Abstract
Background: Patients with locally advanced gastric cancer (GC) and/or peritoneal metastases have a poor prognosis despite systemic chemotherapy or palliative surgery. The aim of this retrospective comparative non-randomised study was to evaluate aggressive cytoreduction in combination with hyperthermic intraperitoneal chemoperfusion (HIPEC) as a novel treatment strategy for patients with intraperitoneal disseminated and locally advanced GC.
Patients and methods: Forty-nine GC patients with serosal invasion (n = 19), limited peritoneal metastases (n = 20), or disseminated peritoneal metastases and tense ascites (n = 10) underwent combination therapy with HIPEC. Three matched control groups undergoing standard therapies were retrospectively identified.
Results: Combination therapy for serosa-invasive GC reduced the level of metachronous peritoneal carcinomatosis and increased median survival from 12 months to 22.5 months (p = 0.001). The median and 1-year survival rates for intraperitoneal disseminated GC patients undergoing therapy with the use of HIPEC were 12 months and 68.8% compared with 8 months and 25%, respectively (p = 0.004) for control subgroup patients (palliative chemotherapy). The symptomatic use of HIPEC allows effective elimination of recurrent ascites in GC patients.
Conclusion: HIPEC is a well-tolerated and effective method of adjuvant therapy for gastric cancer with high risk of intraperitoneal progression. Cytoreduction followed by HIPEC improves survival in patients with limited peritoneal carcinomatosis of gastric origin.
Introduction
The past two decades have witnessed a change in the treatment paradigm for intraperitoneal disseminated tumours with the introduction of new combined modality treatments, namely those based on cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion (HIPEC). This approach to treatment has proven effective for patients suffering from colorectal cancer with peritoneal carcinomatosis, peritoneal pseudomyxoma and peritoneal mesothelioma, which accounts for its inclusion in the national standards of some EU countries [Citation1,Citation2]. However, evidence for the use of combined therapy based on HIPEC for gastric cancer (GC) still remains limited to the clinical experience of separate clinics in Japan and Europe, therefore the issue of advisability and effectiveness of HIPEC in the treatment of GC patients remains unanswered [Citation3–5].
Peritoneal dissemination is one of the most common forms of GC metastasis [Citation6] and is diagnosed in 30% of all GC patients [Citation7]. Intraperitoneal progression of the disease after radical interventions (metachronous carcinomatosis) develops in 34–60% of patients and is the main cause of GC death [Citation8]. Systemic palliative chemotherapy in GC patients with peritoneal implants is ineffective [Citation9]. The use of target medications is in most cases restricted to intestinal type GC, characterised by haematogenous cancer spread [Citation10].
Cytoreductive surgical procedures (in cases of GC with peritoneal implants: gastrectomy + lymphadenectomy D2 + partial peritonectomy) are based on the principle of aggressive cytoreductive surgery in order to minimise the intraperitoneal tumour load and ensure that the subsequent chemohyperthermic treatment of residual microscopic tumour elements is effective. The fundamental difference between cytoreductive procedures and palliative ones is the excision of not only the loco-regional segment of a disseminated tumour, but also of distant metastases. On completion of the main stage of the surgery and in order to carry out a HIPEC treatment, a closed sterile circuit is created by connecting major catheters positioned in the peritoneum with automatic thermostatic equipment, which allows perfusion of the peritoneum with a solution of cytostatic drugs in the hyperthermic mode with permanent thermal monitoring of the patient’s body at different levels.
The aim of this study was to evaluate the effect of combined modality treatment, consisting of aggressive cytoreduction in combination with HIPEC, in three subsets of patients (those with serosal invasion, those with disseminated peritoneal carcinomatosis, those with peritoneal carcinomatosis and tense ascites) and to define prognostic factors for advanced GC patients who received combination therapy.
Patients and methods
Outcomes for 98 advanced GC patients who were treated at the clinic between 2008–2012 were analysed in this retrospective non-randomised clinical study. Of those patients, 66.3% were male (65) and 33.7% were female (33). The ages of the patients ranged from 22 to 74 years, mean age 56.6 ± 10.2 years. All patients had GC verified morphologically prior to treatment and gave their informed consent to participation in the study. The study was approved by the local ethical committee. GC was staged in the patients according to the criteria of the TNM Classification of Malignant Tumours, 7th edition [Citation11].
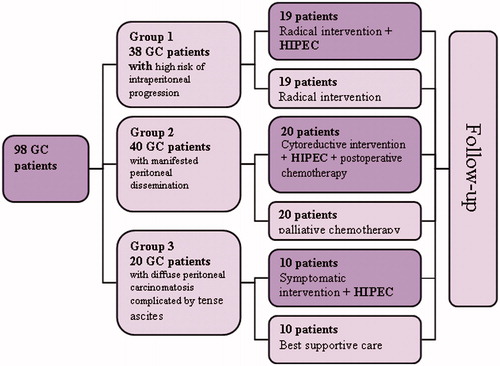
The patients included in the study were divided into three groups. Group 1 consisted of 38 localised or locally advanced GC patients with serosa invasion and consequent high-risk of intraperitoneal progression. Group 1 patients were divided into two subgroups: the study subgroup – these patients underwent standard radical surgery including gastrectomy or subtotal gastrectomy followed by intra-operational HIPEC with adjuvant purposes; and a surgical control subgroup – these patients underwent only standard surgical treatment without adjuvant therapy because it was not confirmed by significant results in randomised trials [Citation12,Citation13]. All anastomoses were hand sewn.
Group 2 consisted of 40 GC patients with apparent peritoneal dissemination. Group 2 patients were divided into two subgroups: the study subgroup – the patients underwent cytoreductive surgery, including gastrectomy or subtotal gastrectomy and partial peritonectomy of peritoneal sections affected by implants, followed by intra-operational HIPEC and systemic post-operative chemotherapy, and a control subgroup – the patients received only standard treatment – systemic palliative chemotherapy.
Group 3 consisted of disseminated intraperitoneal GC patients with diffuse peritoneal carcinomatosis complicated by tense ascites. Group 3 patients were divided into two subgroups: the study subgroup – the patients underwent symptomatic surgery including laparotomy, ascites evacuation and symptomatic HIPEC in order to eliminate recurrent ascites; and a control subgroup – the patients were given best supportive care. The patients of the study group were not given cytoreductive surgery because of the massive character of peritoneal dissemination and the impossibility to achieve complete cytoreduction ().
Patients who had all eligibility criteria for modality treatment with HIPEC were prospectively included. These patients were selected preoperatively according to the following criteria: a confirmed diagnosis of gastric adenocarcinoma, age of less than 80 years, good performance status (Eastern Cooperative Oncology Group score (ECOG) < 2), absence of decompensated co-morbidity. Criteria specific to study subgroup 1 included resectable primary tumour and infiltration of gastric serosa. Criteria specific to study subgroup 2 included resectable primary tumour, no distant extraperitoneal metastasis, degree of intraperitoneal spread according to the Japanese Gastric Cancer Association (JGCA) from CY1 to P2, peritoneal cancer index (PCI) less than 14 and completeness of cytoreduction score (CC) preferably CC-0 and CC-1.
The HIPEC procedure lasted 90 min at the medium intra-abdominal temperature of 42.3 ± 1.3 °C using mitomycin C (MMC) at a dose of 12.5 mg/m2 and cisplatin at a dose of 75 mg/m2. Three trial patients were given bidirectional chemotherapy (HIPEC plus intra-operative intravenous 5-FU).
We performed a retrospective case-control study on highly selected patients with locally advanced and intraperitoneal disseminated GC treated with standard treatment (surgery, palliative chemotherapy or best supportive care) in the clinic during the same period. The control group was selected from the 343 patients who did not receive HIPEC. These patients met eligibility criteria of a confirmed diagnosis of gastric adenocarcinoma, age of less than 80 years, good performance status (ECOG less 2), and absence of decompensated co-morbidity. Criteria specific to control subgroup 1 included resectable primary tumour and infiltration of gastric serosa. Criteria specific to control subgroup 2 included: resectable primary tumour, no distant extraperitoneal metastasis, degree of intraperitoneal spread according to JGCA from CY1 to P2, and PCI of less than 14. This control subgroup of comparable patients had not benefited from HIPEC because the technique was not available from the majority of surgical teams at the clinic. Patients were highly selected to ensure comparability with the study subgroups in accordance with the main clinicopathological parameters.
The degree of dissemination among the patients assigned to the control subgroups was determined according to the results of the diagnostic laparoscopy or explorative laparotomy.
The classification suggested by JGCA is a reliable method of evaluating the degree of intraperitoneal spread of the metastatic process [Citation14]: P0 – no implants on the peritoneum, P1 – isolated disseminates in the upper peritoneum (above the transverse colon level), P2 – isolated disseminates in all parts of the peritoneum, P3 – diffuse carcinomatosis of the peritoneum, including ascites and CY1 – the presence of malignant cells in peritoneal lavage without macroscopic carcinomatosis.
PCI is calculated as follows: the peritoneum is divided into 13 tentative sections, the degree of carcinomatosis being evaluated in each of them depending on the size of implants (1 to 3 points) with a further summing of points for the whole peritoneum [Citation15].
CC is an important prognostic index of cytoreductive operation effectiveness [Citation15]: CC-0 – no macroscopic residual tumour nodules on the peritoneum after cytoreductive operation, CC-1 – residual nodules less than 2.5 mm in diameter, CC-2 – residual nodules from 2.5 mm to 2.5 cm in diameter and CC-3 – the diameter of residual tumour nodules is larger than 2.5 cm.
Source data were processed using the Statistica program version 8 for Windows (StatSoft, Tulsa, OK). The Kaplan-Meier method was used to estimate the cumulative survival. The log-rank significance test was applied to determine the difference in survival between the various groups. Multivariate analysis was conducted by means of discriminative research.
Results
The patients in Group 1 were comparable in accordance with the main clinicopathological parameters ().
Table I. Characteristics of patients in Group 1.
shows the main clinicopathologic parameters of the patients ingroup 2.
Table II. Characteristics of patients in Group 2.
The scores of cytoreduction completeness among 20 patients in Group 2 were as follows: 15 patients (75%) CC-0, three patients (15%) CC-1, and two patients (10%) CC-2.
In subgroup II, 12 (60%) out of 20 patients receiving HIPEC as part of the combined modality treatment at the post-operative stage underwent chemotherapy according to the following schemes: ECF four patients (20%), CF four patients (20%), CAF two patients (10%), 5-FU one patient (5%) and tegafur one patient (5%). From the control subgroup II, 17 patients (85%) underwent systemic palliative chemotherapy according to the following schemes: XELOX one patient (5%), CF six patients (30%), CAF four patients (20%), 5-FU four patients (20%), tegafur two patients (10%); three (15%) patients received best supportive care.
The average amount of peritoneal fluid in the abdominal cavity among patients from Group 3 equalled 5.5 ± 1.4 L (from 3.5 to 8 L).
The average hospital stay was 24.3 ± 5.7 days (range 16–48 days).
At the completion of best surgical effort at cytoreduction using HIPEC, 13 out of 49 patients (26.5%) developed post-operative complications. Among them, seven patients (14.3%) developed surgical complications, six patients (12.2%) had complications related to HIPEC and one patient (2%) experienced somatic complications. Surgical complications included: two (4.1%) patients subhepatic abscess, two (4.1%) infected pancreatic necrosis with purulent-septic complications, one (2%) anastomositis, one (2%) mesenteric thrombosis, and one (2%) gastrointestinal anastomotic leak. The complications related to HIPEC included: one (2.6%) patient grade III–IV nephrotoxicity (according to the Clinical Toxicity Criteria of the National Cancer Institute of Canada (CTC NCIC) [Citation16]), one (2.6%) grade III leukopenia, one (2.6%) significant intestinal distention, one (2.6%) systemic increase in body temperature during the HIPEC procedure to 40.2 °C, one (2.6%) acute enterocolitis, one (2.6%) patient combination of grade III nephrotoxicity and long-term enterocolitis. As for general somatic complications, nosocomial pneumonia was seen in one patient (2.6%).
Following combined modality treatment with HIPEC, post-operative mortality occurred in two (4.1%) out of 49 patients: mesenteric thrombosis was seen in one patient with generalised atherosclerosis, and necrotic pancreatitis with purulent-septic complications was seen in one patient as well.
Minimal follow-up time in patients receiving cytoreductive surgery and HIPEC was 12 months.
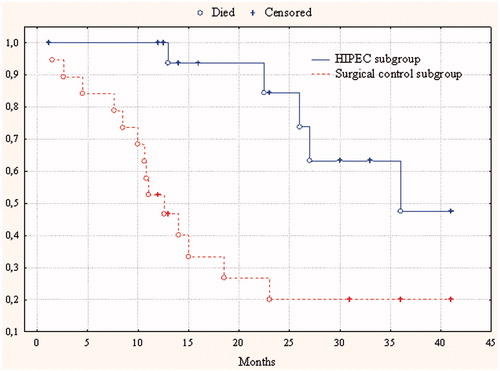
In Group 1, 19 patients were diagnosed as at high risk of intraperitoneal progression and underwent HIPEC with adjuvant purposes. Median and 1-year survival rates were 22.5 ± 6.5 months (95%CI 9.7–35.3) and 100%, respectively, and in 19 patients from the surgical control subgroup, 12 ± 1.3 months (95%CI 9.4–14.6) (p = 0.002) and 52.6% respectively ().
Figure 2. Cumulative censored overall survival in gastric cancer patients with a high risk of intraperitoneal disease progression after the HIPEC procedure in adjuvant regime and in the surgical control subgroup.
The intraperitoneal recurrence rate in patients in Group 1 receiving combined treatment with HIPEC was11.1% and in patients assigned to the surgical control subgroup it was 73.7% (p < 0.001).
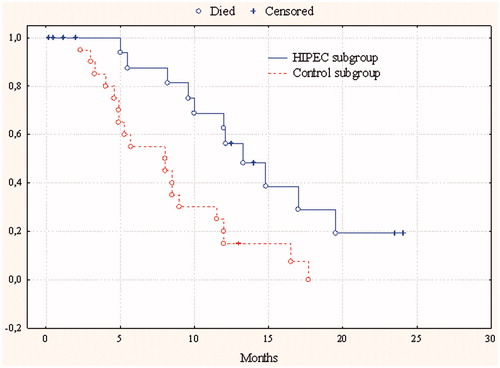
In 20 patients from Group 2 (with implant-associated manifestation), after combined treatment with HIPEC, median and 1-year survival were 12 ± 1.6 months (95%CI 8.9–15.1) and 68.8% respectively, in 20 patients from the control subgroup receiving palliative chemotherapy, 8 ± 2.6 months (95%CI 2.99–13) (p = 0.004) and 25% respectively ().
Figure 3. Cumulative censored overall survival in gastric cancer patients with peritoneal dissemination after combined therapy with HIPEC and in the control subgroup.
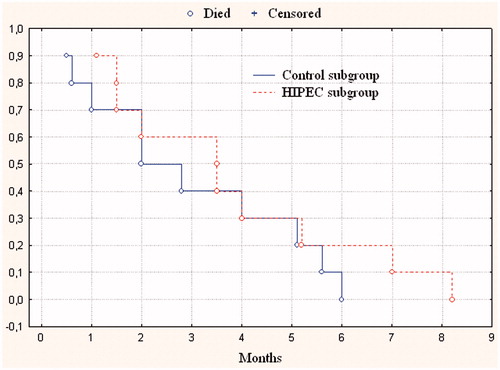
In 10 patients with tense malignant ascites from Group 3 undergoing combination therapy with the use of HIPEC, median survival was 3.5 months, and in 10 patients from the control subgroup it was 2.4 months, the difference in survival was not statistically significant (p = 0.49) ().
Figure 4. Cumulative censored overall survival in gastric cancer patients with diffuse peritoneal carcinomatosis with symptomatic ascites after use of HIPEC and after best supportive care.
The repetitive procedures (from 1 to 9) of laparocentesis and ascites evacuation were conducted in order to improve the quality of life of all patients in the control subgroup. The average number of laparocentesis procedures was 3.6 ± 2.1. In the HIPEC subgroup only two patients (20%) had to undergo laparocentesis procedure due to ascites recurrence.
The independent prognostic factors in patients with disseminated intraperitoneal gastric cancer after aggressive surgical cytoreduction and HIPEC were identified by means of multivariate analysis according to the classification of the Japanese Gastric Cancer Association a degree of peritoneal dissemination (p = 0.004), and a score of cytoreduction completeness (p = 0.031).
Discussion
Serosa-invasive GC exhibits a poor prognosis and a high-risk of metachronous peritoneal carcinomatosis, which develops as a result of intraperitoneal dissemination of the microscopic intraperitoneal tumour cell pool before or during surgery.
For a long time the use of adjuvant chemotherapy in patients with resectable GC was not supported by significant data from randomised trials; however, intraperitoneal chemohyperthermia led to increased survival rates in serosa-invasion GC patients. At present, the results of three meta-analyses on this issue [Citation17–19] have already been published. However, this approach has not been accepted as a standard treatment used in the daily practice of oncology surgeons. Our study results show a doubling of survival rate in patients undergoing HIPEC in adjuvant regime and reduction of peritoneal recurrence from 73.7% to 11.1%. In the study subgroup intraperitoneal progression was seen in two patients, bearing in mind that one of them had surgery and HIPEC 3 years ago.
Cytoreduction surgery involves maximum excision of tumour mass from the patient’s body as well as metastatic lesions in order to minimise the intraperitoneal tumour cell pool level and ensuring that the subsequent treatment of residual microscopic tumour cell proliferation with the use of cytotoxic agents is effective. HIPEC therapy aims at the destruction of the residual microscopic intraperitoneal tumour cell pool by means of intraperitoneal application of two synergistic anti-tumour factors – chemotherapy and hyperthermia [Citation20].
In 1996, Yonemura in collaboration with his colleagues published the results of the first large clinical trial [Citation21] on the efficacy of HIPEC (mitomycin 30 mg + cisplatin 300 mg +etoposide 150 mg, 60 min at 42–43 °C) in combination with aggressive cytoreductive surgery, including gastrectomy, extended regional lymphadenectomy and partial or subtotal peritonectomy in GC patients with peritoneal carcinomatosis. As a result they achieved 1-year survival in 43% of patients and for the first time 5-year survival was seen for 11% of patients with such a poor prognosis.
In 2010, Glehen and his colleagues from Lyon published the summarised retrospective results of a French national clinical study [Citation22] of the results gained from the treatment of 159 patients from 15 surgery centres. Median overall survival was 9.2 months, and 1-year, 3-year and 5-year survival rates were 43%, 18% and 13%, respectively. The score of cytoreduction completeness was considered to be the only independent prognostic factor identified by means of multivariate analysis. Those patients with CC-0 had better results: the median was 15.0 months, 1-, 3- and 5-year survival time of 61%, 30% and 23% respectively.
Our study results also showed an advantage in terms of survival in GC patients with peritoneal metastases after aggressive cytoreductive surgery in combination with HIPEC and achievements in long-term survival among selected patients. Two of our patients are alive more than 2 years after treatment and one of them has no sign of disease.
The applicability of PCI as a prognostic factor for metastatic GC and the selection criteria for combination therapy have been presented at international conferences on intraperitoneally disseminated tumours (Milan, 2006; Uppsala, 2010; Berlin, 2012). It has been suggested that cytoreductive therapy and HIPEC should be used in patients with PCI 10–13. In Glehen’s study [Citation22], patients with PCI of greater than 12 did not reach the 3-year survival end point, and patients with PCI exceeding 19 did not reach 6-month survival. We achieved improved survival using combination therapy in patients with PCI below 14. The question of patient selection, therefore, remains important, and further studies on the boundary level peritoneal dissemination of the GC are required.
Despite the failure to improve survival in patients with symptomatic ascites, HIPEC with symptomatic purposes allows effective elimination of recurrent ascites.
This way, the use of HIPEC in combined treatment of advanced GC demonstrates positive results with extended survival time among selected patients. However, clear understanding of various regimes of HIPEC use allows objective evaluation of treatment results in different GC patient groups.
Conclusions
The use of combination therapy with HIPEC in advanced GC patients is considered to be a safe treatment with acceptable levels of post-operative complications and mortality.
HIPEC in an adjuvant regimen used to treat GC patients with a high risk of intraperitoneal progression allowed reduction of peritoneal metachronous carcinomatosis from 73.7% in the surgical control subgroup to 11.1% (p < 0.001), and significantly improved patients’ survival.
Cytoreductive surgery, HIPEC and systemic post-operative chemotherapy used to treat GC patients with limited extent of peritoneal carcinomatosis resulted in median and 1-year survival times equal to 12 months and 68.8%, whereas palliative chemotherapy applied to those control subgroup patients resulted in median survival times of 8 months and 25%, respectively (p = 0.004).
The symptomatic use of HIPEC in GC patients with diffuse peritoneal carcinomatosis complicated by symptomatic ascites did not significantly increase survival, it allowed effective elimination of recurrent ascites.
The independent prognostic factors in GC patients with peritoneal metastases undergoing combined treatment with HIPEC are the stage of peritoneal dissemination in compliance with the classification of the Japanese Gastric Cancer Association and the score of cytoreduction completeness.
Declaration of interest
The authors declare that they have no proprietary, financial or other personal interest associated with this article. The authors alone are responsible for the content and writing of the paper.
References
- Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, et al. Pseudomyxoma peritonei: A French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2010;36:456–62
- Baratti D, Scivales A, Balestra MR, Ponzi P, DiStasi F, Kusamura S, et al. Cost analysis of the combined procedure of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol 2010;36:463–9
- Sommariva A, Pilati P, Rossi CR. Cytoreductive surgery combined with hyperthermic intra-peritoneal chemotherapy for peritoneal surface malignancies: Current treatment and results. Cancer Treat Rev 2012;38:258–68
- Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J SurgOncol 2010;101:713–24
- Nissan A, Garofalo A, Esquivel J. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy (HIPEC) for gastric adenocarcinoma: Why haven’t we reached the promised land? J Surg Oncol 2010;102:359–60
- Yatsenko LD. Peculiarities of stage IV metastatic gastric cancer. Oncology (Ukraine) 2007;9:139–44
- Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg 1999;178:256–62
- Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236–42
- Macdonald JS. Advances in the management of gastric cancer. New York: CMP Medica, 2006
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 7th edition. Chichester: Wiley-Blackwell, 2010
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97
- Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: Meta-analysis of randomized trials. J Clin Oncol 1993;11:1441–7
- Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur J Surg 2002;168:597–608
- Japanese Gastric Cancer Association. Japanese classification of gastric cancer, 2nd ed. Gastric Cancer 1998;1:10–24
- Sugarbaker PH. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbeck’s Arch Surg 1999;384:576–87
- Cassidy J, Bissett D, Spence R, Payne M. Oxford Handbook of Oncology, 3rd ed. Oxford: Oxford University Press, 2010
- Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng OR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004;10:2727–30
- Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for respectable gastric cancer. Ann Surg Oncol 2007;14:2702–13
- Mi DH, Li Z, Yang KH, Cao N, Lethaby A, Tian JH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: A systematic review and meta-analysis of randomised controlled trials. Int J Hyperthermia 2013;29:156–67
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hypertherm 2007;23:431–42
- Yonemura Y, Fujimura T, Nishimura G, Falla R, Sawa T, Katayama K, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996;119:437–44
- Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370–7