Abstract
Introduction: Vapour ablation is used to create lung volume reduction for emphysema patients to improve lung function and quality of life. This study characterises effects of vapour ablation treatment in lung segments within a lobe that are adjacent to lung segments previously treated with vapour in a healthy canine model. Because emphysema is a progressive disease, subsequent treatments could offer continued benefit to the patient. Method: Six healthy canines were treated with vapour at 8.5 cal/g in one upper lobe segment. After a 4-week healing period, the adjacent segment was treated. After a second 4-week healing period, necropsy was performed and the tissue inspected. Clinical effects were monitored during each healing period. Results: Each treatment was well tolerated and no significant abnormalities were observed during the healing phases, including death, pneumothorax, or major decline in health status. Animal health, oxygenation changes, pathology, and airway changes were monitored during the study. Analysis of these end points showed no difference in changes after treatment 2 as compared to changes after treatment 1. Conclusion: In this model, there was no evidence of increased or different clinical observations after a second adjacent vapour ablation. It was not possible to differentiate between the clinical effects of treatment 1 and the clinical effects of treatment 2. These results support investigation of sequential adjacent segmental vapour treatments in humans.
Introduction
The lungs of emphysema patients are hyperinflated and as a result pulmonary function is restricted. There is presently no cure for emphysema; however, the interventional treatment lung volume reduction (LVR) has been shown to improve pulmonary function, shortness of breath and quality of life in certain patients. By removing a portion of the lungs, the remaining lung has more room for respiration.
Vapour ablation is a minimally invasive procedure that uses energy in the form of heated water vapour to achieve lung volume reduction in patients with severe emphysema. The procedure utilises a reusable vapour generator with a disposable balloon catheter for vapour delivery. The catheter is guided with a bronchoscope to deliver vapour to targeted emphysematous lung regions. Vapour ablation in the lung has been shown to safely and effectively improve pulmonary function and quality-of-life in human patients with heterogeneous emphysema [Citation1–3] when an entire upper lobe is treated at once.
Because emphysema is a progressive disorder, improvements from any therapy eventually subside as the condition worsens [Citation4]. There is some evidence from lung volume reduction surgery (LVRS) that sequential single-sided upper lobe resection may extend the benefit of LVRS [Citation5]. In a current human study of vapour ablation, one segment of an upper lobe will be treated followed by treatment of two segments in the opposite upper lobe three months later. The strategy is to optimise safety and efficacy by targeting only the most diseased segments. This randomised controlled study of sequential segmental treatment with vapour ablation in patients with upper lobe predominant heterogeneous emphysema will prospectively document 12-month FEV1 (forced expiratory volume) and health-related quality of life 12-month changes (ClinicalTrials.gov Identifier NCT01719263).
However, to provide yet further benefit to the patient with vapour ablation, additional treatments could theoretically be delivered to different segments of the same lobe at a later date. Ablating a segment in a lobe already treated previously has never been attempted surgically or via the various other bronchoscopic lung volume reduction techniques. It is unknown whether recent inflammatory changes in an adjacent segment contribute to an enhanced inflammatory response, or enhanced therapeutic volume loss, following the second treatment.
Therefore, the present study aimed to establish whether additional vapour ablation treatments could be successfully delivered to the remaining untreated segments in the upper lobe. The primary objective was to characterise the safety of vapour ablation in lung segments that are adjacent to previously treated lung segments within the same lobe in a healthy canine model. This canine model has been used successfully previously to demonstrate the effect of vapour ablation in the lung [Citation6–8]. The acquired data will be used to support sequential segmental bronchoscopic treatments in the same lobe in humans.
Methods
Study population and design
Vapour ablation has been studied extensively in the canine model due to its unique collateral ventilation characteristic [Citation6–9], and was therefore chosen as the model in the present study. Six healthy adult mongrel canines (mean weight = 26.0 kg) were treated with vapour (treatment 1), allowed to survive and heal, and then subsequently treated a second time in a separate segment within the same lobe and adjacent to the previous treatment (treatment 2). Treatments occurred in the left upper lobe. Half of the animals were randomly assigned to be treated first in the LB1D1 segment followed by the LB1-distal segment (all tissue of the LB1 segment distal to LB1D1). The nomenclature of the segments is based on the method established by Nakakuki [Citation10]. The remaining animals were treated in the reverse order.
In this study, end points from each canine post-treatment 1 were compared to the end points post-treatment 2 (except for necropsy end points). Treatment 1 end points were the baseline expected outcome control for comparison to treatment 2 end points. The difference between end points post-treatment 2 compared to post treatment 1 was the subject of this investigation. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of American Preclinical Sciences (Coon Rapids, MN). InterVapor by Uptake Medical (Tustin, CA) was used for the vapour ablation treatments.
Study procedures and techniques
Prior to treatment the animal health was assessed through physical examination, clinical pathology, oxygenation, and airway assessment. The vapour ablation treatment was performed on anaesthetised and intubated animals. The vapour catheter was introduced to the target segment through a bronchoscope and the airway was occluded with the balloon. The catheter was attached to the vapour generator and a predetermined dose of 8.5 cal/g of lung tissue was delivered [Citation1–3].
To determine the appropriate treatment time for the 8.5 cal/g dose, it was necessary to estimate the segment tissue mass. A previous canine study used CT imaging to determine upper lobe density as well as helium dilution measurements to determine lung functional residual capacity (FRC) for each of the 19 animals measured [Citation9]. The canines in this previous study were of similar age, weight, and breed as the present study. This previously collected data was leveraged to estimate segment mass in this study with the equation
where average lung density = 0.211 g/mL, based on the density of the upper lobe region as measured by CT analysis, FRC = function residual capacity as estimated by an FRC to bodyweight correlation (), relative segment size = percentage of the entire canine lung as measured with canine lung casts (LB1D1 = 3.3%, LB1-distal 6.4%) [Citation9].
Figure 1. In a previous study [Citation8] FRC and body weight were measured for each animal prior to treatment. A linear regression was done through this dataset and the resultant function (FRC = 40.26 × Weight + 112.21) was used to estimate FRC of each canine prior to treatment. This FRC estimate was used in the calculation of the vapour dose.
![Figure 1. In a previous study [Citation8] FRC and body weight were measured for each animal prior to treatment. A linear regression was done through this dataset and the resultant function (FRC = 40.26 × Weight + 112.21) was used to estimate FRC of each canine prior to treatment. This FRC estimate was used in the calculation of the vapour dose.](/cms/asset/a605ef11-3ea8-4f23-bc9b-74197ff58b09/ihyt_a_925145_f0001_b.jpg)
After an 8.5 cal/g vapour treatment to one segment of the left upper lobe, the animal was recovered from anaesthesia and returned to its housing where it was monitored. Clinical observations and vital signs (temperature, heart rate, respiratory rate) were documented daily by trained animal care personnel, and blood panels were collected and analysed at American Preclinical Services (Minneapolis, MN) and Marshfield Labs (Marshfield, WI).
Four weeks later, once the animals were presumed to have recovered from the first treatment and assessed to be healthy, the second treatment was delivered. Each was anaesthetised and the degree of stenosis in the previously treated segment airway was characterised bronchoscopically. Each treated airway was given a score (+1 dilated, 0 no change from baseline, −1 stenosed (narrowed but not fully occluded), −2 occluded). Following the same method as the first treatment, 8.5 cal/g was delivered to the left upper lobe segment that was not treated previously. Again the animal was returned to its housing where it was monitored for 4 additional weeks.
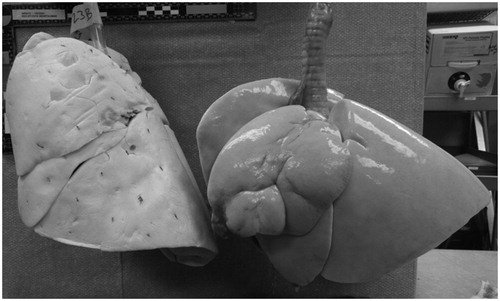
Eight weeks after the first treatment, the airways were again inspected via bronchoscopy and the degree of stenosis characterised. While still under general anaesthesia, the animals were euthanised. At necropsy, the lungs and chest cavity were inspected for any abnormal findings with specific attention given to the phrenic nerves, lungs, oesophagus and trachea, chest wall, and abdominal viscera. The lungs were then removed and inflated with air to a pressure of 20–30 cm H2O and further examined. Two observers visually verified the presence of upper lobe volume reduction observed in the treated lungs as compared to reference lungs from a normal untreated dog of similar size whose lungs had been dried at a similar inflation pressure (). The treatment zones were then examined for differences between the first and second treatment. The examiner was blinded as to which segment was treated first.
Results
All animals were successfully treated in both upper lobe segments. The average LB1-distal treatment time was 3.9 s (range 3.7–4.1 s) and the average LB1D1 treatment time was 2.3 s (range 2.1–2.4 s) resulting in an average delivery of 200 ± 10 calories per animal. Airway stenosis or deformation as a result of the first treatment did not hinder or prevent any of the second treatments. All animals survived to the scheduled termination date. No acute cardiovascular or respiratory adverse events were observed.
Clinical observations
All animals were generally healthy through the duration of the study. Most animals experienced a mild amount of post-operative recovery discomfort and acute loss of appetite following treatment and follow-up procedures. These observations are typical of bronchoscopy and resolved as expected [Citation9]. No difference in clinical observations following treatment 2 as compared to treatment 1 was noted. These observations are summarized in .
Table 1. Clinical observations in the period following treatment 1 (P1) and the period following treatment 2 (P2).
Clinical pathology
Beginning with the first treatment, standard haematology and clinical pathology was performed every 14 days. Only mild excursions from reference ranges established by Marshfield Clinical Laboratories and American Preclinical Services were observed. These are summarised in . The excursions were all considered mild and clinically insignificant. Additionally, no increase in excursion was observed following the second treatment as compared to the first.
Table 2. Clinical pathology excursions from reference range.
Lobar volume reduction and gross pathology
No abnormalities were observed on macroscopic inspection, including pneumothorax, adhesions, fibrosis or inflammation in neighbouring lobes.
In 100% of the lobes treated with vapour, volume reduction was observed. The average amount of visual lobar reduction was consistent with the 19 ± 15% lobar reduction that was observed in the left upper lobe in a previous study with a comparable dose [Citation9]. An example of lobar volume reduction four weeks after the final vapour ablation treatment is shown in .
The lungs were evaluated for differences between treatment 1 and 2. Deformation, stenosis, and atelectasis were generally more pronounced in the LB1-distal segment as compared to the LB1D1 segment. However, no consistent physical differences between treatment 1 and treatment 2 were observed.
Four weeks following each treatment, the degree of airway stenosis was characterised. In most instances the treated airway was stenosed, but not fully occluded, after four weeks these observations are summarized in . This degree of stenosis is similar to what is observed clinically in humans [Citation1,Citation2].
Table 3. Summary of airway score, lobar volume reduction, and abnormality results from the gross pathology. (+1 dilated, 0 no change from baseline, −1 stenosed (narrowed but not fully occluded), −2 occluded).
Discussion
While there are a number of techniques for causing an inflammatory response in lung tissue [Citation11,Citation12], it was not known whether the response is different when treating near previously treated tissue that has recently healed. The current study confirms that vapour ablation effects on lung tissue are not exaggerated in the setting of recent adjacent inflammation. In fact, all vapour treatments were well tolerated and no significant procedural complications were observed during the healing phases, including death or pneumothorax. Analysis of the detailed results found no difference in changes post-treatment 2 as compared to changes post-treatment 1 in terms of major decline in health status, oxygenation changes, pathology and airway changes.
Vapour ablation reproducibly causes a localised inflammatory response in the regions treated. The healing process following this response leads to a permanent remodelling of the lung tissue yielding lobar volume reduction [Citation13]. This inflammatory response does not appear to have effects in regions of the lung outside of the targeted segments. However, in previous work neighbouring tissue has always been untreated and it was unknown whether treating adjacent to previously treated tissue could cause an unanticipated different result. An enhanced inflammatory response in the second segment could potentially have resulted from the supply of increased local mediators and inflammatory cells migrating through inter-segmental collateral ventilation pores. Alternatively, airway deformation and residual inflammation in the first treated segment could have seen an enhanced inflammatory process in the first segment following treatment of the second. Pre-experiment concern had been that either of these potential mechanisms could have produced clinical manifestations, including pneumonitis (sterile or infective) or tissue destruction and pneumothorax.
While vapour ablation is the only energy-based method of achieving lung volume reduction, energy is being used to address other conditions in the lung. Thermoplasty uses radiofrequency heat to induce structural changes in the lung to improve breathing in asthma patients [Citation11]. Radiofrequency, microwave, and laser are being explored as means of ablating pulmonary tumours [Citation12]. It is probable similar experiments utilising these energy sources delivered focally would produce similar results.
Glues or biologically active substances delivered directly into airway segments to induce a local inflammatory response have been investigated as an alternative form of bronchoscopic LVR [Citation14]. The physical persistence of these agents in treated segments and the possibility of transfer through collateral pores has been previously linked to the potential for infection [Citation15] and spillover into adjacent segments. The current study results may therefore not necessarily hold for this form of LVR.
A limitation of this study is that the canine model has healthy tissue with functionally normal collateral ventilation pathways. The clinical target population are patients with emphysema, a disease characterised by enlarged airspaces, gas trapping, variable (although likely increased) collateral ventilation, and decreased lung vasculature. These differences could potentially alter the local inflammatory responses up or down. While a canine emphysema model exists and has been used in the past to investigate the effects of vapour ablation, it is in principle an injury that causes an inflammatory reaction in the lung that leads to pulmonary functional changes consistent with chronic obstructive pulmonary disease (COPD) [Citation16]. The effects of this artificial injury in the canine model on collateral ventilation are unclear. Considering both these points, and noting that vapour therapy itself has an inflammatory effect, it was determined that utilising the diseased model could be even more confounding.
In conclusion, sequential vapour ablation treatments to adjacent lung segments do not produce unexpected inflammatory consequences. These results give reasonable confidence that sequential vapour treatments in adjacent segments of human lungs should be expected to be safe and therapeutic in terms of lung volume reduction. Long-term clinical follow-up in patients with emphysema will be needed to confirm the positive clinical implications of this study in healthy dog lungs.
Declaration of interest
This study was funded by Uptake Medical Corporation. E.H., R.B., and R.M. are employees of Uptake Medical. G.S. is on the Uptake Medical Scientific Advisory Board. E.H. was involved with study design, study execution, data collection, statistical analysis, and manuscript preparation. R.B. and R. M. were involved with study design, data interpretation and manuscript preparation. G.S. was involved with data interpretation and manuscript preparation.
References
- Snell GI, Hopkins P, Westall G, Holsworth L, Carle A, Williams TJ. A feasibility and safety study of bronchoscopic thermal vapor ablation: A novel emphysema therapy. Ann Thorac Surg 2009;88:1993–8
- Snell G, Herth FJ, Hopkins P, Baker KM, Witt C, Gotfried MH, et al. Bronchoscopic thermal vapor ablation therapy in the management of heterogeneous emphysema. Eur Resp J 2012;39:1326–33
- Herth FJ, Ernst A, Baker KM, Egan JJ, Gotfried MH, Hopkins P, et al. Characterization of outcomes one year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema. Int J COPD 2012;7:397–405
- Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J 2010;35:676–80
- Soon SY, Saidi G, Ong MLH, Syed A, Codispoti M, Walker WS. Sequential VATS lung volume reduction surgery: Prolongation of benefits derived after the initial operation. Eur J Cardiothorac Surg 2003;24:149–53
- Emery MJ, Eveland RL, Eveland K, Couetil LL, Hildebrandt J, Swenson ER. Lung volume reduction by bronchoscopic administration of steam. Am J Respir Crit Care Med 2010;182:1282–91
- Tuck SA, Anderson JC, Lopes-Berkas V. The safety and efficacy of bronchoscopic thermal vapor ablation (BTVA) in an emphysematous canine model. Am J Respir Crit Care Med 2011;183:A1552
- Tuck SA, Lopes-Berkas V, Beam S, Anderson JC. Bronchoscopic thermal vapor ablation in a canine model of emphysema. Int J COPD 2012;7:21–31
- Kaplan J, Koehler RC, Terry PB, Menkes HA, Traystman RJ. Effect of lung volume on collateral ventilation in the dog. J Appl Physiol Respir Environ Exerc Physiol 1980;49:9–15
- Nakakuki S. The bronchial tree and lobular division of the dog lung. J Vet Med Sci 1994;56:455–8
- Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: A multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116–24
- Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin A. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations – Review article. Eur J Radiol 2011;77:346–57
- Gompelmann D, Eberhardt R, Ernst A, Hopkins P, Egan J, Stanzel F, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation. Respiration 2013;86:324–31
- Herth FJ, Gompelmann D, Stanzel F, Bonnet R, Behr J, Schmidt B, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®). Respiration 2011;82:36–45
- Ingenito EP, Reilly JJ, Mentzer SJ, Swanson SJ, Vin R, Keuhn H, et al. Bronchoscopic volume reduction: A safe and effective alternative to surgical therapy for emphysema. Am J Respir Crit Car Med 2001;164:295–301
- Rosenthal FS, Wright F. Assessment of papain-induced lung injury in isolated lungs by measurements of aerosol deposition and mixing. J Appl Physiol 1992;72:459–67