ultrasound fields at key locations over the respiratory cycle. During the fast treatment simulations in the interactive treatment planning, a look-up is performed and the pre-computed fields are loaded and transformed to best fit the current motion state. Thus we replace computations of the pressure fields by relatively quick memory look-ups and interpolation. Validation is currently being performed against MR thermometry of ex vivo experiments and will be continued with data from animal studies.
Software demonstrator
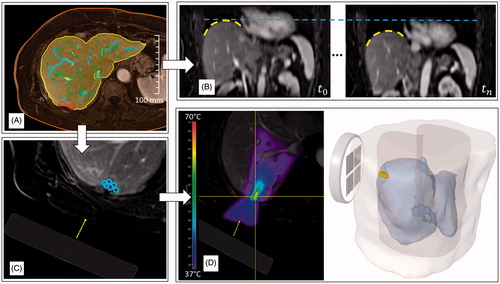
We have developed a software demonstrator for patient-specific planning and simulation of the FUS treatment under free breathing of a virtual patient. The software demonstrator can be seen as a proof-of-concept prototype for an actual treatment system, but instead of being connected to real hardware the software simulates the hardware system and the effects of the treatment on the patient. It integrates the simulation models to predict the treatment outcome and it parameterises the models with the corresponding patient-specific imaging data that are acquired before and during the treatment. The results of the integration are presented in the following section and the parameterisation with imaging data beyond geometrical modelling is work in progress. shows results for an example patient case.
Figure 4. Workflow-steps within the software demonstrator for an example patient case. Based on image segmentation methods the anatomical patient model is defined: (A) shows contours of the skin line, liver, portal and hepatic vein and the tumour. After the motion model adaption to the patient the motion can be assessed in a live preview: (B) shows two example motion states of the preview with superimposed dashed lines to highlight the difference of liver position. Afterwards an interactive treatment planning can be performed: (C) shows the virtual transducer placed in the image and the sonications currently planned. Based on the planning, the therapy outcome can be predicted using the numerical simulation: (D) hows the temperature distribution in 2D superimposed on the planning image and a 3D visualisation of the tissue damage.
Workflow guidance
The interaction with the software is based on workflow steps for the different tasks that need user input. The user is thereby guided through the process of planning the FUS treatment.
Anatomy from images
Based on a patient-specific MR planning image, the anatomy of the patient is modelled using semi-automatic segmentation methods. This includes the liver, vascular structures, the target volume and other structures relevant to the treatment. shows this for the example patient.
Risk analysis
Based on an analysis of the biliary system in the liver, a risk analysis can be performed. Analysing the hierarchical tree structure of the biliary system, parts of the liver are highlighted that might be influenced by any damage to the bile ducts draining that part of the liver. Potential risks of biliary occlusion can thereby be estimated based on the location of the target volume with respect to the bile ducts.
Motion model set-up and motion preview
The next step focuses on the respiratory motion of the specific patient. The user is guided through a landmark setting tool that asks for defined anatomical positions in the patient image. The patient-specific motion model is then set up and the user is presented with a real-time preview of the abdominal motion of the patient. Two distinct motion states of the preview are visualised in .
Virtual treatment planning
When entering the planning step, the user is asked to place the virtual transducer in a proper position. The user defines the transducer location and orientation in the planning image and a sequence of sonications, see .
Simulation of outcome
The outcome can then be predicted using the numerical simulations. The results of the simulation are displayed super-imposed on the planning scan to give direct feedback to the clinician, see . In case of any discrepancy between the intended outcome and the predicted outcome, the clinican can return to the planning step and alter the treatment plan.
Visualisation
The results of the simulation are displayed in 2D and 3D views, see . The temperature and tissue damage predictions can furthermore be inspected both directly in the breathing patient and also mapped to the static planning scan. The latter approach makes it possible to analyse the outcome without distraction by patient motion. Furthermore, temperature history curves can be displayed to assess the temperature over time for locations of interest. The treatment progress is visualised for the clinician, as shown in , by displaying the tissue damage distribution within the patient.
Experimental validation
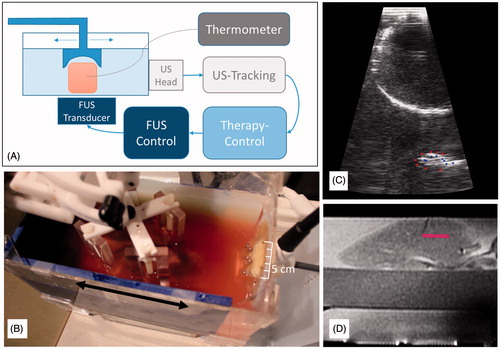
We developed a proof-of-concept system for the sonication of moving targets. To evaluate the capacity of the system we developed an experimental set-up for the ablation of ex vivo ovine liver phantoms under controlled motion, see .
Figure 5. Experimental system for the sonication of a moving target. Schematic (A) and a photograph (B) of the experimental set-up. The ultrasound tracking and the underlying image is shown in (C). The planning scan of the experimental set-up can be seen in (D) superimposed with the target points that the system selected during the sonication.
Motion phantom
We modelled an organ under motion by periodically moving a fresh ovine liver in a water tank with an MR-compatible robotic arm (Innomotion Robot, IBSmm, Brno, Czech Republic) as can be seen in .
Steered FUS
To achieve target-tracked beam steering, the motion of the organ is tracked using an MR compatible diagnostic ultrasound device (DiPhAS, Fraunhofer IBMT, St Ingbert, Germany) and ultrasound tracking software (SonoPlan, Mediri, Heidelberg, Germany), see . Vessels within the liver serve as feature points and are tracked with the software [Citation65,Citation66]. The tracking information is sent to our therapy control system that predicts the current target position and adjusts the focal spot of a steerable FUS device (ExAblate 2100, InSightec). displays the target points the system uses for compensating the motion (from left to right with respect to the displayed planning image). The FUS is operated in a pulsed fashion, interleaved with the diagnostic ultrasound imaging for motion tracking.
Evaluation
To validate the ability of the system to compensate motion, the temperature evolution in a target spot is compared in moving and static scenarios. A thermocouple is inserted into the phantom to measure temperature curves while sonicating with varying output powers (15 W, 30 W, 45 W) each with a static and a moving phantom and over 1 min. To compare corresponding experiments we compute the mean temperature difference (MTD), the temperature peak difference (PTD), and the difference in the baseline corrected area under the curve (AUCD) as a measure for the thermal dose delivered (see ). From these minor differences in the achieved temperatures, we conclude that the system is capable of compensating the target motion. To be able to model more complex motion we are currently developing a phantom mover based on a ventilation machine.
Table 1. Comparing the motion experiments with static sonications. Mean temperature difference (MTD), peak temperature difference (PTD) and difference of area under the curve (AUCD) as a measure for the delivered thermal dose compared to the static experiment.
In order to validate the system in a scenario close to the clinical setting, a human model based on Thiel soft embalmed cadavers was developed. The Thiel embalming process leaves tissue in a state that is close to the physiological condition in terms of colour, texture and elasticity [Citation67]. Liver perfusion and respiratory motion have been established in the cadavers and the MR temperature mapping adjusted to the tissue coefficient altered by the Thiel embalming process [Citation68,Citation69]. This model will be used in the pre-clinical validation.
Summary and conclusions
The software described in this article is based on three mathematical models that describe the motion of the organ during breathing, the propagation of the ultrasound wave through the rib cage and into the moving organ, and the functional state of the tissue in terms of perfusion and thermal damage. On one hand, these models are used to predict and plan the treatment by simulation of the respective patient-specific processes. On the other hand, the models are also used to augment imaging data and observations of the motion and tissue heating. In the latter sense, our models may bridge the gaps in temporal and spatial resolution of the imaging modalities. We have set up an experimental proof-of-concept system for sonication of moving targets, which has shown that our system comprising ultrasound target tracking in combination with electronic beam steering yields temperatures similar to static target heating. Currently, one of the main challenges is the development of a clinically applicable integrated treatment system that combines available solutions to specific problems in abdominal FUS treatments. We address this by an ongoing translation of our software system to the clinical setting. Clinical feasibility studies are planned to validate the system. Through the combination of such an integrated real-time system with accurate treatment planning, improved monitoring and treatment efficiency, FUS can become an effective and clinically competitive alternative to current surgical and minimally invasive treatment for liver lesions. It has the potential to obviate the need for surgical excision or any transcutaneous access with the associated complications of possible intra-abdominal bleeding and tumour cell spread. There is therefore a potential long-term impact in improving the treatment of primary cancer and metastases in a variety of abdominal organs and for a wide range of patients.
Acknowledgements
The authors acknowledge the whole FUSIMO and TRANS-FUSIMO teams and management. We thank Matthias Matzko for his support in the observation studies and the team at Fraunhofer IBMT for their work on the DiPhAS ultrasound device. Helen McLeod and Karen French are acknowledged with gratitude for preparing and conducting the volunteer MRI imaging study at IMSaT Dundee University. Osnat Dogakin and Alexander Volovick (InSightec, Israel) have kindly supported focused ultrasound procedures.
Declaration of interest
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreements no. 270186 (FUSIMO, www.fusimo.eu) and no. 611889 (TRANS-FUSIMO, www.trans-fusimo.eu). M.S., J.S., S.H., C.T., T.L., A.L., S.B. and T.P. declare no competing interests. J.J. is partly employed by Mediri. Y.L. is employed by InSightec. G.S. is employed by GE. A.M. and M.B. acknowledge research collaborations with GE and InSightec. A.M. further acknowledges research collaborations with IBSmm and support from the Northern Research Partnership. M.G. is CEO and Co-Owner of Mediri. The authors alone are responsible for the content and writing of the paper.
References
- Kennedy J, ter Haar G, Cranston D. High intensity focused ultrasound: Surgery of the future? Br J Radiol 2003;76:590–9
- Foley J, Eames M, Snell J, Hananel A, Kassell N, Aubry JF. Image-guided focused ultrasound: State of the technology and the challen ges that lie ahead. Imaging Med 2013;5:357–70
- Aubry JF, Pauly K, Moonen C, Haar G, Ries M, Salomir R, et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: Technical and clinical consensus. J Ther Ultrasound 2013;1:13
- Tanter M, Pernot M, Aubry JF, Montaldo G, Marquet F, Fink M. Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound. Int J Hyperthermia 2007;23:141–51
- Dorr LN, Hynynen K. The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia 1992;8:45–59
- Hamacher HW, Küfer KH. Inverse radiation therapy planning – A multiple objective optimization approach. Discrete Appl Math 2002;118:145–61
- Appelbaum L, Sosna J, Pearson R, Perez S, Nissenbaum Y, Mertyna P, et al. Algorithm optimization for multitined radiofrequency ablation: Comparative study in ex vivo and in vivo bovine liver. Radiology 2010;254:430–40
- Kröger T, Altrogge I, Preusser T, Pereira PL, Schmidt D, Weihusen A, et al. Numerical simulation of radio frequency ablation with state dependent material parameters in three space dimensions. In: Larsen R, Nielsen M, Sporring J, editors. Medical Image Computing and Computer-Assisted Intervention, MICCAI 2006. Lecture Notes in Computer Science 4191. Berlin: Springer, 2006, pp. 380–8
- Zhai W, Xu J, Zhao Y, Song Y, Sheng L, Jia P. Preoperative surgery planning for percutaneous hepatic microwave ablation. In: Metaxas DN, Axel L, Fichtinger G, Szekely G, editors. Medical Image Computing and Computer-Assisted Intervention, MICCAI 2008. Lecture Notes in Computer Science 5242. Berlin: Springer, 2008, pp. 569–77
- Coon J, Todd N, Roemer R. HIFU treatment time reduction through heating approach optimisation. Int J Hyperthermia 2012;28:799–820
- Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason Sonochem 2004;11:149–54
- Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol 2004;11:1061–9
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a western population. Br J Cancer 2005;93:890–5
- Park MY, Jung SE, Cho SH, Piao XH, Hahn ST, Han JY, et al. Preliminary experience using high intensity focused ultrasound for treating liver metastasis from colon and stomach cancer. Int J Hyperthermia 2009;25:180–8
- Jung SE, Cho SH, Jang JH, Han JY. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: Complications. Abdom Imaging 2011;36:185–95
- Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev 2012;38:346–53
- Li JJ, Gu MF, Luo GY, Liu LZ, Zhang R, Xu GL. Complications of high intensity focused ultrasound for patients with hepatocellular carcinoma. Technol Cancer Res Treat 2009;8:217–24
- Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci 2006;5:167–71
- Anzidei M, Marincola BC, Bezzi M, Brachetti G, Nudo F, Cortesi E, et al. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: Preliminary experience for pain palliation and local tumor control. Invest Radiol 2014;49:759–65
- Anzidei M, Napoli A, Sandolo F, Marincola BC, Di Martino M, Berloco P, et al. Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs: A feasibility study in selected cases of pancreatic and liver cancer. Cardiovasc Intervent Radiol 2014;37:1611–16
- Aubry JF, Pernot M, Marquet F, Tanter M, Fink M. Transcostal high-intensity-focused ultrasound: Ex vivo adaptive focusing feasibility study. Phys Med Biol 2008;53:2937–51
- Botros YY, Volakis JL, VanBaren P, Ebbini ES. A hybrid computational model for ultrasound phased-array heating in presence of strongly scattering obstacles. IEEE Trans Biomed Eng 1997;44:1039–50
- Bobkova S, Gavrilov L, Khokhlova V, Shaw A, Hand J. Focusing of high-intensity ultrasound through the rib cage using a therapeutic random phased array. Ultrasound Med Biol 2010;36:888–906
- Gelat P, Ter Haar G, Saffari N. Modelling of the acoustic field of a multi-element HIFU array scattered by human ribs. Phys Med Biol 2011;56:5553–81
- Gélat P, ter Haar G, Saffari N. A comparison of methods for focusing the field of a HIFU array transducer through human ribs. Phys Med Biol 2014;59:3139
- Salomir R, Petrusca L, Auboiroux V, Muller A, Vargas MI, Morel DR, et al. Magnetic resonance-guided shielding of prefocal acoustic obstacles in focused ultrasound therapy: Application to intercostal ablation in liver. Invest Radiol 2013;48:366–80
- Pernot M, Tanter M, Fink M. 3-D real-time motion correction in high-intensity focused ultrasound therapy. Ultrasound Med Biol. 2004;30:1239–49
- Marquet F, Aubry JF, Pernot M, Fink M, Tanter M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys Med Biol 2011;56:7061–80
- Auboiroux V, Petrusca L, Viallon M, Goget T, Becker CD, Salomir R. Ultrasonography-based 2D motion-compensated HIFU sonication integrated with reference-free MR temperature monitoring: A feasibility study ex vivo. Phys Med Biol 2012;57:N159–71
- Ries M, de Senneville BD, Roujol S, Berber Y, Quesson B, Moonen C. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med 2010;64:1704–12
- Amini AN, Ebbini ES, Georgiou TT. Noninvasive estimation of tissue temperature via high-resolution spectral analysis techniques. IEEE Trans Biomed Eng 2005;52:221–8
- Yuan J, Mei CS, Panych LP, McDannold NJ, Madore B. Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry. Quant Imaging Med Surg 2012;2:21–32
- Rieke V, Vigen KK, Sommer G, Daniel BL, Pauly JM, Butts K. Referenceless PRF shift thermometry. Magn Reson Med 2004;51:1223–31
- Kuroda K, Kokuryo D, Kumamoto E, Suzuki K, Matsuoka Y, Keserci B. Optimization of self-reference thermometry using complex field estimation. Magn Reson Med 2006;56:835–43
- Salomir R, Viallon M, Kickhefel A, Roland J, Morel DR, Petrusca L, et al. Reference-free PRFS MR-thermometry using near-harmonic 2-D reconstruction of the background phase. IEEE Trans Med Imaging 2012;31:287–301
- Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, et al. Elastography: Imaging the elastic properties of soft tissues with ultrasound. J Med Ultrasonics 2002;29:155–71
- Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: A review. Clin Anat 2010;23:497–511
- Mougenot C, Salomir R, Palussiere J, Grenier N, Moonen CT. Automatic spatial and temporal temperature control for MR-guided focused ultrasound using fast 3D MR thermometry and multispiral trajectory of the focal point. Magn Reson Med 2004;52:1005–15
- Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol 2010;36:250–67
- European Association for the Study of the Liver and European Organisation for Research and Treatment of Cancer. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–43
- Park E, Kim H, Kim C, Hur Y, Koh Y, Kim J, et al. A comparison between surgical resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Ann Surg Treat Res 2014;87:72–80
- Fernandez-Gutierrez F. Workflow analysis, modelling and simulation for improving conventional and MRI-guided vascular interventions. PhD Thesis, University of Dundee, 2014, pp 84–97. Available from: http://hdl.handle.net/10588/6b7fca49-19ba-47b0-831a-ca9677084a7a
- Tanner C, Boye D, Samei G, Székely G. Review on 4D models for organ motion compensation. CR Rev Biom Eng 2012;40:135–54
- McClelland J, Hawkes D, Schaeffter T, King A. Respiratory motion models: A review. Med Image Anal 2013;17:19–42
- Todd N, Payne A, Parker DL. Model predictive filtering for improved temporal resolution in MRI temperature imaging. Mag Reson Med 2010;63:1269–79
- Zeng X, McGough RJ. Optimal simulations of ultrasonic fields produced by large thermal therapy arrays using the angular spectrum approach. J Acoust Soc Am 2009;125:2967–77
- Vyas U, Christensen D. Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:1093–100
- Bakhvalov NS, Zhileikin YM, Zabolotskaya EA. Nonlinear Theory of Sound Beams. American Institute of Physics, 1987
- Scott SJ, Salgaonkar V, Prakash P, Burdette EC, Diederich CJ. Interstitial ultrasound ablation of vertebral and paraspinal tumours: Parametric and patient-specific simulations. Int J Hyperthermia 2014;30:228–44
- Soneson JE. A user-friendly software package for HIFU simulation. AIP Conf Proc 2009;1113:165–9
- Treeby BE, Jaros J, Rendell AP, Cox BT. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k-space pseudospectral method. J Acoust Soc Am 2012;131:4324–36
- Georgii J, von Dresky C, Meier S, Demedts D, Schumann C, Preusser T. Focused Ultrasound – Efficient GPU simulation methods for therapy planning. In: Bender J, Erleben K, Galin E, eds. Proceedings of the 8th Workshop on Virtual Reality Interaction and Physical Simulation, VRIPHYS 2011, Lyon, France. Darmstadt, Germany: Eurographics Association, 2011, pp. 119–28
- Kok HP, Gellermann J, van den Berg CAT, Stauffer PR, Hand JW, Crezee J. Thermal modelling using discrete vasculature for thermal therapy: A review. Int J Hyperthermia 2013;29:336–45
- Liu Z, Ahmed M, Sabir A, Humphries S, Goldberg SN. Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 2007;23:49–58
- Tadmor E. A review of numerical methods for nonlinear partial differential equations. Bull Am Math Soc 2012;49:507–54
- Samei G, Tanner C, Székely G. Predicting liver motion using exemplar models. In: Ayache N, Delingette H, Golland P, Mori K, editors. Abdominal Imaging: Computational and Clinical Applications. MICCAI 2012. Lecture notes in Computer Science 7601. Berlin: Springer, 2012, pp. 147–57
- von Siebenthal M, Székely G, Gamper U, Boesiger P, Lomax A, Cattin P. 4D MR imaging of respiratory organ motion and its variability. Phys Med Biol 2007;52:1547–64
- Tanner C, Eppenhof K, Gelderblom J, Székely G. Decision fusion for temporal prediction of respiratory liver motion. In: Proceedings of the 2014 IEEE 11th International Symposium on Biomedical Imaging, ISBI 2014, pp. 698–701
- Preiswerk F, De Luca V, Arnold P, Celicanin Z, Petrusca L, Tanner C, et al. Model-guided respiratory organ motion prediction of the liver from 2D ultrasound. Med Image Anal 2014;18:740–51
- Samei G, Székely G, Tanner C. Detection and registration of ribs in MRI using geometric and appearance models. In: Golland P, Hata N, Barillot C, Hornegger J, Howe J, editors. Medical Image Computing: Computer Assisted Interventions, MICCAI 2014. Lecture Notes in Computer Science 8673. Berlin: Springer, 2014, pp. 706–13
- Zemp RJ. Modeling Nonlinear Ultrasound Propagation in Tissue (master's thesis). University of Toronto, Toronto, 2000
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948;1:93–122
- Selle D, Preim B, Schenk A, Peitgen HO. Analysis of vasculature for liver surgical planning. IEEE Trans Med Imag 2002;21:1344–57
- Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia 2013;29:262–80
- Rothlübbers S, Schwaab J, Jenne J, Günther M. MICCAI CLUST 2014: Bayesian real-time liver feature ultrasound tracking. In: De Luca V, Cifor A, Lediju Bell MA, Tanner C, editors. Challenge on Liver Ultrasound Tracking. MICCAI 2014. pp. 45–52
- CLUST, Challenge on Liver Ultrasound Tracking, Results, November, 2014. Available from: http://clust14.ethz.ch/results.html
- Thiel W. An arterial substance for subsequent injection during the preservation of the whole corpse. Ann Anat 1992;174:197–200
- Karakitsios I, Bobeica M, Saliev T, Rube M, Melzer A. Thermometry during MR-guided focused ultrasound in a preclinical model based on Thiel embalmed tissue. Minim Invasive Ther Allied Technol 2013;23:120–6
- Karakitsios I, Dogadkin O, Le N, Melzer A. Measurement of proton resonance frequency shift coefficient during MR-guided focused ultrasound on Thiel embalmed tissue. Mag Reson Med 2014. doi: 10.1002/mrm.25378