Abstract
Purpose: The aim of this study was to evaluate the safety and efficiency of ultrasound-guided percutaneous microwave (MW) ablation for the control of locally recurrent papillary thyroid carcinoma (LR-PTC) in patients for whom surgery is not viable. Materials and methods: The inclusion criteria for MW ablation were three or fewer LR-PTCs and no recurrence beyond the neck, with ineligibility or refusal to undergo surgery. MW ablation was carried out using a 16-gauge MW antenna under local anaesthesia. Patients were then followed at 1, 3, 6 and 12 months after treatment and every 6 months thereafter. Technical success usually meant volume reduction more than 50%. Results: Between October 2010 to March 2013 a total of 17 patients (14 women, 3 men; average age 54.1 years) with 23 LR-PTCs, were treated with MW ablation in our department. All the LR-PTCs were technical successes with the number of treatment sessions for one tumour ranging from 1 to 4 (mean, 2.3 ± 0.9). The mean volume reduction ratio of the LR-PTCs was 1 ± 86%, 47 ± 12%, 70 ± 33%, 91 ± 14% at the 1, 3, 6 and 18 months follow-up visit respectively (all p < 0.05). All treated nodules decreased in size: 30.4% nodules (7/23) had completely disappeared, 52.2% nodules (12/23) remained as small scar-like lesions. One patient experienced transient dysphonia immediately after MW ablation. No other severe and permanent complications occurred. Conclusion: Although with some limitations, our preliminary results are encouraging and show MW ablation may be an alternative treatment option for the control of LR-PTCs in selected patients for whom surgery is not viable.
Introduction
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, accounting for more than 80% of all cases of thyroid cancers [Citation1]. The disease follows an indolent course and has a relatively low mortality rate [Citation2,Citation3], although it frequently spreads locally and recurs by metastasising to local cervical lymph nodes. The overall recurrence and mortality rates for well-differentiated thyroid carcinoma are 20.5% and 8.4% respectively, at an average follow-up rate of 11.3 years [Citation4]. Currently, the standard treatment regimen for a locally recurrent lesion is surgery. However, for those who have undergone previous neck dissections reoperation can be challenging because distortion of normal tissue planes by scar tissue formation within the surgical bed puts the patients at risk of a higher rate of complications [Citation5].
If a minimally invasive technique could eradicate these small nodal recurrences, then in some cases surgery can be avoided. Therefore, both radiofrequency ablation (RFA) and ethanol ablation treatment have been attempted, yielding good results [Citation6,Citation7].
Ultrasound-guided microwave (MW) ablation is a minimally invasive technique that has been described as a method for treating liver [Citation8,Citation9], lung, renal and adrenal malignancies [Citation10–12]. Towards the thyroid gland, MW has shown promise as an effective treatment for benign thyroid nodules and primary thyroid cancers [Citation13,Citation14]. However, to our knowledge, application of MW energy to recurrent thyroid cancers has not yet been reported. We have therefore tested the efficiency and safety of ultrasound-guided MW ablation in selected patients with locally recurrent papillary thyroid carcinomas (LR-PTC).
Materials and methods
Patients
This study was a prospective study design and was approved by our hospital ethics committee. Prior to the MW ablation we obtained informed consent from each patient, and we explained the necessity of reoperation if MW ablation was not successful.
The inclusion criteria for MW ablation were as follows: (1) three or fewer LR-PTCs and no recurrence beyond the neck at the time of MW ablation, (2) ineligibility or refusal to undergo surgery for high thyroid surgical risk (poor surgical candidates, general anaesthesia due to a medical condition, repeated neck dissection) or other reasons. The exclusion criteria were (1) LR-PTCs close to vessels, trachea or oesophagus, (2) no puncture route on US, and (3) supraclavicular lymph nodes and lymph nodes below the clavicle; these were not included in this study.
Pre-ablation assessment
Before treatment, all ultrasound examinations, contrast-enhanced ultrasound (SonoVue, Bracco, Switzerland) and biopsies were performed by two radiologists, and an appropriate puncture route was chosen on ultrasound. The size, volume, solid component, vascularity, enhancement modality and characteristics of all the LR-PTCs were carefully evaluated by ultrasound examination. Three diameters of the tumours were measured, and the tumour volume was calculated with the equation V = πabc/6 (where V is volume, a is the largest diameter, and b and c are the other two perpendicular diameters) and the volume reduction = [(initial volume − final volume) × 100]/initial volume.
Laboratory tests included thyroid function test, serum concentration of thyroglobulin, complete blood count and blood coagulation test. All medical records and the ultrasonography images were recorded by one radiologist who did not perform the procedure.
Equipment
Microwave ablation instrument
A MW ablation instrument (KY-2000, Kangyou Medical Instruments, Nanjing, China) was used to administer MW energy. The MW ablation system also consists of a flexible low-loss coaxial cable and a thyroid-dedicated cooled shaft antenna. The generator is capable of producing 1–100 W of power at 2450 MHz, pulse or continuous. The MW shaft antenna was developed and modified specially for treating superficial neck organ diseases – 16-gauge, needle type (1.9 mm in diameter and10 cm in length), coated with polytetrafluoroethylene to prevent tissue adhesion. To prevent the shaft overheating, distilled water is circulated through dual channels inside the antenna shaft, continuously cooling the shaft.
Ultrasound system
Sonograms for the LR-PTCs were performed with Toshiba SSA 790 A (Toshiba, Italy) and GE Logiq E9 to guide the MW ablation procedure and evaluate the tumours before ablation and at each follow-up, in terms of 2D colour Doppler ultrasound and ultrasonic contrast.
Procedure
All treatments were performed at our institution on an inpatient basis. All the microwave ablations were carried out by the same radiologist under ultrasound control. Before the treatment, all patients were premedicated with 100 mg of bucinnazine hydrochloride injection and 1 U haemocoagulase injected intramuscularly (i.m.) to relieve the discomfort and reduce bleeding during the procedure. The patient was placed in the supine position with hyperextended neck, and a multiparametric monitor was connected to the patient to monitor continuous electrocardiogram, breath rate, PO2 and blood pressure.
Local anaesthesia with 2% lidocaine was performed subcutaneously on the puncture site. A small incision less than 2 mm in length was made after local anaesthesia. To prevent serious haemorrhage the vessels along the approach route were carefully evaluated. We then usually injected a mixture of 0.9% lidocaine and physiological saline solution between the mass and the expected location of the nerve, using the hydrodissection technique, to provide a safe thermal barrier to the MW energy [Citation13,Citation14]. After that, the 16-gauge MW antenna was percutaneously inserted into the tumour under ultrasound guidance (). Based on the previous experience for MW ablation of benign thyroid nodules, a power output of 40 W was used for LR-PTCs, and the therapy was not stopped until the hyperechoic treatment zone covered the whole tumour. Usually for the small tumours (≤1 cm diameter), monosection ablation was enough, but for larger ones we adjusted the ablation plane to achieve complete necrosis. When withdrawing the antenna, the needle track was coagulated to prevent tumour cell seeding.
Figure 1. A 47-year-old woman had a recurrent lymph nodule in the right neck level 3 confirmed with ultrasound-guided biopsy. (A) Ultrasound examination revealed the nodule to be 0.29 mL in volume before microwave ablation, with inhomogenous internal echoes and microcalcification. (B) Under the guidance of ultrasound, a thyroid-dedicated cooled shaft antenna (16 gauge) was positioned in the tumour. The sonogram obtained during treatment shows a typical hyperechoic region (arrow) surrounding antenna. (C) A transverse ultrasound image of the treated lymph node immediately after treatment shows increased internal echogenicity (arrow). The echogenic area (1.06 mL) was larger than the initial lymph node, suggesting the ablation zone includes surrounding normal tissue. (D) A transverse ultrasound image 3 months after treatment shows the decreased size of the ablated lymph node (arrow). (E) The transverse ultrasound image 12 months after treatment shows the ablated lymph node remaining as small scar-like tumour (arrow).
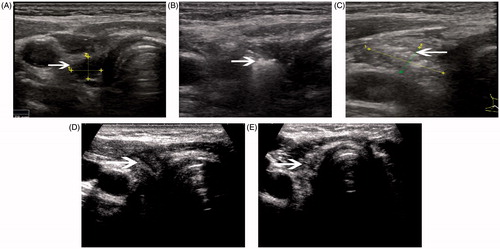
For the LR-PTCs, MW ablation was usually performed on both the LR-PTC and the surrounding normal tissue to extend the range of ablation (a ≥5-mm ablative margin around the entire tumour [Citation12]) to decrease the chance of relapse and to improve the effect of operation. For the LR-PTC, we recognised achieving slightly more necrosis than the pre-operation nodule as a technical success, evaluated with contrast-enhanced ultrasound at the end of each procedure, based on the previous experience. After MW ablation, patients were closely monitored for 30 min with compression of the neck lasting 15–20 min.
Post-procedural observation and follow-up
After ablation, the possible complications such as skin burns, status of voice, tracheal injury and oesophageal perforation were evaluated carefully. All the patients were advised to be treated with levothyroxine after MW ablation to maintain thyroid stimulating hormone (TSH) levels below 0.1 mU/L.
Clinical follow-up consisted of a physical examination, laboratory examination and an ultrasound, evaluated at 1, 3, 6 and 12 months after treatment and every 6 months thereafter. At ultrasound examination we evaluated changes in tumour size, intratumoural vascularity, and development of new recurrent tumours, and technical success usually meant volume reduction of more than 50%.
Analysis and statistics
Data were analysed with statistical software (SPSS, version 17.0). Post-MW ablation nodule volume recorded was compared with pretreatment volume by means of the paired sample t-test. Data were reported as mean ± standard deviation (SD), and the level of significance was defined as p < 0.05.
Results
From October 2010 to March 2013, a total of 17 patients (3 male and 14 female; mean age 54.1 ± 13.6 years, range 33–80 years) with 23 lesions were treated with ultrasound-guided MW ablation in our department. Follow-up was conducted to December 2013. Thirteen patients were treated for one tumour each, two patients for two tumours each and two patients for three tumours each. The mean number of operations before MW ablation was 2.1 (range 1–4). Three of 23 LR-PTC s were located in the surgical bed, and the remaining 20 LR-PTCs were in the lateral neck (5 level II, 10 level III, 4 level IV, 1 level V). No patients were taking antiplatelet or anticoagulant medications for at least 1 week before the procedure.
The treatment outcome after MW ablation is summarised in . All LR-PTC s were confirmed by ultrasonography-guided biopsy prior to the procedure. All patients underwent thyroidectomy and central lymph node resection as primary surgery. All patients received post-operative radioiodine therapy and thyrotropin (TSH)-suppressing therapy using levothyroxine. After MW ablation, all 23 LR-PTCs decreased in size. The numbers of follow-up cases at 1, 3, 6, 12 and 18 months were 17(100%), 17(100%), 15(88.2%), 12(70.6%) and 9 (52.9%), respectively. The mean largest diameter of the treated lesions had decreased significantly from 14.4 ± 7.7 to 4.1 ± 1.3 mm by the 18-month follow-up visit (all p < 0.05, and ). Also, the mean volume of the treated tumours had decreased significantly from 1.45 ± 2.3 to 0.13 ± 0.22 mL (). The mean volume reduction ratio of the tumours was 1 ± 86%, 47 ± 12%, 70 ± 33%, 91 ± 14% at the 1-month, 3-month, 6-month and 18-month follow-up visits respectively (p < 0.05). All differences were statistically significant. Seven nodules had completely disappeared, 12 nodules remained as small scar-like lesions ( and ; ). At ultrasound examination well-treated tumours exhibited change to a hypoechoic nature, a marked decrease in size, and loss of internal vascularity.
Figure 2. A 62-year-old woman with previous thyroidectomy for papillary carcinoma developed two recurrent lymph nodules in the left neck, level IV. The patient refused surgery. (A) Transverse greyscale ultrasound imaging prior to ablation revealed two rounded hypoechoic recurrent masses (measured volume = 0.10 mL, 1.17 mL, arrow) in the left neck, level IV. (B) A follow-up ultrasonogram 1 month after the ablation shows that the larger one has decreased in volume (0.37 mL, arrow), and we cannot find any evidence of the smaller one at the previous site.
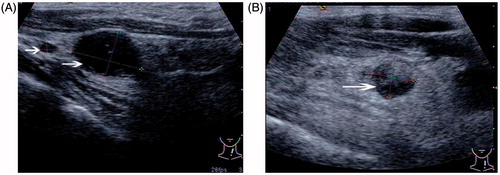
Figure 3. The mean volume change of all neck recurrences of papillary thyroid carcinoma after ultrasound-guided percutaneous microwave ablation.
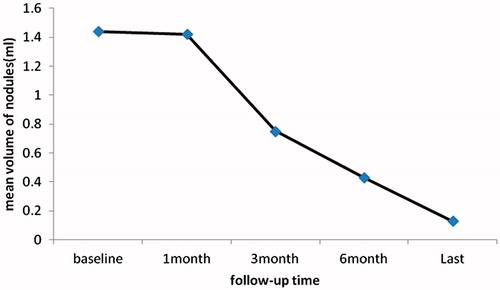
Table 1. Outcome of microwave ablation.
All the LR-PTCs were technical successes with the number of treatment sessions for one tumour ranging from 1 to 4 (mean 2.3 ± 0.9). During follow-up, except for one patient who had a new recurrent tumour (≤5 mm) in the lateral aspect of the neck (level IV), no patient had a recurrence at the treatment site. One patient refused further treatment and was undergoing observation at the time of this writing.
At the 18-month follow-up evaluation, thyroglobulin levels for nine of the 17 patients demonstrated a drop from a mean pretreatment value of 5.3 ± 4.1 ng/mL to 1.3 ± 1.0 ng/mL, and the difference was statistically significant (p = 0.015). Immediately after MW ablation, ultrasound imaging revealed that hypoechoic tumours had changed to hyperechoic on greyscale imaging, but it was lower than that observed pre-ablation ( and ).
All of the patients tolerated the MW ablation procedure well. Most patients reported a burning sensation, pain, or both, although no one asked for the procedure to stop. The symptoms were relieved without treatment within 12 h after the MW ablation. Average time in hospital was 3.7 days (range 3–8 days). In one patient in whom MW ablation was performed for the mass in the surgical bed, dysphonia occurred immediately after the procedure, but completely recovered within 3 months spontaneously. No tranquilliser medicines were given before or after ablation. No procedure-related major complications such as local infection, skin burn or damage to the vital structures of the neck were observed.
Discussion
Surgical treatment of patients with LR-PTCs can be challenging because of scar and adhesion formation from previous operations. The scar tissue often causes marked distortion of the anatomical planes, making surgical dissection difficult even for experienced surgeons, and the rate of the surgical morbidity can be higher as well, making minimally invasive ultrasound-guided ablation therapies (RFA, ethanol ablation) an attractive treatment option. Since the first trial of RFA for regional recurrence of well-differentiated thyroid malignancy in 2001 [Citation15], reports by many papers have followed. They reveal that RFA appeared to have significant clinical application with recurrent well-differentiated papillary carcinoma. The ethanol ablation method has also been used effectively and widely [Citation17–20]. However, the ethanol injection has some drawbacks. Multiple sessions of treatment are needed to obtain a total cure. With increasing sessions, the risk of complications increases [Citation20]. Furthermore, alcohol leakage can cause extra glandular fibrosis and local pain [Citation19].
MW ablation is a relatively novel technique that has been used to treat benign and malignant liver, lung, renal and adrenal tumours, both primary and metastatic [Citation8–12]. For small liver cancers especially, the long-term efficacy is similar to that of hepatectomy. In particular, compared with RFA, MW ablation may offer a larger ablation zone, less treatment time, more complete tumour kill, and is less affected by the heat-sink effect that is thought to contribute to local recurrence after RFA [Citation21,Citation22]. MW has shown promise as an effective treatment for benign thyroid nodules. However, its use in thyroid cancers has not been reported. With this in mind, we applied MW ablation guided with ultrasound in a small group of patients with LR-PTCs. For the LR-PTCs, we adopted an expanded ablation method, and the expected necrosis includes some surrounding normal tissue.
Volume reduction after RFA has been reported to range from 49–100%, 50% of tumours completely disappearing [Citation16], and 37.5–96%, 31–44.7% after ultrasound-guided ethanol treatment [Citation17–19]. In our study the mean nodule volume reduction ratio was 91 ± 14% at the last follow-up with 30.4% (n = 7) index nodules disappearing and the volume decreasing more slowly during initial follow-up, with a mean volume reduction ratio of 0.01 ± 0.86 at the 1-month follow-up. However, in the later follow-ups the volume of the tumours reduced quickly; the mean volume reduction radios at the 3-month, 6-month and 12-month follow-up visit were 47 ± 12%, 70 ± 33% and 91 ± 14%, respectively. The efficacy we observed was similar to that reported in previous studies of RFA (49–100%, 50%) and ethanol (37.5–96%, 31–44.7%) ablation. Furthermore, in this study we also assessed the efficacy of this method with the new recurrence rate (5.9%) and the decreased serum thyroglobulin concentration.
Thermal injury to the recurrent laryngeal nerve is a serious complication and the complication after RFA was less, but unavoidable. Dupuy et al. [Citation15] and Monchik et al. [Citation23] reported that one patient had dysphonia after treatment of a surgical bed tumour. In our study, there was also one patient who experienced transient dysphonia after treatment of a tumour in the surgical bed. These findings suggest that thermal ablation therapy may be less safe in the surgical bed than in the lateral aspect of the neck. There was no laryngoscopy performed on him so it is supposed that the MW ablation treatment most likely caused thermal injury to the recurrent laryngeal nerve. At ultrasound examination, the recurrent laryngeal nerve cannot be well visualised and the nerve is more vulnerable to the thermal injury. Dupuy et al. [Citation15] and Monchik et al. [Citation23] reported one case of skin burn at the treatment site. However, no patients in our study experienced skin burn. No other procedure-related major complications occurred.
There are five factors to note that may have contributed to our results: (1) we have had extensive experience in the MW ablation of benign thyroid nodules, (2) the cooled shaft antenna used in this study is capable of generating a large enough ablation zone to encompass the entire tumour, (3) we adopted an extended ablation method and the ablation zone includes the surrounding normal tissue, (4) all patients were treated with levothyroxine after MW ablation to maintain TSH levels below 0.1 mU/L, and (5) the mixed solution we injected between the mass and the expected location of the nerve played an important part in our success. During MW ablation of the lateral aspect of the neck, similar caution towards the vagus nerve also helped minimise complications.
However, this study has some limitations: (1) only 17 patients were included in this study, and (2) follow-up was relatively short and the long-term results are not certain. Also, the prognosis of PTC was related to many factors, such as patient age, tumour size, UICC TNM stage, treatment modality (surgery, radiotherapy, and/or combination therapy, with or without TSH suppression therapy) and so on. Given all of this, single factor variance analysis should be made to estimate the effect of MW ablation on the long-term outcome or efficacy for treatment of patients with LR-PTC.
Conclusion
Although with some limitations, our preliminary results are encouraging and show MW ablation was effective for controlling the LR-PTCs. The results of this study suggest that MW ablation may be an alternative treatment option for control of LR-PTCs in selected patients who are not promising surgical candidates.
Declaration of interest
This work was supported by the Shandong Province Science and Technology Development projects (item numbers: 2011YD18028) and the Yantai Science and Technique Plan (item numbers: 2010156). The authors alone are responsible for the content and writing of the paper.
References
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. Cancer J Clin 2001;51:15–36
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30
- Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 2002;26:879–85
- Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumour-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: A retrospective analysis of 700 patients. J Clin Endocrinol Metab 1997;82:3553–62
- Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: A retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20
- Heilo A, Sigstad E, Fagerlid KH, Håskjold OI, Grøholt KK, Berner A, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab 2011;96:2750–5
- Guenette JP, Monchik JM, Dupuy DE. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol 2013;24:672–9
- Swan RZ, Sindram D, Martinie JB, Iannitti DA. Operative microwave ablation for hepatocellular carcinoma: Complications, recurrence, and long-term outcomes. J Gastrointest Surg 2013;17:719–29
- Li X, Fan WJ, Zhang L, Zhang XP, Jiang H, Zhang JL, et al. CT-guided percutaneous microwave ablation of liver metastases from nasopharyngeal carcinoma. J Vasc Interv Radiol 2013;24:680–4
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urol 2008;180:844–8; discussion 848
- Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, et al. Microwave ablation of pancreatic head cancer: Safety and efficacy. J Vasc Interv Radiol 2013;24:1513–20
- Belfiore G, Ronza F, Belfiore MP, Serao N, di Ronza G, Grassi R, et al. Patients’ survival in lung malignancies treated by microwave ablation: Our experience on 56 patients. Eur J Radiol 2013;82:177–81
- Yue W, Wang S, Wang B, Xu Q, Yu S, Yonglin Z, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: Safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11–16
- Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: Initial experience. Int J Hyperthermia 2014;30:150–7
- Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001;130:971–7
- Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. Am J Roentgenol 2011;197:W331–6
- Lim CY, Yun JS, Lee J, Nam KH, Chung WY, Park CS. Percutaneous ethanol injection therapy for locally recurrent papillary thyroid carcinoma. Thyroid 2007;17:347–50
- Lewis BD, Hay ID, Charboneau JW, McIver B, Reading CC, Goellner JR. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704
- Kim BM, Kim MJ, Kim EK, Park SI, Park CS, Chung WY. Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. Eur Radiol 2008;18:835–42
- Bennedbak FN, Hegedus L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: A randomized trial comparing one injection with three injections. Thyroid 1999;9:225–33
- Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012;22:1983–90
- Dodd GD III, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 2013;267:129–36
- Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 2006;244:296–304

