Abstract
Purpose: The radioprotectors currently available are generally poorly tolerated in human beings; thus, their use has been restricted due to their side effects and their limited clinical tolerance. In a search for fewerand/or without side effects agents, the radioprotective effects of partial body hyperthermia (PBH) were tested on Wistar rats of both sexes at different ages. Materials and methods: PBH (43 °C, 1 h) was carried out by immersion of each animal’s lower parts and legs in a thermostatically controlled water bath 20 h prior to irradiation with a lethal single exposure dose of 9 Gy of gamma irradiation. Irradiated PBH pretreated animals were monitored for 30 days post-irradiation and survival percentages were calculated. Results: The data obtained provide evidence that PBH treatment prolonged the irradiated rats’ lifespans and the mortality rates varied significantly with animal age and sex. In addition, PBH treatment significantly enhanced bone marrow recovery of irradiated rats of both genders. Conclusions: Partial body hyperthermia prior to radiation proved to have beneficial effects on gamma irradiated rats.
Introduction
Ionising radiation has been used in a large number of diverse applications over recent decades. Exposures to radiation may occur due to radiotherapy in cancer treatment or during accidents in nuclear power plants. Consequently, it seems especially important to understand the mechanisms of radiation damage to cells, tissues, and living organisms and its possible prevention by chemical or physical means [Citation1,Citation2].
Heat therapy has been recognised for its beneficial effects on health since antiquity. Induced fever or hyperthermia, where the temperature of the tissue of the body is elevated artificially, has been studied for the treatment of chronic pain, recurrent respiratory infections, asthma, urinary tract infections, malignant diseases, and immune deficiency [Citation3,Citation4]. Hyperthermia is usually classified as local, partial (regional), or whole body hyperthermia (WBH), and treatment has been applied as a freestanding therapy or in conjunction with conventional treatments including radiotherapy, chemotherapy, hormonal, and biological therapies [Citation5].
In recent years there has been increased emphasis in combining ionising irradiation with hyperthermia in radiotherapy [Citation6–8]. In fact it has been found that hyperthermia has the ability to selectively kill some heat-sensitive malignancies and/or to reduce tumour growth [Citation9]. Moreover, it has been shown that WBH can be considered as a potent radioprotector [Citation10]. Accordingly, Zhao et al. found that pretreatment of mice with 39.5 °C enhanced lipopolysaccharide and induced tumour necrosis factor-alpha (TNF-α) and IL-6 production which might activate the innate immune response by promoting TLR4 expression [Citation11]. Vartak et al. demonstrated that local body hyperthermia (contralateral leg of mice, 41–43 °C for 40 min) prior to transplantation of fibrosarcoma reduced its growth [Citation12]. Meanwhile, Robins et al. reported that WBH induced elevated plasma levels of granulocyte-colony stimulating factor (G-CSF), a great range of interleukins, and TNF-α within hours of whole body hyperthermia [Citation13].
Currently, a variety of radioprotective agents are available for cancer patients receiving radiation therapy during their course of illness [Citation14]. However, the administration of these radioprotectors has always been associated with significant adverse effects and the search for efficient radioprotectants with acceptable side-effect profiles is needed. Thus, the present study was conducted to elucidate the role of partial body hyperthermia (PBH) as a non-toxic radioprotective agent before whole body exposure to a lethal dose of gamma irradiation in male and female Wistar rats of different age groups.
Materials and methods
Animals
A total of 396 Wistar male and female rats divided into five groups; control (n = 100) and four treatments groups (n = 296) (purchased from Center R. Janvier, St Berthevain, France) weighing 200–250 g were used. Animals were housed five per cage in transparent Plexiglas boxes (45 × 30 × 30 cm) and maintained on a standard stock laboratory diet and water ad libitum. The rats were kept in a temperature-controlled room (22 ± 1 °C) with a standard 12:12 h light/dark cycle. The use and care of animals conformed strictly to the Institutional Animal Ethics Committee guidelines.
Partial body hyperthermia
PBH was carried out by immersion of each animal’s lower parts and legs in a thermostatically controlled water bath. The animals were maintained in a vertical position during heat treatment by using specially designed perforated Plexiglas cylinders fixed on a platform calibrated to allow immersion of the animal’s lower parts and legs in the water bath. The temperature of the water bath was maintained at 43 ± 0.2 °C by covering the water surface with a stereo-board plate (local manufacturing; Starpack, Aleppo-Syria) to homogenise temperature and reduce the maximum temperature fluctuation through the duration of the heat treatment. In order to avoid hypoxia, the animals were exposed to a high ventilation rate during heat treatment. After 1 h of heat treatment, the animals were removed from the Plexiglas cylinders, dried with tissues and maintained at room temperature (for 2 h, in order to adapt to the temperature differences) before returning to the animal house.
Four groups of male and female rats were prepared: (1) group I: 50 mature males of average body weight of 175–200 g, 8–10 weeks of age; (2) group II: 79 males weighing ≥250 g, 12–14 weeks of age; (3) group III: 95 females, average body weight of 175–200 g, 8–10 weeks of age, and (4) group IV: 72 females weighing ≥250 g, 12–16 weeks of age.
Irradiation
Irradiation of PBH-treated rats was performed 20 h after heat treatment in a gamma cell source irradiator with 60Co (ROBO-Techsnabexport, www.tenes.ru). After dosimetry and dose estimates, points in which the dose rate was 0.8–0.9 Gy min−1 were determined. Control and PBH-treated animals placed in specially designed Plexiglas containers were irradiated with a single dose of 9 Gy and monitored for 30 days.
Preparation of bone marrow cell suspensions
After heat and irradiation treatments the animals’ bone marrow cell (BMC) counts were periodically monitored for at least 8 weeks following the treatment. BMC were prepared from femurs and tibias of male and female rats according to Bloom et al. [Citation15]. Cells were counted using classic blood counting slides (Neubauer) used in haematology and the results were expressed as the number of cells × 104/mL.
Statistical analyses
Time–mortality responses of the irradiated rats were estimated by probit analysis. Lethal times to achieve 99% mortality (LT99) were considered significantly different if their 95% fiducial limits did not overlap. Kaplan-Meier curves and estimates of survival data were constructed. The log-rank test was performed to detect differences between the survival curves. The significance level was set at α = 0.05 [Citation16]. Differences in mean bone marrow cell counts were determined using analysis of variance (ANOVA) at the 5% level (p < 0.05). Significant ANOVA was followed by the protected least significant difference (PLSD) at α < 0.05, StatView Version 4.02 [Citation17].
Results
The time–mortality responses of Wistar rats (LT99 values) to gamma irradiation and PBH varied significantly according to the tested treatments and the sex of the animals (e.g. LT99 values were 13.67 and 8.63 days for gamma-irradiated male and female rats, respectively). Moreover, significant χ2 values were found for five of the six time–mortality regressions, indicating that the response of Wistar rats to gamma irradiation and PBH treatments was heterogeneous (). In terms of survival curves calculated by the Kaplan-Meier method, we found that the PBH treatment prolonged the lifespan of gamma-treated rats of both sexes. For instance, the 200 -g PBH-treated male group the survival proportion was almost 35% by day 30, while all 9-Gy-irradiated males died within 16 days post-irradiation. According to the log-rank test, PBH treatment significantly improved survival (p < 0.0001) in both male and female rats compared to the 9-Gy irradiated male and female groups ().
Figure 1. Kaplan-Meiersurvival curves illustrating 30 days survival of 200 -g Wistar male and female rats subjected to 9-Gy whole body gamma irradiation and treated with partial body hyperthermia (PBH) 20 h prior to irradiation. According to Log-rank (Mantel-Cox) test, control vs. 9 Gy male, p < 0.0001; control vs. 9 Gy female, p < 0.0001; 9 Gy 200g male vs. PBH male, p < 0.001; 9 Gy 200g female vs. PBH female, p < 0.001.
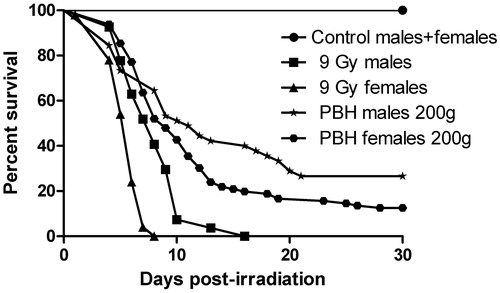
Table 1. Time-mortality responses of Wistar male and female rats subjected to 9 Gy whole body gamma irradiation and treated with partial body hyperthermia (PBH) 20 h prior to irradiation.
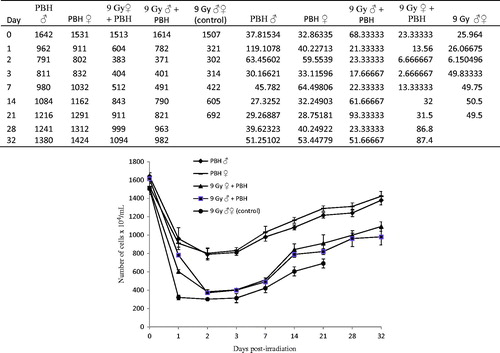
Significant decreases in the total BMC counts occurred in the PBH rat groups following heat treatment and then gradually recovered back towards the pretreatment baseline level on week 8, especially for PBH-treated male and female rats (). The decreases in BMC counts of the 9-Gy irradiated rat groups were significantly more pronounced compared to the PBH rat groups (F = 5737; df = 4, 70; p < 0.0001 on day 1 after treatment), thereafter the BMC counts began to rise as time passed after the exposure. Recovery of BMC after PBH and irradiation was relatively lower in male than female rats during the entire 8-week recovery.
Figure 2. Recovery of bone marrow cells of Wistar male and female rats subjected to 9-Gy whole body gamma irradiation and treated with partial body hyperthermia (PBH) 20 h prior to irradiation. Error bars represent the mean ± SEM. Significant differences were found between treatments on day 21 post-irradiation (F = 1936; df = 4, 70; p < 0.0001), ANOVA followed by PLSD.
Discussion
Hyperthermia is an ancient therapeutic approach, dating back to around 3000 BC However, despite this long history, several methods of extracorporeal hyperthermia are still under investigation in order to enhance the effects of radio-chemotherapy combination or to treat certain infectious diseases [Citation18–21].
In general, younger Wistar rats (≤200 g) appear to be more radio-resistant than older ones, and female rats are more sensitive to ionising radiation than males. Our data revealed that PBH of animal legs played an important role in extending survival of male and female rats subjected to a lethal dose of gamma irradiation and this effect was correlated with the animals’ age and sex. For instance, more than 25% and 10% of PBH-treated males and females, respectively, survived up to 30 days post-lethal irradiation. This finding corroborates results that WBH (40 °C, 1 h) treatment given to mice 20–48 h prior to whole body irradiation provided 80% protection as estimated by monitoring animal mortality rates [Citation22].
At high radiation doses, it is well documented that the vital systems of haematopoiesis and immunity are the most susceptible to lethal radiation damage [Citation23,Citation24]. Our results showed that the BMC counts decreased 24 h post-irradiation and PBH treatments, but the decrease rate was more pronounced for irradiation than PBH treatment. A possible reason for the decrease of bone marrow cell count 24 h post-PBH treatment could be attributed to the direct haemolytic effects of hyperthermia on the cell membrane resulting in increased membrane fluidity and consequently cell fragility [Citation25]. However, the PBH gradually accelerated the recovery of both irradiated male and female rats, and their BMC counts attained values higher than those of irradiated animals that did not receive PBH treatment. These data are in agreement with several studies claiming that mild WBH applied to mice before their total-body gamma irradiation significantly enhanced the radio resistance of the haematopoietic stem cells in their bone marrow [Citation26,Citation27].
Rhind et al. reported that cytokine induction by WBH was able to induce elevated plasma levels of G-CSF and certain interleukins (IL1β, IL6, IL8 and IL10) and TNF-α within hours of heat treatment of patients [Citation28]. IL6 is known as a multifunctional cytokine regulating immune response, acute phase reactions and haematopoiesis [Citation29]. Furthermore, there is evidence that IL6 as well as IL1β contributes to generating febrile seizures in developing rats [Citation30].
Finally, it is worth noting that we did not notice any obvious changes in the vigour or behaviour of the PBH-treated rats. Moreover, the ulceration seen in eyes of the irradiated animals, even before death, did not occur, indicating the beneficial effects of PBH.
In conclusion and as a future direction, it will be interesting to carry out more studies on hyperthermia as a radioprotective agent and examine the possibility of developing this approach at a clinically acceptable scale for people having radiotherapy, exposed to diagnostic radiation or who are in certain professional activities.
Acknowledgements
The authors would like to thank Ibrahim Othman (General Director) and Nizar Mirali for their help and support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hosseinimehr SJ, Tavakoli H, Pourheidari G, Sobhani A, Shafiee A. Radioprotective effects of citrus extract against gamma-irradiation in mouse bone marrow cells. J Radiat Res 2003;44:237–41
- Mauryia DK, Devasagayan TP, Nair CK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol 2006;44:93–114
- Kerner T, Deja M, Ahlers O, Löffel J, Hildebrandt B, Wust P, et al. Whole-body hyperthermia: A secure procedure for patients with various malignancies. Intensive Care Med 1999;25:959–65
- Fiorentini G, Szasz A. Hyperthermia today: Electric energy, a new opportunity in cancer treatment. J Cancer Res Ther 2006;2:41–6
- Franckena M, Lutgens LC, Koper PC, Kleynen CE, Steen-Banasik EM, Van der Steen-Banasik EM, et al. Radiotherapy and hyperthermia for treatment of primary locally advanced cervix cancer: Results in 378 patients. Int J Radiat Oncol Biol Phys 2009;73:242–50
- Kaur P, Hurwitz MD, Krishnan S, Asea A. Combined hyperthermia and radiotherapy for the treatment of cancer. Cancers 2011;3:3799–823
- Zhao C, Dai C, Chen X. Whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy for the treatment of stage IV advanced gastric cancer. Int J Hyperthermia 2012;28:735–41
- Tang Y, McGoron AJ. Increasing the rate of heating: A potential therapeutic approach for achieving synergistic tumour killing in combined hyperthermia and chemotherapy. Int J Hyperthermia 2013;29:145–55
- Westermann AM, Wiedemann GJ, Jager E, Jager D, Katschinski DM, Knuth A, et al. A Systemic Hyperthermia Oncologic Working Group trial. Ifosfamide, carboplatin, and etoposide combined with 41.8 °C whole-body hyperthermia for metastatic soft tissue sarcoma. Oncology 2003;64:312–21
- Shen RN, Hornback NB, Shidnia H, Wu B, Lu L, Broxmeyer HE. Whole body hyperthermia: A potent radioprotector in vivo. Int J Radiat Oncol Biol Phys 1991;20:525–30
- Zhao W, An H, Zhou J, Xu H, Yu Y, Cao X. Hyperthermia differentially regulates TLR4 and TLR2-mediated innate immune response. Immunol Lett 2007;108:137–42
- Vartak S, George KC, Singh BB. Antitumor effects of local hyperthermia on a mouse fibrosarcoma. Anticancer Res 1993;13:727–30
- Robins HI, Kutz M, Wiedemann GJ, Katschinski DM, Paul D, Grosen E, et al. Cytokine induction by 41.8 °C whole body hyperthermia. Cancer Lett 1995;2:195–201
- Wang K, Liu C, Di CJ, Ma C, Han CG, Yuan MR, et al. Kojic acid protects C57BL/6 mice from gamma-irradiation induced damage. Asian Pac J Cancer Prev 2014;15:291–7
- Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol 2004;12:1163–72
- SPSS. IBM SPSS Statistics 19, Chicago, IL, 2010
- StatView version 4.02. Abacus Concepts, Berkeley, CA, 1994
- Singh BB. Hyperthermia: An ancient science in India. Int J Hyperthermia 1991;7:1–6
- Raaphorst GP, Feeley MM. Hyperthermia radiosensitization in human glioma cells, comparison of recovery of polymerase activity survival and potentially lethal damage repair. Int J Rad Oncol Biol Phys 1994;29:133–9
- Hildebrandt B, Rau B, Gellermann J, Wust P, Riess H. Hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinosis. J Clin Oncol 2004;22:1527–29
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncology 2007;6:418–26
- Patil MS, Kaklij GS, Poduval TB, Singh BB. Radioprotective effect of whole-body hyperthermia on mice exposed to lethal doses of total-body gamma irradiation. Indian J Exp Biol 1996;34:842–4
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9
- Wang GJ, Cai L. Induction of cell-proliferation hormesis and cell-survival adaptive response in mouse hematopoietic cells by whole-body low-dose radiation. Toxicological Sci 2000;53:369–76
- Hassan AI, Abd El-Rahim AH. Effect of hyperthermia at different ages and mode of recovery on the chromosomal aberrations and biological parameters in female rats. J Am Sci 2010;6:153–66
- Zaidi AK, Bagewadikar RS, Subramanian M, Kaklij GS, Patil MS. Effect of whole body hyperthermia (39 °C, 1 h) on radiation-induced apoptosis in Swiss mice. J Thermal Bio 2004;29:3–8
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42
- Rhind SG, Gannon GA, Shephard RJ, Buguet A, Shek PN, Radomski MW. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: Neuroendocrine regulatory mechanisms. Int J Hyperthermia 2004;20:503–16
- Hirano T. Interleukin 6 and its receptor: Ten years later. Int Rev Immunol 1998;16:249–84
- Fukuda M, Morimoto T, Suzuki Y, Shinonaga C, Ishida Y. Interleukin-6 attenuates hyperthermia-induced seizures in developing rats. Brain Dev 2007;10:644–48

