Abstract
Purpose: The study was performed to assess the safety and efficacy of ultrasound (US)-guided percutaneous microwave (MW) ablation for hepatic malignancy adjacent to the gallbladder. Materials and methods: From January 2011 to December 2013, 49 patients with 51 hepatic tumours adjacent to the gallbladder who underwent US-guided percutaneous MW ablation were included in the study group. A total of 106 patients with 117 hepatic tumours not adjacent to the gallbladder who underwent US-guided percutaneous MW ablation were included in the control group. In the study group the temperature of marginal ablation tissue proximal to the gallbladder was monitored and controlled at 45–54 °C for 5–10 min during the ablation. Ethanol (4.5–13 mL) was injected into the marginal tissue in 27 of 51 tumours of the study group. We compared the results of ablation between the two groups. Results: All patients were successfully treated. A total of 47 of 51 tumours in the study group (92.2%) and 110 of 117 tumours in the control group (94.0%) achieved complete ablation (p = 0.93). Local tumour progression was found in nine (17.6%) tumours in the study group and 15 (12.8%) tumours in the control group during follow-up after MW ablation (p = 0.41). No peri-procedural major complications occurred in either group. Conclusions: Under strict temperature monitoring, US-guided percutaneous MW ablation assisted with ethanol injection appears to be safe and can achieve a high rate of complete ablation for the treatment of hepatic malignant tumours adjacent to the gallbladder.
Introduction
Surgical resection of hepatic malignant tumours has been the primary curative treatment. However, most hepatic tumours are not eligible for surgical resection because of poor hepatic function and unresectable locations [Citation1–3]. For the past few years, non-surgical treatment modalities have been developed in treating primary and metastatic hepatic tumours [Citation4,Citation5]. Image-guided thermal ablation using different energy sources, such as radiofrequency (RF), microwave (MW), cryoablation, high intensity focused ultrasound (HIFU) or laser has been proven to play an important role against hepatic tumours [Citation6–19]. The benefits of thermal ablation include low morbidity, few complications and repeatability for recurrence [Citation20–26].
Although image-guided thermal ablation has been considered a safe technique, a broad spectrum of complications has been reported in several large studies [Citation8,Citation27–30]. Collateral thermal damage after ablation of hepatic tumour tissue has been reported in various organs [Citation1,Citation5,Citation8,Citation20–22]. Among them, the most important major complication due to thermal damage was perforation of the gastrointestinal tract and gallbladder. Several clinical reports have shown complications of the gallbladder after thermal ablation including acute cholecystitis and perforation of the gallbladder [Citation31–33]. Some authors have recommended that percutaneous thermal ablation should be avoided when treating liver tumours adjacent to the gallbladder [Citation34,Citation35]. Some have noted that when treating tumours adjacent to the gallbladder, an open or laparoscopic technique provides the ability to mobilise adjacent vital structures away from the treatment area to decrease the risk [Citation36,Citation37]. Our previous in vivo experimental study showed that MW ablation of liver tissue adjacent to the gallbladder is safe by monitoring the temperature of the ablation area fluctuating between 45 and 54 °C [Citation38]. Thermal injury may be prevented by strict temperature monitoring of hepatic marginal tissue adjacent to the gallbladder. This prospective study was undertaken to assess the safety and efficacy of MW ablation with temperature monitoring assisted with a small dose of ethanol infusion for such tumours adjacent to the gallbladder.
Materials and methods
Patients
From January 2011 to December 2013, 451 patients with hepatocellular carcinoma (HCC) or hepatic metastases underwent US-guided percutaneous MW ablation with curative intention at Chinese PLA General Hospital. Indication criteria for percutaneous MW ablation were unresectable tumour or patient’s refusal to undergo surgery, tumour accessible via a US-guided percutaneous approach, single nodular lesions of 5 cm or smaller, three or fewer multiple nodular lesions with a maximum diameter of 3 cm or less in each nodule, absence of portal vein thrombosis or extrahepatic metastases, prothrombin time of less than 25 s, platelet count higher than 40 × 109/L, and prothrombin activity higher than 40%. This investigation was approved by our institutional ethics committee. Written informed consent was obtained from all patients.
Of 451 patients who underwent MW ablation, 296 were excluded from the study for the following reasons: tumours treated after transcatheter arterial chemoembolisation (TACE), RF ablation, radiotherapy, or other therapy including immunotherapy, traditional Chinese medicine therapy and biological targeted therapy, local tumour progression after previous MW ablation but no indications for the study, tumours adjacent to the diaphragm and gastrointestinal tract, patients were lost to follow-up. Thus, 155 patients with 168 hepatic lesions were included in our study. A total of 49 patients with 51 lesions adjacent to the gallbladder (the shortest distance from the lesion margin to the gallbladder being less than 5 mm) who underwent US-guided percutaneous MW ablation were included in the study group. A total of 106 patients with 117 hepatic lesions not adjacent to the gallbladder (the shortest distance from the lesion margin to the gallbladder and the first or second branch of the hepatic vessels being more than 10 mm), who underwent US-guided percutaneous MW ablation, were included in the control group. The patients with liver metastasis had not received other therapy before. According to ‘expert consensus on local ablation therapy for liver cancer’ in China [Citation39], the security boundary of local tumour ablation was 5–10 mm, and 10 mm was especially necessary for the lesions with no clear boundary, of irregular shape, or with metastatic carcinoma. In order to make sure the tumours were completely ablated we defined the control group as greater than 10 mm from the gallbladder, and excluded those tumours between 5 and 10 mm. The shortest distance from the lesion margin to the gallbladder was confirmed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) in the transverse plane and coronal plane.
In total 31 men and 18 women were included in the study group, with a mean age of 59.1 ± 10.2 years (range 37–72 years); 71 men and 35 women were included in the control group, with a mean age of 57.6 ± 12.1 years (range 34–81 years). In the study group, 33 patients with 33 lesions had HCC and 16 patients with 18 lesions had hepatic metastases. In the control group, 64 patients with 71 lesions had HCC and 42 patients with 46 lesions had metastatic liver disease. Among the 64 metastatic liver lesions, 21 were from colorectal cancer, 14 from gynaecologic cancer, nine from lung cancer, eight from breast cancer, seven from gastric cancer, two from prostate cancer and one from lymphoma. Before MW ablation, Child-Pugh classification was performed in all patients: 37 patients (75.5%) in the study group and 88 patients (83.0%) in the control group had class A disease, and 12 patients (24.5%) in the study group and 18 patients (17.0%) in the control group had class B disease. The median follow-up periods of the study group and the control group were 16.5 and 17 months, respectively.
There was no significant difference in clinical backgrounds between the study group and the control group ().
Table 1. Comparison of clinical data in the study and control groups.
Pre-ablation imaging work-up and histological diagnosis
Pretreatment examination included sonography, contrast-enhanced sonography (CEUS), contrast-enhanced CT and/or contrast-enhanced MR, and tumour marker assay in all patients. Sonography and contrast-enhanced sonography were performed using an Acuson Sequoia 512 (Mountain View, CA) with 3.5–5.0 MHz 4V1 transducer. Ultrasound contrast agent was SonoVue, sulphur hexafluoride microbubbles for injection (Bracco, Milan, Italy). The tumour was examined from different scanning sections on CEUS and the maximum diameter was found and measured. After ablation, we examined the ablation area in the same scanning section and observed whether the ablation area had covered the tumour.
Histological diagnosis was obtained by US-guided tumour biopsy using an 18-gauge needle (Bard Medical, Covington, GA, USA) in all patients. In patients with multiple tumours, at least one biopsy was performed. If new tumours emerged after ablation, biopsies for the new nodules were performed. We made diagnosis by means of needle biopsy not only in hepatic metastatic lesions but also in HCC, mainly due to the analysis of the histological type of HCC and to study the impact of the pathological types on ablation efficacy and long-term efficacy.
Laboratory data
Since an increase in serum alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA125 and CA199 levels may indicate recurrence or new lesions, tumour marker assays were performed in all patients before and after MW ablation. The levels of tumour markers were abnormal in 41 patients (83.7%) in the study group and 85 patients (80.2%) in the control group. The levels of tumour markers were normal in the remaining 29 patients (18.7%). Serum tumour marker assays were performed 1 month after MW ablation and follow-up was performed at an interval of 3 months. The total levels of bilirubin before and after MW ablation were also measured in all cases to evaluate the liver function.
Microwave ablation procedures
All treatments were performed in our institution and were carried out under US guidance with the patients under unconscious intravenous anaesthesia (propofol 6–12 mg/kg/h, ketamine 1–2 mg/kg) in the operating room. The MW unit (KangYou-2000, KangYou Medical, Nanjing, China) consisted of three independent MW generators, three flexible coaxial cables, and three water-pumping machines, which could drive three cool-tip needle antennae. The generator was capable of producing 1–100 W of power at 2450 MHz and with a 15-gauge cool-shaft antenna. Under US guidance, a single antenna or multiple antennae were used depending on the tumour size. All therapy was performed by two experienced radiologists with more than 20 years of experience in interventional procedures. A detailed protocol, which included the placement of the antennae, power output setting, MW emission time, and appropriate approach, was worked out for each patient on an individual basis before treatment. All patients were placed in a supine or left-lateral position depending on the operation plan and tumour location. After the tumours were visualised and the puncture points were decided by US, the skin marks were made, and the puncture sites were routinely disinfected. Then, the antennae were inserted into the tumours. To ensure the accuracy of all insertions, the ultrasound probe holder and guide groove were used. MW generators were started when the ends of the antennae were located in the expected positions of the lesions. In general, for tumours less than 2 cm in diameter a single antenna was used, and for tumours 2 cm or larger, multiple antennae were required. A power output setting between 40 W and 60 W was used during ablation, with continuous or intermittent emission. During the therapy, the hyperechoic area of ablation was monitored by greyscale sonography and thermal monitoring to decide the end point of treatment. After ablation of the tumour, the antennae were slowly withdrawn, and MW emission was continued until the antennae were pulled to just under the skin entrance site. This method adopted needle track cauterisation to prevent tumour seeding and to minimise bleeding after ablation. The MW ablation was performed under real-time US guidance (Acuson Sequoia 512) by two experienced radiologists. Within 3 days after ablation, all patients received contrast-enhanced sonography to evaluate the ablation area. Additional MW ablation treatment was performed if residual tumour or unablated therapeutic margin was detected.
Thermal monitoring
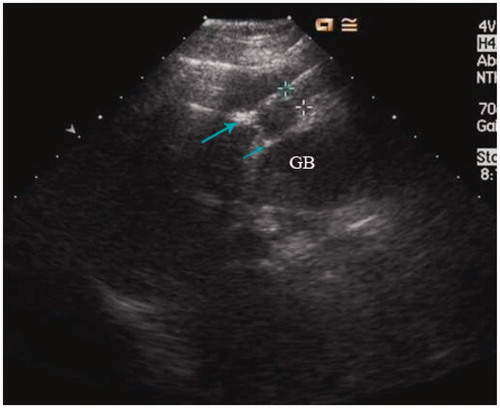
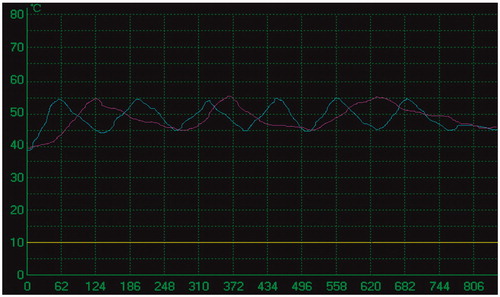
A thermal monitoring system attached to the MW unit was used during treatment in the study group. The threshold of coagulation necrosis for thermal ablation was 60 °C or 54 °C for 3 min [Citation40]. To avoid thermal injury to the gallbladder during ablation for the tumours in the study group, the temperature of marginal tumour or liver tissue proximal to the gallbladder was monitored. Under US guidance, one or two 21-gauge thermal monitoring needles (KangYou Medical) were placed into the marginal tumour or liver tissue proximal to the gallbladder for real-time temperature monitoring during the ablation to protect the gallbladder from thermal mediated injury (). Based on our experimental evidence and clinical experience, the temperature cut-off point for ablation therapy was set at 54 °C in the patients. If the temperature measured reached 54 °C, emission of MW antenna was stopped immediately and was restarted after the temperature had decreased to 45 °C. The temperature measured was controlled between 45 °C and 54 °C for 5–10 min ().
Adjuvant therapy with percutaneous ethanol injection
Among 51 lesions in the study group, 27 (52.9%) were protruding or in contact with the gallbladder. For those 27 lesions, one or two 21-gauge PTC needles were percutaneously placed into marginal tumour tissue proximal to the gallbladder under US guidance. Dehydrated, sterile, 99.5% ethanol was slowly injected into the marginal tumour tissue during ablation treatment, with total sessions of 1.3 ± 0.4 and a total dose of 5.5 ± 3.6 mL (4.5–13 mL) by the end of the ablation procedures. For eight tumours near the third branch of bile ducts, 2.4 ± 1.7 mL (1.5–6 mL) ethanol was injected into the tissue proximal to the bile duct. All ethanol injections were planned beforehand.
Follow-up imaging
Modified RECIST (mRECIST) evaluation was used for follow-up [Citation41]. The follow-up period was calculated starting from the beginning of MW ablation in all patients. The follow-up period in both groups are shown in . Therapeutic efficacy was assessed on the basis of an integrative evaluation of contrast-enhanced imaging and serum tumour marker levels. Contrast-enhanced CT or MRI and CEUS were repeated at 1-month and 3-month intervals within 1 year after MW ablation treatment and then at 6-month intervals.
Assessment of safety
For the assessment of safety, we evaluated whether any complications occurred during the therapy procedure and follow-up period. The description of complications in this study followed the classification of the Society of Interventional Radiology [Citation7]. Major complications were described as events that led to substantial morbidity and disability, increased the level of care, or resulted in hospital admission or substantially lengthened hospital stay.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 for windows and the data were expressed as mean ± standard deviation (SD). The independent samples t-test was used to compare the means between the groups and the chi-square test was undertaken to compare the proportions. p < 0.05 was considered to indicate a significant difference.
Results
Outcomes of MW ablation
All patients were successfully treated. No more than three sessions were performed to complete the treatment (one session for 38 patients, two sessions for 11 patients, and three sessions for two patients; mean 1.3 ± 0.4 sessions per patient) in the study group and (one session for 81 patients, two sessions for 27 patients, and three sessions for nine patients; mean 1.2 ± 0.3 sessions per patient) in the control group. No statistically significant differences in the number of sessions was found between the two groups (p = 0.08). A total of 47 tumours (92.2%) in the study group and 110 tumours (94.0%) in the control group achieved complete ablation as confirmed at 1month after MW ablation by contrast CT or MRI (). No statistically significant difference in the rate of complete ablation was found between the study group and the control group (p = 0.93). In the study group, 27 tumours were treated with MW ablation and percutaneous ethanol injection (PEI) and 24 tumours achieved complete ablation, whereas 24 tumours were treated with MW ablation alone and 23 tumours achieved complete ablation. There was no significant difference between the two treatments (p = 0.36). In all 168 tumours under study, 101 of 104 HCCs (97.1%) and 56 of 64 metastatic hepatic tumours (87.5%) achieved complete ablation, and significant statistical difference was found between HCC and metastatic hepatic tumours (p = 0.03). The total duration of treatment for one nodule was 540–1760 s in the study group and 210–1680 s in the control group. Although the size of tumours showed no significant difference between the study group and the control group, lesions in the study group required longer duration of ablation than those in the control group; however, they did not require a larger number of treatment sessions ().
Figure 3. A 68-year-old man treated with MW ablation for HCC adjacent to the gallbladder. (A) Before ablation the tumour (arrowhead) is seen in MRI scans. (B) Six months after ablation, the ablation zone (arrowhead) has no arterial enhancing in CT scans (left) and contrast enhanced ultrasound (right). (C) MRI scans (left) and contrast enhanced ultrasound (right) from 1-year follow up show the ablation zone (arrowhead) had no local tumour progression.
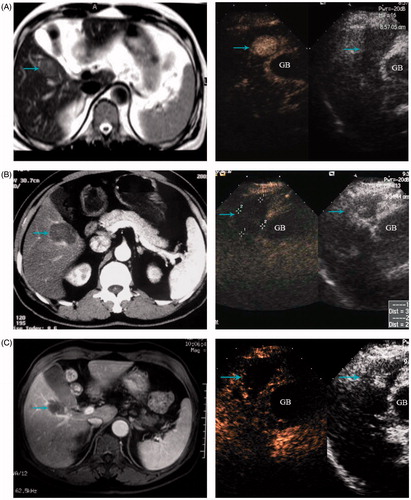
Table 2. Comparison of therapeutic data in the study and control groups.
Changes in tumour markers
The levels of tumour markers were abnormal in 41 patients (83.7%) in the study group and 85 patients (80.2%) in the control group. Among the 41 patients, 25 (61.0%) had a completely negative result in tumour marker assays, and 11 (26.8%) had the tumour markers decrease by more than 50% 1month after the ablation. Among the 85 control patients, 57 (67.1%) had a completely negative result in tumour marker assays, and 22 (25.9%) had the tumour markers decrease by more than 50% 1 month after the ablation. There was no significant difference in the rate of tumour marker level decrease between the two groups (p = 0.34).
Complications and side effects
There were neither immediate nor peri-procedural major complications in either group. We also found neither an increase in the total level of bilirubin nor a deterioration of hepatic reserve in either group. In the study group, on a scale of mild, moderate, or severe, nine (20.4%) cases suffered mild or moderate abdominal pain that ranged in duration from 0.5 to 8 days, with a mean duration of 3.5 days, one case suffered severe abdominal pain and needed analgesics to control, and the pain disappeared within 11 days. Fever was reported in six (12.2%) cases, the highest temperature was 38–39 °C, and the duration of fever was 2–7 days, three patients (6.1%) developed a small amount of pleural effusion and had the symptoms self-relived in 3–7 days. Nausea and vomiting occurred in seven cases (14.3%) and disappeared within 1–2 days after ablation. In the control group, four cases (3.8%) suffered mild abdominal pain after ablation, three (2.8%) cases suffered fever after ablation, and the highest temperature was 37.5–38.2 °C. Five (4.7%) cases developed a small amount of pleural effusion that ranged in duration from 3–8 days, two cases (1.9%) developed nausea and vomiting after ablation. The study group had significantly higher rates of symptoms such as fever, abdominal pain, and nausea and vomiting than the control group ().
Table 3. Complications after MW ablation between the study group and control groups.
Local tumour progression
Local tumour progression was found in nine of 51 tumours (17.6%) in the study group and in 15 of 117 tumours (12.8%) in the control group by follow-up contrast-enhanced imaging. There was no significant difference in the rate of local tumour progression between the two groups (p = 0.41). The maximum diameter of tumours and the number of sessions were not significant predictors of local tumour progression (). In the study group, 27 tumours were treated with MW ablation and PEI and six tumours developed local tumour progression, whereas 24 tumours were treated with MW ablation alone and four tumours developed local tumour progression. There was no significant difference in the rate of local tumour progression between the patients treated with the two treatments in the study group (p = 0.27). Nine (8.9%) of 104 HCCs and 16 (25.0%) of 64 metastatic hepatic tumours had local tumour progression, with a significant difference between HCCs and metastatic tumours (p = 0.01).
Table 4. Comparison of the maximum diameter and number of sessions in the completely ablated lesions and local tumour progression lesions.
Discussion
US-guided percutaneous thermal ablation is a widely accepted method for treating malignant liver tumours. Although thermal ablation is considered safe, several complications are associated with all thermal therapies. The major concern for thermal ablation for tumours adjacent to the gallbladder other than for tumours in other sites was gallbladder perforation or acute cholecystitis. Percutaneous ablation for tumours adjacent to the gallbladder has not been well accepted in the past and remains controversial mainly due to RF ablation systems which have some unfavourable factors for percutaneous ablation. On the one hand, some types of RF ablation systems incorporate multi-tined expandable electrodes, for which it is not possible to confirm the positions of retractable curved electrodes by ultrasound, so the electrodes may pierce the gallbladder to directly heat it. On the other hand, some RF ablation electrodes are not internally cooled and the temperature of the ablation area is hard to control and monitor [Citation42]. These factors may lead to gallbladder injury and incomplete ablation of the tumours. Some authors have reported complications of percutaneous RF ablation [Citation31,Citation37,Citation38,Citation42–44]. Additionally, animal studies have shown that hepatic RF ablation abutting the gallbladder could produce substantial thermal injury of the gallbladder wall, including perforation, especially when performed without a safe margin [Citation45]. Although gallbladder injuries are rarely life-threatening, they can be quite distressing to patients. Therefore, any method that can reduce the risk of gallbladder injury during ablation would help decrease complications and increase the number of patients who are eligible to undergo the procedure. Recently Orlacchio [Citation46] successfully treated eight cases of HCC adjacent to the gallbladder with RFA, using an expandable multi-electrode system, noting that it is easier to evaluate the position of the hooks with this system and to predict the volume of thermal effect more accurately with the multi-electrode system than with a cool-tip needle, and also the needle position was not influenced by respiratory movements. Jiang et al. [Citation47] reported their experience in treating HCC adjacent to the gallbladder safely with RFA by a laparoscopic approach, thus bringing new ideas to the treatment of liver tumours adjacent to the gallbladder.
MW could offer more direct heating than other energies, making MW ablation more potent in organs with high blood perfusion or near vascular heat sinks as compared with other thermoablative modalities, and the MW ablation zones are uniform in shape and size and remain unaffected by convective heat loss [Citation48,Citation49]. These advantages have made MW ablation widely used for treatment of hepatic tumours over recent years [Citation4,Citation9,Citation24,Citation25].
Our results provide evidence that under strict temperature monitoring, MW ablation for tumours adjacent to the gallbladder can be performed safely. Some authors have reported that gallbladder wall thickening happened on the immediate post-procedure CT scans and the gallbladder wall thickening after ablation was associated with symptoms [Citation31]. In our study group, gallbladder wall thickening was not observed on follow-up imaging after ablation.
Although the size of tumours showed no significant difference between the study group and the control group, tumours in the study group required a longer duration of ablation than those in the control group because of intermittent emission of MW antenna to avoid thermal damage to the adjacent gallbladder. The number of treatment sessions was not increased in our study, which benefited from detailed treatment protocol and accurate placement of antenna, tissue thermal monitoring needle, and PEI needle. Based on our prior study of adult dogs model, when the temperature fluctuation between 50 °C and 60 °C in the liver margin adjacent to the gallbladder lasted for 5–10 min, the gallbladder was not obviously hurt, as demonstrated by the gross anatomy and histopathological examination performed immediately and in the follow-up period after MW ablation. We intended to control the temperature of marginal tissue of tumour or liver tissue proximal to the gallbladder to below 54 °C, the threshold temperature of coagulation, to achieve the following two goals: to avoid thermal damage of the adjacent gallbladder and to ensure the thermal field covered the marginal field of the tumour. During the procedure we observed especially the site of the thermal monitoring needle by real-time sonography to prevent it from moving due to patients’ respiration.
The results of our technical success may also be attributable to the fact that all MW ablation sessions were performed by two experienced radiologists with more than 20 years of experience in interventional procedures, who ensured accurate placement of the needle in the tumours. In our opinion, the placement of the needle is of utmost importance in the procedure. In addition, to protect the gallbladder from being punctured unintentionally, the ablation electrode we adopted was an internally cooled straight-needle electrode and the advantage of the straight-needle electrode was greater visibility of the emission tip. Equally important, the straight needle was easily drawn back during the ablation if the patient felt pain resulting from the gallbladder. These favourable factors can reduce the risk of major complications. In our study, we found no significant difference between the study group and the control group either in major complications or in the rate of local tumour progression. Although there were no controlled studies of MW ablation vs. other methods of treatment for tumours adjacent to the gallbladder, our results of technical safety and therapeutic efficacy were close to the results in other studies [Citation31,Citation37].
For those tumours protruding or in contact with the gallbladder, the temperature of the marginal tumour tissue was controlled lower than the threshold temperature of coagulation. We added adjuvant therapy with a small dose of ethanol injection in the vicinity of the adjacent gallbladder to achieve complete necrosis of the marginal tumour tissue. Ethanol injection has two effects: procuring chemical ablation for the marginal cells of the tumour, and achieving a synergistic necrotising effect with combined use of ethanol and MW ablation. Experimental and clinical reports have shown that combined use of ethanol and RF or MW ablation causes a synergistic necrotising effect, with coagulation volumes clearly larger than those usually obtained with PEI, RF ablation, or MW ablation alone [Citation50–52]. In the study, there were no significant differences in the rate of complete ablation and local tumour progression between the patients treated with MW ablation alone and those treated with MW ablation and PEI.
For the study, a higher complete ablation rate and a lower local tumour progression rate were achieved in HCC than in metastatic hepatic lesions. The outcomes may be attributed to the pathological features of the tumours. Different from metastatic hepatic lesions, HCC is more prone to form a pseudo-capsule, which consists mainly of peritumoural hepatic sinusoids and/or fibrosis [Citation53]. As an important pathological feature of HCC, pseudo-capsule may prevent free invasion of HCC to the host liver and also indicates a relatively positive prognosis after tumour therapy [Citation54]. In terms of thermal ablation for tumours, the pseudo-capsule could make the heat from the electrodes congregate in the lesions and thus achieve a higher complete ablation rate compared with metastatic lesions.
This study also had some limitations. First, we evaluated the thermal injury of the gallbladder based only on symptomatology and imaging features, and no pathological proof of changes in the gallbladder after ablation was available. Second, the number of patients in our single study was small and the follow-up periods are relatively short. A multicentre study with a larger number of patients and a prolonged follow-up time is required to evaluate the further curative effect. Third, at present a thermal monitor can get the temperature at only one point. A study with temperature monitoring for multiple points with the thermal monitoring system may be more precise for the evaluation of ablation effect in the future. In addition, this study was our experience with MW ablation. It may need to be further confirmed whether it can be used in RF ablation or not.
Conclusion
In conclusion, under strict temperature monitoring, US-guided percutaneous MW ablation assisted with ethanol injection appears to be safe and can achieve a high rate of complete ablation for the treatment of hepatic tumours adjacent to the gallbladder. This modality may provide a new way for the treatment of hepatic tumours adjacent to the gallbladder.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bowles BJ, Machi J, Limm WM, Severino R, Oishi AJ, Furunoto NL, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg 2001;136:864–9
- Head HW, Dodd GD III. Thermal ablation for hepatocellular carcinoma. Gastroenterology 2004;127:S167–78
- Cady B, Jenkins RL, Steele GD Jr, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: A critical and improvable determinant of outcome. Ann Surg 1998;227:566–71
- Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: From percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology 2002;62:S64–8
- Haemmerich D, Laeseke PF. Thermal tumor ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005;21:755–60
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions. Radiology 2000;214:761–8
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD III, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005;235:728–39
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210–24
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8
- Liu N, Gao J, Liu Y, Li T, Feng K, Ma K, et al. Determining a minimal safe distance to prevent thermal injury to intrahepatic bile ducts in radiofrequency ablation of the liver: A study in dogs. Int J Hyperthermia 2012;28:210–17
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010;26:305–15
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994;74:817–25
- Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. Int J Hyperthermia 2004;20:671–7
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299–307
- Orlacchio A, Bazzocchi G, Pastorelli D, Bolacchi F, Angelico M, Almerighi C, et al. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: Initial experience. Cardiovasc Intervent Radiol 2008;31:587–94
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2014;30:1–8
- Kitchin D, Lubner M, Ziemlewicz T, Hinshaw JL, Alexander M, Brace CL, et al. Microwave ablation of malignant hepatic tumours: Intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 2014;30:299–305
- Liu F, Liang P, Yu X, Lu T, Cheng Z, Lei C, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: A preliminary clinical application. Int J Hyperthermia 2013;29:671–7
- Saccomandi P, Schena E, Silvestri S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int J Hyperthermia 2013;29:609–19
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001;221:159–66
- Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: Long-term results. Eur Radiol 2001;11:914–21
- Rossi S, Distasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol 1996;167:759–68
- Ren H, Liang P, Yu X, Wang Y, Lu T, Li X. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: A pilot study. Int J Hyperthermia 2011;27:249–54
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Thermal ablation therapy for hepatocellular carcinoma: Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology 2006;53:651–4
- Ryan TP, Turner PF, Hamilton B. Interstitial microwave transition from hyperthermia to ablation: Historical perspectives and current trends in thermal therapy. Int J Hyperthermia 2010;26:415–33
- Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003;226:441–51
- Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound 2002;15:73–6
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003;180:1547–55
- Livraghi T. Radiofrequency ablation of hepatocellular carcinoma. Surg Oncol Clin N Am 2011;20:281–99
- Chopra S, Dodd GD III, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. Am J Roentgenol 2003;180:697–701
- Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: Imaging spectrum and management. Radiographics 2005;25:S57–68
- Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 2013;29:558–68
- Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound 2001;13:159–66
- Sobiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, et al. Percutaneous US-guided radiofrequency tissue ablation of liver metastases: Treatment and follow-up in 16 patients. Radiology 1997;202:195–203
- McGahanl JP, Dodd GD III. Radiofrequency ablation of the liver. Am J Roentgenol 2001;176:3–16
- Kim SW, Rhim H, Park M, Kim H, Kim YS, Choi D, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: Assessment of safety and therapeutic efficacy. Korean J Radiol 2009;10:366–76
- Zhang F, Wu G, Sun H, Ding J, Xia F, Li X, et al. Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the Milan criteria: A single centre with 13 years experience. Int J Hyperthermia 2014;30:471–9
- Chen MS, Chen MH. Expert consensus on the norms of local ablation therapy for hepatocellular carcinoma. Chin J Hepatol 2011;19:257–9
- Zervas NT, Kuwayama A. Pathological characteristics of experimental thermal lesions. Comparison of induction heating and radiofrequency electrocoagulation. J Neurosurg 1972;37:418–22
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60
- Teratani T, Yoshida H, Shiina S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006;43:1101–8
- Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: Tailored approach. Abdom Imaging 2008;33:428–36
- Zhang F, Wu G, Sun H, Ding J, Xia F, Li X, et al. Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the Milan criteria: A single centre with 13 years experience. Int J Hyperthermia 2014;30:471–9
- Lee J, Rhim H, Jeon YH, Lim HK, Lee WJ, Choi D, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: Histopathologic changes of gallbladder wall in a pig model. Am J Roentgenol 2008;190:418–25
- Orlacchio A, Chegai F, Del Giudice C, Massaccesi M, Costanzo E, Di Caprera E, et al. Radiofrequency thermoablation of HCC larger than 3 cm and less than 5 cm proximal to the gallbladder without gallbladder isolation: A single center experience. Biomed Res Int 2014;2014:896527
- Jiang K, Su M, Zhao X, Chen Y, Zhang W, Wang J, et al. ‘One-off’ complete radiofrequency ablation of hepatocellular carcinoma adjacent to the gallbladder by a novel laparoscopic technique without gallbladder isolation. Cell Biochem Biophys 2013;68:547–54
- Brace CL. Microwave tissue ablation: Biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38:65–78
- Garrean S, Hering J, Saied A, Hoopes PJ, Helton WS, Ryan TP, et al. Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: Lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J Gastrointest Surg 2009;13:334–40
- Goldberg SN, Kruskal JB, Oliver BS, Clouse ME, Gazelle GS. Percutaneous tumor ablation: Increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology 2000;217:827–31
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72:S124–31
- Vallone P, Catalano O, Izzo F, Siani A. Combined ethanol therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intevent Radiol 2006;29:544–51
- Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: Correlation with histopathologic findings. Radiology 2009;250:435–43
- Xu W, Li JD, Shi G, Li JS, Dai Y, Wang XF. Different prognostic factors are associated with early and late intrahepatic recurrence following curative hepatectomy for patients with hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi 2010;48:806–11