Abstract
Background: Peritoneal carcinomatosis (PC) arising from colorectal cancer is associated with poor prognosis and few treatment options are currently available. Laparoscopic CO2 insufflation stimulates the progression and metastatic potential of gastrointestinal carcinomas. However, heated and humidified CO2 pneumoperitoneum (HH-CO2) is a promising treatment for PC, although its effects and mechanism of action in human colon cancer cells remain unclear. This study evaluated the anti-tumour effects of HH-CO2 on human colon cancer in vitro. Methods: Cell viability was assessed using the WST-8 assay in two colon cancer cell lines. Apoptosis was assessed by Annexin V PI flow cytometry, and migration and invasion were examined using wound healing and Transwell® invasion assays. The expressions of Bcl-2, Bax, matrix metalloproteinase-2 (MMP-2), E-cadherin, ICAM-1, and CD44 were detected by western blotting. Results: HH-CO2 significantly inhibited cell proliferation, migration, invasion and adhesion. HH-CO2 induced apoptosis and significantly inhibited the expression of Bcl-2, MMP-2, ICAM-1 and CD44, and increased Bax and E-cadherin expression in colon cancer cells. Conclusions: HH-CO2 induces apoptosis and inhibits proliferation, migration, invasion and adhesion of human colon cancer cells. Our results suggest that HH-CO2 may serve as a potential candidate for the treatment and/or prevention of peritoneal carcinomatosis from colorectal cancer and warrant further in vivo investigation.
Introduction
Peritoneal carcinomatosis (PC) from colorectal cancer arises from the intra-peritoneal seeding of cancer cells that are shed from a primary tumour either spontaneously or as a result of spillage during surgical procedures [Citation1]. In the past, patients with PC were treated with palliative methods because of the poor prognosis and fatal outcomes associated with this disease [Citation2,Citation3]. However, in recent years, the development of aggressive surgical procedures in combination with intraperitoneal chemotherapy resulted in prolonged survival and even curative effects, as reported in several studies [Citation4–6]. Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemoperfusion (HIPEC) has become popular in the last two decades [Citation7,Citation8], and patient survival after CRS and HIPEC, which is associated with the extent of cytoreduction, was shown to be superior to that achieved with palliative surgery and systemic chemotherapy [Citation9–11]. However, the combination of CRS and intraperitoneal chemotherapy is associated with a high rate of morbidity and mortality and only patients with low-volume, low-grade peritoneal disease benefit from this approach [Citation12,Citation13], indicating that new therapeutic strategies for the treatment of PC are needed.
The use of hyperthermia as a therapeutic tool for the treatment of malignancies including colorectal cancer has been well documented [Citation14–16]. Hyperthermia itself exerts several cellular and molecular effects that enhance anti-tumour immune responses [Citation17,Citation18]. In addition, thermotherapy increases the sensitivity of cancer cells to chemotherapeutic agents [Citation19,Citation20] and studies have shown that hyperthermia enhances the cytotoxic effects of certain chemotherapeutic agents in colorectal cancer [Citation21]. In pneumoperitoneum, which is generated for adequate visualisation and manipulation during surgery, changing the conditions of the gas to physiological conditions regarding temperature and humidity maintains a physiological environment important to improve patient outcomes [Citation22–24].
In the present study we investigated the anti-tumour effects of heated and humidified CO2 (HH-CO2) pneumoperitoneum on colon cancer cells in vitro. We used two different human colon cancer cell lines and showed that the creation of a HH-CO2 pneumoperitoneum at different temperatures inhibited cell proliferation, induced apoptosis, and inhibited the migration, invasion and adhesion of colorectal cancer cells, suggesting that HH-CO2 pneumoperitoneum may serve as a potential new strategy for the treatment or prevention of PC from colorectal cancer.
Materials and methods
Cell lines and culture
The human CRC cell lines COLO 205, and HCT 116 were obtained from the American Type Culture Collection (Rockville, MD) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco, UK), and maintained at 37 °C in a humidified 95% atmosphere containing 5% CO2.
HH-CO2 study model
An in vitro HH-CO2 model was designed to stimulate the conditions of hyperthermic CO2 pneumoperitoneum in the human body (patent under consideration by the State Intellectual Property Office, Shanghai, China, patent numbers 200620047773.6, 200620047772.1, 200610118324.0). The details of the model have been described previously [Citation25]. In this system, cells are exposed to HH-CO2 at constant temperature, pressure, flow rate and humidity under stable conditions (±0.3 °C, ±1 mmHg, ±0.5 l/min and ±5%, respectively).
Experimental design
The cells were divided into three groups and treatments were as follows: 1) heated and humidified CO2 pneumoperitoneum (HH-CO2): the cells were treated with the in vitro HH-CO2 study model and exposed to heated CO2 pneumoperitoneum at various temperatures (42–44 °C), with 15 mmHg pressure, 10 L/min flow rate and >95% humidity for 3 h; 2) normo-thermic CO2 pneumoperitoneum: CO2 at room temperature was insufflated into the pneumoperitoneum chamber using Stryker’s 40-L insufflator (Stryker, Kalamazoo, MI). Cells grown in culture dishes were placed inside the chamber and exposed to CO2 at room temperature (25 °C) with 15 mmHg pressure and 10 L/min flow rate for 3 h; 3) conventional incubation: the cells were incubated conventionally (37 °C, >95% humidity, 5% CO2).
Cell viability assay
Cell viability was determined with the WST-8 cytotoxicity assay using a cell Counting Kit-8 (CCK-8) (Beyotime Inst Biotech, Beijing, China) according to the manufacturer’s instructions. Briefly, cells were seeded onto a 96-well microplate at a density of 5 × 103 cells/well. Cells were treated as indicated and further incubated for 6 h. A volume of 10 μL of the CCK-8 solution was then added to each well, incubated for 4 h and the absorbance was read in a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm. Values were calculated using the following formula: cell viability (%) = (mean treated absorbance/mean untreated absorbance) × 100%.
Annexin V and PI flow cytometry
Apoptosis was assessed by Annexin V and PI flow cytometry using an Annexin V FITC apoptosis detection kit I (BD Biosciences, San Diego, CA) following the manufacturer’s instructions. Briefly, cells were treated as indicated, harvested, washed twice with ice-cold PBS and resuspended in 1× Annexin V binding buffer at a density of 1 × 106 cells/mL. Then, Annexin V FITC (5 μL) and PI (5 μL) were added, incubated for 15 min at room temperature in the dark and analysed with a FACS Calibur (FACScan, Becton Dickinson, Mountain View, CA). Data were analysed using Cellquest software (Becton Dickinson).
Western blot analysis
Cells were harvested at the indicated times after treatment, and protein was extracted using the M-PER™ Mammalian Protein Extraction Reagent (Santa Cruz Biotechnology, Santa Cruz, CA) and a Nuclear Extract Kit (Active Motif, Carlsbad, CA). Proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride membranes, blocked and probed with the following antibodies: rabbit anti-Bcl-2, anti-Bax, anti-ICAM-1, anti-E-cadherin, anti-CD44 (Santa Cruz Biotechnology), anti-MMP-2 (Cell Signaling Technology, Danvers, MA), and anti- β-actin as a loading control (Sigma-Aldrich, St Louis, MO). Blots were then incubated with peroxidase-conjugated anti-mouse/rabbit secondary antibodies (Santa Cruz Biotechnology) and protein bands were visualised using the Super Enhanced chemiluminescence visualisation kit (Applygen Technologies, China). Bands were quantified using densitometric image analysis software (Quantity One, Bio-Rad, Hercules, CA). The relative expressions of Bcl-2, Bax, E-cadherin, ICAM-1, CD44 and MMP-2 were normalised to that of β-actin.
In vitro wound healing assay
For wound healing migration assays, COLO 205 and HCT 116 cells were grown in a monolayer to approximately 60% confluency. A cell-less strip was generated by scraping the cells off the middle of the monolayer with a 200-μL pipette tip. Wound closure was monitored at the time of wounding (0 h) and at 36 h after wounding using microphotography (100× magnification) under a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions, Cambridge, UK). The assay was repeated three times. Wound healing rate was calculated according to the following formula.
Experiments were repeated at least three times.
In vitro Transwell® invasion assays
For Transwell invasion assays, Matrigel (BD Biosciences, Franklin Lakes, NJ) was allowed to polymerise at the base of the top chamber of a 24-well Transwell plate (8 μm, Corning Costar, Corning, NY) for 45 min at 37 °C. COLO 205 and HCT 116 cells (1 × 105 cells/well) were serum and growth factor starved for 24 h, treated with HH-CO2 at 42 °C, 43 °C, 44 °C for 3 h and added to the top chambers. The bottom chambers were filled with serum-containing medium. Non-invading cells were removed from the upper surface of the membrane with cotton swabs. Invading cells were fixed in methanol, stained with 0.1% crystal violet for 15 min at room temperature and photographed at 200× magnification. Invading cells were counted in 10 fields and the mean ± standard deviation (SD) was calculated. Assays were performed in triplicate wells and repeated twice.
Statistical analysis
The data are expressed as mean ± SD. The differences between groups were determined by one-way analysis of variance (ANOVA) or two-tailed paired Student’s t-test with SPSS 11.5 software (Chicago, IL). Differences were considered statistically significant at p < 0.05.
Results
HH-CO2 inhibits colon cancer cell viability and induces apoptosis
The effect of HH-CO2 on cell viability was assessed in the human colorectal cancer cell lines COLO 205 and HCT 116 using the WST-8 assay (). The results showed that HH-CO2 reduced cell viability in both cell lines in a time- and temperature-dependent manner. Treatment of cells at 44 °C for 4 h reduced cell viability to approximately 35% and 50% in COLO 205 and HCT 116 cells, respectively.
Figure 1. HH-CO2 inhibits colon cancer cell proliferation and induces apoptosis. Temperature- and time-dependent effects of HH-CO2 on COLO 205 and HCT 116 cell proliferation. (B) Flow cytometry-based Annexin V FITC PI labelling of apoptotic cells. (C) Histograms showing apoptosis rates. Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
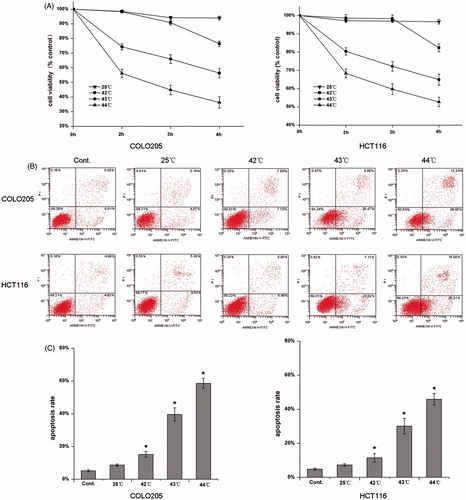
To determine whether the cytotoxic effect of HH-CO2 is mediated by the induction of apoptosis, COLO 205 and HCT 116 cells were analysed by Annexin V and PI flow cytometry after 3 h of exposure to HH-CO2 at different temperatures (). The results showed that HH-CO2 increased the percentage of apoptotic cells from approximately 8% in the controls to approximately 60% in COLO 205 and 45% in HCT 116 cells after 4 h of exposure at 44 °C (p < 0.05), suggesting that the cytotoxic effect of HH-CO2 is mediated by the induction of apoptosis. Representative cytograms showing the temperature-dependent induction of apoptosis by HH-CO2 in COLO 205 and HCT 116 are shown in .
HH-CO2 cytotoxicity is mediated by the Bax mitochondrial apoptosis pathway
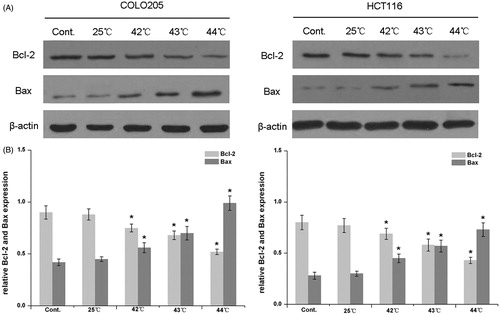
The involvement of apoptosis-related proteins in the cytotoxic effect of HH-CO2 was examined by western blot analysis of the expression levels of Bcl-2 and Bax in COLO 205 and HCT 116 cells exposed to HH-CO2 at different temperatures (). The results showed that HH-CO2 treatment significantly up-regulated the expression of Bax and down-regulated the expression of Bcl-2 in both cell lines in a temperature-dependent manner (p < 0.05). The relative Bcl-2/Bax expression after exposure to HH-CO2 at different temperatures is shown in . These results indicate that HH-CO2 exerts its cytotoxic effect on colorectal carcinoma cells via the Bax mitochondrial apoptosis pathway.
Figure 2. Effect of HH-CO2 on Bcl-2 and Bax expression. The expressions of Bcl-2 and Bax in COLO 205 and HCT 116 cells were assessed by western blotting. (B) Histograms showing the expressions of Bcl-2 and Bax relative to that of β-actin. Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
HH-CO2 inhibits the migration and invasion of colon cancer cells
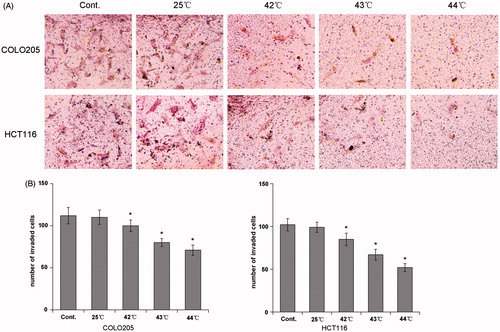
To determine the effect of HH-CO2 on the migration of colon cancer cells, COLO 205 and HCT 116 cells were treated with HH-CO2 at 25 °C and 43 °C, and migration was assessed using the wound healing assay. The results showed that HH-CO2 inhibited cell migration in both cell lines, although the effect was only significant at 43 °C (). Histograms showing the wound healing rate in COLO 205 and HCT 116 cells are shown in . The effect of HH-CO2 on cell invasion was assessed using the Transwell assay, which showed that treatment with HH-CO2 significantly inhibited cell migration in COLO 205 and HCT 116 cells in a temperature-dependent manner (p < 0.05) ().
Figure 3. HH-CO2 inhibits monolayer wound healing of colon cancer cells. (A) Phase micrographs of COLO 205 and HCT 116 cells at various times after monolayer wounding (100× original magnification). (B) Quantification of COLO 205 and HCT 116 cell migration using the monolayer wound healing assay. Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
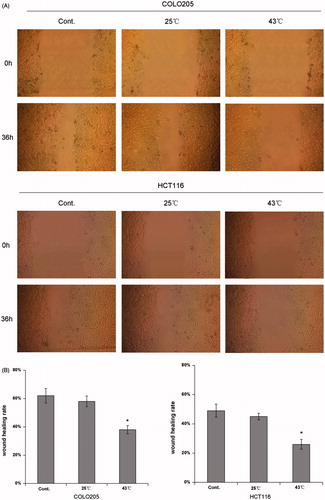
Figure 4. HH-CO2 inhibits colon cancer cell invasion. (A) Phase micrograph of invading COLO 205 and HCT 116 cells. COLO 205 and HCT 116 cells were treated with HH-CO2 at 42 °C, 43 °C, 44 °C for 3 h. Invading cells were fixed with methanol and stained with crystal violet and photographed at 200× magnification. (B) Quantification of cell invasion shown in (A). Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
HH-CO2 inhibits the expression of invasion- and adhesion-related proteins
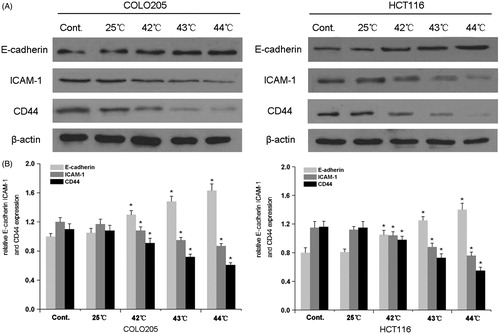
To further examine the mechanisms underlying the effect of HH-CO2 on migration and invasion in colon cancer, the expression of matrix-metalloproteinase-2 (MMP-2), which is an important marker associated with the invasive and metastatic potential of cells, was assessed by western blotting in COLO 205 and HCT 116 cells. The results showed a temperature-dependent down-regulation of MMP-2 in both cell lines, which was statistically significant at temperatures ≥ 42 °C (p < 0.05) (). Because reduced intercellular adhesion is implicated in the development of metastasis, we examined the expression of the adhesion molecules E-cadherin, intracellular adhesion molecule 1 (ICAM-1), and CD44 in response to HH-CO2 in COLO 205 and HCT 116 cells. The results showed that HH-CO2 significantly up-regulated E-cadherin and down-regulated ICAM-1 and CD44 in a temperature dependent manner at temperatures ≥42 °C in both cell lines (p < 0.05 for all), indicating that HH-CO2 decreased the metastatic potential of colon cancer cells. and show the results of densitometric quantification of bands in the respective western blots showing the levels of expression of MMP-2, E-cadherin, ICAM-1, and CD44 relative to the levels of β-actin.
Figure 5. HH-CO2 inhibits MMP-2 expression in colon cancer cells. (A) MMP-2 expression in COLO 205 and HCT 116 cells was detected by western blotting. β-actin was used as the loading control. (B) Histograms showing the expression of MMP-2 relative to that of β-actin. Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
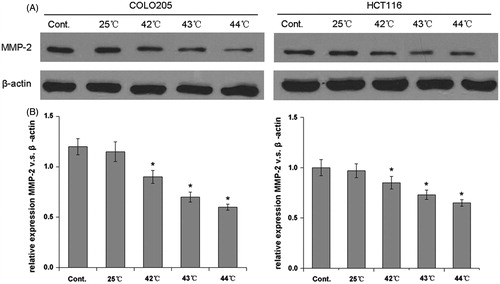
Figure 6. Effect of HH-CO2 on the expression of adhesion molecules. E-cadherin, ICAM-1 and CD44 expressions in COLO 205 and HCT 116 cells were detected by western blot analysis. (B) Histograms showing the expression of E-cadherin, ICAM-1 and CD44 relative to that of β-actin. Each data point represents the mean ± SD from three independent experiments. *p < 0.05 versus control.
Discussion
The creation of a pneumoperitoneum with CO2 as the insufflation gas is used in laparoscopic surgery to facilitate visualisation and operative manipulation. The induction of hyperthermia has many applications, among them hyperthermic intraperitoneal chemotherapy, which in combination with cytoreductive surgery has shown favourable results in the treatment of residual microscopic PC. In the present study, we examined the effect of heated and humidified CO2 pneumoperitoneum as a potential new strategy for the treatment of PC from colorectal cancer. Our results show that HH-CO2 had cytotoxic effects on colon cancer cells mediated by the induction of apoptosis. Furthermore, HH-CO2 decreased the invasive and metastatic potential of colon cancer cells as determined by wound healing assay, Transwell invasion assay and changes in the expression of MMP-2 and adhesion molecules in cells exposed to HH-CO2.
PC as a form of locoregional cancer dissemination as opposed to systemic metastases was traditionally treated with cytoreductive surgery in combination with perioperative intraperitoneal chemotherapy [Citation26]. However, the intraperitoneal administration of high doses of chemotherapeutic drugs is associated with high morbidity because of the systemic toxicity of these agents. The use of hyperthermia with intraperitoneal chemotherapy increases drug penetration in addition to having an anti-tumour effect itself [Citation27], and its application in combination with cytoreductive surgery has been reported extensively [Citation28–31]. Furthermore, acidification has been shown to enhance the cytotoxic effect of hyperthermia [Citation32]. In the present study we extended these findings by assessing the effect of humidification in addition to hyperthermia and acidification, and showed that HH-CO2 has a significant cytotoxic effect and it decreases the metastatic potential of colorectal cancer cells. The use of HH-CO2 insufflation has been reported previously as an improved method for the creation of a pneumoperitoneum during laparoscopic surgery. HH-CO2 was reported to prevent hypothermia, reduce post-operative pain, shorten recovery and reduce tumour spread [Citation22,Citation23,Citation33,Citation34]. The cell-killing and metastasis-inhibiting effects of HH-CO2 demonstrated in the present study could help explain its beneficial effects during laparoscopic surgery and suggest that it could be developed as a strategy for the treatment of PC from colorectal cancer.
Hyperthermia sensitises cancer cells to chemotherapy. Our results showing that the cytotoxic effects of HH-CO2 are mediated by the induction of apoptosis may help explain this effect. Furthermore, we showed the involvement of the Bax-associated mitochondrial apoptotic pathway in the cytotoxicity of HH-CO2 in colon cancer cells. The involvement of mitochondrial apoptosis in the effect of hyperthermia has been reported previously [Citation20,Citation25,Citation35]. However, the exact role of p53 and Bax in the hyperthermia-induced cytotoxicy in colorectal cancer is controversial and appears to be cell-line dependent [Citation25]. Whereas heat shock triggers apoptosis in Bax wild-type HCT 116 cells, no effect is observed in Bax−/− cells, indicating the Bax-dependency of hyperthermia-induced apoptosis [Citation36]. Similar results were obtained by Zhang et al., who showed that hyperthermia induces cell cycle arrest at the G0/G1 phase through the up-regulation of p53 and Bax expression [Citation20]. On the other hand, no involvement of Bax or Bcl-2 was observed in wild-type p53 colorectal cancer RKO.C cells or p53 deficient RC10.1 cells subjected to hyperthermia and acidification [Citation32]. In the present study, the effects of HH-CO2 on cell viability were shown to be mediated by the induction of apoptosis via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway in colon cancer cells. Further investigation of the mechanisms underlying the effect of HH-CO2 in a comparative analysis of different cell lines may reveal specific molecules involved in this process, which could be of value for the application of HH-CO2 pneumoperitoneum in the treatment of PC.
In the present study, the results of wound healing and Transwell assays show that HH-CO2 reduced the migration and invasion of colon cancer cells. Furthermore, HH-CO2 down-regulated MMP-2, ICAM-1 and CD44 expression and up-regulated E-cadherin expression, which indicated that the induction of a HH-CO2 pneumoperitoneum reduces the metastatic and invasive potential of colon cancer cells. Similar results were reported recently by Zhou et al., who showed that hyperthermic CO2 pneumoperitoneum suppressed gastric cancer cell invasion and metastasis [Citation37]. CO2 insufflation was also shown to alter the expression of E-cadherin, ICAM-1 and CD44 in colon cancer cells, consistent with a reduction in the adhesive and invasive capacity of these cells [Citation38], which supports the results of the present study.
In conclusion, in the present study we investigated HH-CO2 as a potential new strategy for the treatment of PC from colorectal cancer. Our results showed that HH-CO2 significantly reduced the cell viability of colorectal cancer cells via the induction of mitochondrial apoptosis and reduced the invasive and metastatic potential of colon cancer cells. Our results suggest that HH-CO2 could be developed as a novel stand-alone or adjunctive therapeutic strategy for the treatment of PC from colorectal cancer.
Declaration of interest
The study was funded by Shanghai Municipal Science and Technology commission (114119a4900). The authors alone are responsible for the content and writing of the paper.
References
- Ceelen WP, Bracke ME. Peritoneal minimal residual disease in colorectal cancer: Mechanisms, prevention, and treatment. Lancet Oncol 2009;10:72–9
- Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545–50
- Bloemendaal AL, Verwaal VJ, van Ruth S, Boot H, Zoetmulder FA. Conventional surgery and systemic chemotherapy for peritoneal carcinomatosis of colorectal origin: A prospective study. Eur J Surg Oncol 2005;31:1145–51
- Esquivel J, Chua TC. CT versus intraoperative peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: Importance of the difference between statistical significance and clinical relevance. Ann Surg Oncol 2009;16:2662–3
- Kecmanovic DM, Pavlov MJ, Ceranic MS, Sepetkovski AV, Kovacevic PA, Stamenkovic AB. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:147–52
- Sugarbaker PH. Carcinomatosis – Is cure an option? J Clin Oncol 2003;21:762–4
- Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: A consensus statement. Ann Surg Oncol 2007;14:128–33
- Maggiori L, Elias D. Curative treatment of colorectal peritoneal carcinomatosis: Current status and future trends. Eur J Surg Oncol 2010;36:599–603
- Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol 2004;22:3284–92
- Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Experience with 501 procedures. J Am Coll Surg 2007;204:943–53
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43
- Antoun S, Meshaka P, Soltani D, Blot F, Ducreux M, Lasser P, et al. [Complications and tolerance of heated intraperitoneal chemotherapy and cytoreductive surgery for peritoneal carcinomatosis: Results of a phase I-II study of peritoneal carcinomatosis from different sources] [in French]. Bull du Cancer 2000;87:665–70
- Chen MY, Chiles C, Loggie BW, Choplin RH, Perini MA, Fleming RA. Thoracic complications in patients undergoing intraperitoneal heated chemotherapy with mitomycin following cytoreductive surgery. J Surg Oncol 1997;66:19–23
- Hildebrandt B, Drager J, Kerner T, Deja M, Loffel J, Stroszczynski C, et al. Whole-body hyperthermia in the scope of von Ardenne’s systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: A phase I/II study. Int J Hyperthermia 2004;20:317–33
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005;23:3079–85
- Schlemmer M, Lindner LH, Abdel-Rahman S, Issels RD. [Principles, technology and indication of hyperthermia and part body hyperthermia] [in German]. Radiologe 2004; 44:301–9
- Knippertz I, Stein MF, Dorrie J, Schaft N, Muller I, Deinzer A, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011;27:591–603
- Mukhopadhaya A, Mendecki J, Dong X, Liu L, Kalnicki S, Garg M, et al. Localized hyperthermia combined with intratumoral dendritic cells induces systemic antitumor immunity. Cancer Res 2007;67:7798–806
- Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315–25
- Zhang XL, Hu AB, Cui SZ, Wei HB. Thermotherapy enhances oxaliplatin-induced cytotoxicity in human colon carcinoma cells. World J Gastroenterol 2012;18:646–53
- Atallah D, Marsaud V, Radanyi C, Kornprobst M, Rouzier R, Elias D, et al. Thermal enhancement of oxaliplatin-induced inhibition of cell proliferation and cell cycle progression in human carcinoma cell lines. Int J Hyperthermia 2004;20:405–19
- Hamza MA, Schneider BE, White PF, Recart A, Villegas L, Ogunnaike B, et al. Heated and humidified insufflation during laparoscopic gastric bypass surgery: Effect on temperature, postoperative pain, and recovery outcomes. J Laparoendosc Adv Surg Tech A 2005;15:6–12
- Hazebroek EJ, Schreve MA, Visser P, De Bruin RW, Marquet RL, Bonjer HJ. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A 2002;12:355–64
- Savel RH, Balasubramanya S, Lasheen S, Gaprindashvili T, Arabov E, Fazylov RM, et al. Beneficial effects of humidified, warmed carbon dioxide insufflation during laparoscopic bariatric surgery: A randomized clinical trial. Obes Surg 2005;15:64–9
- Peng Y, Zheng M, Feng B, Chen X, Yu B, Lu A, et al. Hyperthermic CO2 pneumoperitoneum induces apoptosis in human colon cancer cells through Bax-associated mitochondrial pathway. Oncol Rep 2008;19:73–9
- Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999;43:S15–25
- Sugarbaker PH. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbecks Arch Surg 1999;384:576–87
- Elias D, Pocard M, Sideris L, Ede C, Ducreux M, Boige V, et al. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg 2004;91:455–6
- Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol 2003;10:508–13
- Schneebaum S, Arnold MW, Staubus A, Young DC, Dumond D, Martin EW Jr. Intraperitoneal hyperthermic perfusion with mitomycin C for colorectal cancer with peritoneal metastases. Ann Surg Oncol 1996;3:44–50
- Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol 2004;11:178–86
- Ohtsubo T, Park HJ, Lyons JC, Ohnishi T, Song CW. Effect of acidic environment and p53 on apoptosis induction by hyperthermia. Int J Hyperthermia 2000;16:481–91
- Margulis V, Matsumoto ED, Tunc L, Taylor G, Duchenne D, Cadeddu JA. Effect of warmed, humidified insufflation gas and anti-inflammatory agents on cytokine response to laparoscopic nephrectomy: Porcine model. J Urol 2005;174:1452–6
- Mouton WG, Bessell JR, Millard SH, Baxter PS, Maddern GJ. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Surg Endosc 1999;13:106–8
- Yuen WF, Fung KP, Lee CY, Choy YM, Kong SK, Ko S, et al. Hyperthermia and tumour necrosis factor-alpha induced apoptosis via mitochondrial damage. Life Sci 2000;67:725–32
- Sturm I, Rau B, Schlag PM, Wust P, Hildebrandt B, Riess H, et al. Genetic dissection of apoptosis and cell cycle control in response of colorectal cancer treated with preoperative radiochemotherapy. BMC Cancer 2006;6:124
- Zhou HM, Feng B, Zhao HC, Zheng MH. Antitumor effects of hyperthermic CO2 pneumoperitoneum on human gastric cancer cells. Asian Pac J Cancer Prev 2012;13:117–22
- Ma JJ, Feng B, Zhang Y, Li JW, Lu AG, Wang ML, et al. Higher CO2-insufflation pressure inhibits the expression of adhesion molecules and the invasion potential of colon cancer cells. World J Gastroenterol 2009;15:2714–22

