Abstract
Purpose: Microwave ablation (MWA) is a new minimally invasive method for thermal ablation of benign thyroid nodules with promising results. The aim of this study was to investigate whether MWA has an impact on thyroid function. Materials and methods: Thirty patients with a total of 34 benign thyroid nodules underwent MWA between January 2013 and July 2014. Serum levels of triiodothyronine (T3), thyroxine (T4), thyrotropin (TSH), thyroglobuline (Tg) and additionally antibodies against Tg (anti-Tg), thyrotropin receptors (TRAb) and thyroid peroxidase (anti-TPO) were measured at enrolment, 24 h after MWA, as well as at the 3-month and 6-month follow-up. Moreover, the nodule volume was evaluated to determine effectiveness. Results: Serum TSH, T4, T3 and Tg levels did not change significantly at the 3-month or 6-month follow-up (p > 0.05); thyroid function was not affected by MWA. Antibody levels did not change significantly either; however, two patients developed antibodies after treatment. A volume reduction of 51.4% or 7.85 mL could be demonstrated after 3 months and a reduction of 55.8% or 14.0 mL after 6 months. Slight complications such as mild pain during the ablation or superficial haematomas emerged. The development of Graves’ disease and mild Horner’s syndrome were observed as more severe side effects. Conclusions: The data suggest MWA as an alternative for the treatment of benign thyroid nodules. While first results for preservation of thyroid function are positive, further measurements of laboratory data and especially antibodies are necessary.
Introduction
Thyroid nodules are frequently detected in patients in about 30% of the German population [Citation1]. Fortunately, most nodules are benign; only 0.1% of all malignant degenerations are thyroid carcinomas [Citation1]. Yet even benign nodules can cause problems, such as cosmetic issues, and subjective symptoms as well as the patient’s fear of malignant transformation [Citation2].
Surgery and radioiodine therapy (RIT) are still the standard therapy options, but especially in recent years novel treatment options for thyroid nodules have been developed. The field of minimally invasive thermal ablation has expanded over recent years and promising results have been achieved for radiofrequency ablation (RFA), laser ablation (LA), high -intensity focused ultrasound (HIFU) [Citation3,Citation4] and ethanol ablation (EA) [Citation2,Citation5–7].
Microwave ablation (MWA) is a new approach for thermal ablation of thyroid nodules, yet has already been used successfully to treat malignancies in liver, lung and kidneys [Citation8,Citation9]. This technique has been proven effective for volume reduction in benign thyroid nodules in various studies [Citation10–12] and has been successfully tested for thyroid cancer [Citation13]. There have been few reports of transient hyperthyroidism after RFA [Citation2,Citation14], hypothyroidism has been reported in two studies [Citation7,Citation15]. Regarding EA, hypothyroidism was provoked more often, all cases happening after treatment of autonomous functioning nodules [Citation16–19]. In terms of HIFU, thyroid function is not affected by the treatment [Citation4].
Regarding MWA, there is currently only one study, by Feng et al. [Citation12], evaluating the impact on thyroid function marginally. As far as we know, antibodies have not been measured in any study. The aim of this study was to examine possible effects of MWA on thyroid function and antibodies for the first time. For this purpose, pre- and post-ablative thyroid hormone status were compared and analysed.
Materials and methods
Patients
Thirty patients with 34 nodules overall (15 men, 15 women, mean age 54.0 years, range 32–79 years) were treated with MWA between January 2013 and July 2014. Inclusion criteria were (1) symptomatic thyroid nodules, (2) cosmetic concerns, and (3) refusal of or contraindications for surgery. Exclusion criteria were: (1) retrosternal growth, (2) histological evidence for malignancy, and (3) conspicuous calcitonin measurement. There was no minimum size for admission. All patients signed an informed consent form. The study was approved by the local ethics committee (No. 243/13).
Pre-ablative assessment
All patients underwent a pre-ablative assessment using laboratory test, ultrasound, scintigraphic imaging and fine needle aspiration biopsy (FNAB). A B-mode ultrasound (Sonix Touch Ultrasound system, Ultrasonix Medical Corporation, Richmond, BC, Canada) was used to evaluate the volume, size, number and composition of the nodules.
Serum levels were determined with commercially available immunoradiometric assay (IRMA) and radioimmunoassay (RIA) kits. Laboratory tests included a complete thyroid hormone status with triiodothyronine (T3, normal range 1.0–3.3 nmol/L) determined by RIA (T3[125I] RIA Kit, Izotop, Budapest, Hungary), thyroxine (T4, normal range 55–170 nmol/L) determined by RIA (T4[125I] RIA Kit, Izotop), thyrotropin (TSH, normal range 0.3–4.0 mIU/L) determined by IRMA (SELco® TSH rapid, Medipan, Dahlewitz, Germany) and thyroglobulin (Tg, normal range 2–70 µg/L) determined by IRMA (Riason® Tg c.t., Iason, Graz-Seiersberg, Austria).
Moreover, calcitonin levels, blood count and coagulation diagnostic were measured and the presence of antibodies was checked. The following antibodies were measured: antibodies against thyroid peroxidase (anti-TPO, positive >50 kIU/L) determined by RIA(anti-TPO magnum, Medipan), thyroglobulin (anti-Tg, positive >50 kIU/L) determined by RIA (anti-Tg magnum, Medipan) and thyrotropin receptor (TRAb, positive >1.5 IU/L) determined by RIA (TRAK Human RIA, Brahms, Henningsdorf, Germany).
All patients received 99 mTc-pertechnetate scans; scintigraphic images were taken 20 min after administration of 75 MBq 99 mTc-pertechnetate and recorded with a scintillation camera (Mediso Nucline® TH/22, Budapest). Patients with ‘cold’ nodules were re-evaluated with a 99 mTc-methoxy-isobutyl-isonitrile (MIBI) scan to exclude malignancy. Scintigraphic images were taken 10 and 60 min after administration of 500 MBq 99 mTc-MIBI.
To exclude malignancy, FNAB and calcitonin measurement took place. No evidence for malignant transformation has been found. Pre-ablative data are summarised in .
Table 1. Clinical data collected prior to MWA.
MWA treatment procedure and equipment
MWA treatment was performed as an outpatient procedure. Patients were placed in a supine position with hyperextended neck. The complete intervention took place in aseptic conditions. Local infiltration anaesthesia (Scandicain 1%) of the puncture site was performed. In cases where cystic components have been detected in pre-ablation ultrasound assessment, fluid was aspirated before ablation. The skin was incised 1–2 mm and the ablation antenna was positioned under sonographic monitoring. A trans-isthmic access was chosen in order to keep the heat impact on vital structures as low as possible [Citation20]. When this access could not be conducted, a cranio-caudal access was chosen. The procedure was conducted in the ‘moving-shot technique’ [Citation2]. If several nodules were found in one patient, the nodules were ablated successively during the same ablation procedure.
During the whole procedure real-time ultrasound was utilised to assure the correct position of the microwave antenna. The microwave system used in this study worked with frequencies of 902–928 MHz and maximum temperatures of approximately 140 °C. Heat is produced by utilisation of the dipole moment of water molecules; microwaves lead to oscillation and thereby heat.
Eventually, antennas with an uncooled tip (14 gauge) were used in agreement with individual aspects of every patient. The target temperature was 60–80 °C; temperatures were monitored to avoid excessive heating. The output varied between 24 and 28 W.
Efficacy evaluation and follow-up
Laboratory tests and scintigraphic imaging were re-assessed 24 h after microwave ablation. Additionally, B-mode ultrasound was performed to exclude focal complications.
After 3 and 6 months, a follow-up examination of both nodule volume and laboratory values took place in which the volume reduction was calculated by the following equation: volume reduction ratio (%) = ((median initial volume − median final volume) × 100)/median initial volume.
Statistical analysis
All statistical analyses were performed with dedicated software (Bias®, Windows, version 10.04, epsilon verlag, 1989–2013). Due to non-parametric distribution, pre-, post-ablative and follow-up variables were compared by use of Wilcoxon’s signed rank test and Friedman test. Spearman’s correlation test was used to analyse a possible correlation between the difference in thyroglobulin parameters and volume reduction.
All laboratory parameters, volume parameters and volume reduction are given as median. The significance level was defined as p < 0.05.
Results
The results demonstrated that in all 30 sessions the complete volume of the 34 nodules could be ablated. The treatment was well tolerated by all patients, an interruption was not necessary. A total of 21 complex nodules (neither cystic nor solid) and 13 solid nodules were treated. The mean time of ablation was 674 ± 336 s (range 300–1500 s), the mean output 29.2 ± 4.38 W (range 24–40 W), the temperature was 80.2 ± 1.6 °C (range 75–85 °C). At the 3-month follow-up 30 patients were assessed, and 11 patients at the 6-month follow-up.
Laboratory tests
Laboratory parameters did not change significantly at the 3-month or 6 month follow-up (p > 0.05). T4 and T3 levels remained within normal limits in all patients (). Five patients had suppressed TSH levels before and/or after treatment, regarding all other patients TSH levels remained in normal limits as well. All of these five patients had a thyroid autonomy in addition to the nodules that were treated with MWA. In four patients TSH levels normalised by 3-month follow-up. Thyroglobulin levels increased significantly within 24 h after ablation, but decreased significantly again by the 3-month follow-up (p < 0.05) (). In 21 of 30 patients thyroglobulin levels fell below initial values at 3-month follow-up; however, altogether the difference between initial values and values at the 3-month follow-up was not significant according to Wilcoxon’s signed rank test. No significant difference between thyroglobulin levels at 3 months and 6 months as well as between initial values and 6 months could be observed. In 7 of 11 patients thyroglobulin levels decreased again by the last follow-up. Serum levels before and 24 h after the treatment as well as at 3-month and 6-month follow-up are summarised in . A correlation between the changes in Tg level and volume reduction could not be allocated.
Figure 1. Serum levels of (a) T3, (b) T4, (c) TSH and (d) Tg prior to microwave ablation (MWA), 24 h after MWA and at 3-month and 6-month follow-up. Tg values were logarithmised for better illustration.
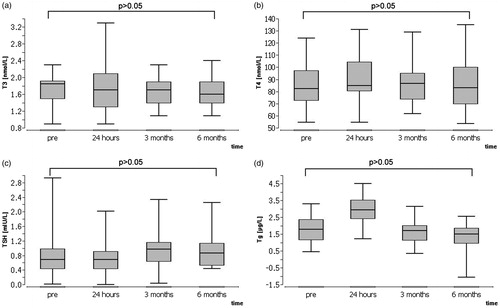
Table 2. Parameters of thyroid function at different dates of examination.
Antibody measurement
One patient presented with elevated anti-TPO and anti-Tg levels before and after MWA, while another patient showed constantly positive anti-TPO levels before and after MWA. These patients did not show any symptoms of Hashimoto’s thyroiditis or Graves’ disease before or after treatment. One patient showed increased anti-Tg levels before MWA; after treatment anti-Tg levels were negative. Another patient presented with increased TRAb, anti-Tg and anti-TPO levels before and after treatment, Graves’ disease was formerly known. This patient was treated with a combination of MWA, for a cold nodule, as well as radioiodine therapy for Graves’ disease.
One patient with formerly known Hashimoto’s thyroiditis had repeatedly elevated anti-TPO levels. Furthermore anti-Tg antibodies developed at 3-month follow-up. Another patient showed positive TRAb levels at the 3-month follow-up, Graves’ disease was diagnosed, yet at the last follow-up, TRAb levels had normalized. This patient was also treated with a combination therapy, MWA was applied for treatment of a cold thyroid nodule, and radioiodine therapy was administered directly after MWA for treatment of goitre. Pathological antibody levels are shown in . None of the other patients were found to develop thyroid antibodies during the follow-up period. Antibody measurement at different dates of examination is summarised in .
Table 3. Patients with pathological findings in antibody measurement.
Volume reduction
Ultrasound examination showed a significant decrease of nodule volume in all patients (). The overall pre-ablative volume was 18.15 mL; post-ablative volume was 8.8 mL after 3 months and 6.2 mL after 6 months. The median volume reduction was 7.85 mL (range 1.5–62.2 mL) or 51.4% (range 14.6–88.4%) at the 3-month follow-up (p < 0.05) and 14.0 mL (range 1.5–64 mL) or 55.8% (41.6–84%) at 6-month follow-up (p < 0.05).
Safety and treatment tolerance
The procedure was tolerated well by all patients, despite some side effects. Light sensations of pressure and mild pain were reported during all ablation sessions, though, none of the patients asked to stop the session. Two patients developed first-degree burns alongside the puncture channel, which did not require any therapy. Mild superficial haematomas appeared in almost every patient due to the pressure of the ultrasound probe, yet faded within the next few days. Sonographic controls assured that the haematomas did not reach deeper tissue layers.
One patient developed a cervical haematoma (approximately 47 × 22 mm) and subsequently Doppler ultrasonography was performed to expulse active bleeding. Haemoglobin parameters were steady. No further treatment was necessary, the haematoma reabsorbed itself.
One patient developed mild Horner’s syndrome with ptosis and miosis. The symptoms disappeared in the further observation period without any treatment; pathologies were no longer noticed at last follow-up. One patient developed TRAb antibodies at 3-month follow up, Graves’ disease was diagnosed. After 6 months, antibodies had normalised without any treatment.
There were no serious complications such as infection, nodule rupture, secondary haemorrhage, hypoparathyroidism or voice change. In the post-therapeutic observation period no other complications were observed.
Discussion
In Germany the prevalence for thyroid nodules is relatively high [Citation21,Citation22]. Thyroidectomy and RIT are still the current standard treatment approaches for thyroid nodules. However, they involve drawbacks and complications such as nerval lesions or scar formation [Citation12,Citation23]. Moreover, both methods have an incontrovertible effect on thyroid function. As far as surgery is concerned, permanent hypothyroidism is mostly inevitable, especially with total thyroidectomy. With this treatment option normal thyroid function cannot be preserved; a lifelong substitution of thyroid hormones is necessary.
RIT can cause hypothyroidism as well. Hypothyroidism develops quite frequently after RIT of autonomous nodules; this contributes to the inevitable impact of RIT on the whole thyroid gland. Spiezia et al. [Citation24] reports that 14% of patients with toxic goitre developed hypothyroidism within 5 years of treatment.
In the past decade, minimal-invasive alternatives to surgery and RIT have been developed, particularly RFA, EA and LA. Regarding the impact of these techniques on thyroid function, few cases of post-ablative hypothyroidism have been described. There are two cases each described for RFA [Citation7,Citation15] and LA [Citation25]. All of the four patients showed a persistent increase of thyroid antibodies before and after RFA. In terms of EA, hypothyroidism has been reported more often, the majority being found after treatment of autonomously functioning nodules [Citation16,Citation17,Citation19]. Transient hyperthyroidism after RFA has been reported in a few patients [Citation2,Citation14]. Ha et al. investigated the possible impact of RFA on thyroid function in patients with previous lobectomy. No effect on thyroid function could be discovered; all serum levels remained within normal limits at the last follow-up, and none of the patients developed new antibodies [Citation26].
Within the group of thermo-ablative techniques, MWA is a new approach to treat benign thyroid nodules; its effectiveness has been proven in several studies [Citation11,Citation12]. Regarding effects on thyroid function, there have not been any other studies evaluating the impact of MWA on the different thyroid hormones and antibodies yet.
The present study demonstrates that MWA does not affect thyroid function in patients with benign thyroid nodules. All hormones and antibodies, except for one patient developing Graves’ disease, remained within normal levels directly after ablation and at the follow-up examinations.
Overall, effects on thyroid function due to minimally invasive ablation methods are rarely discovered. This can surely be attributed to the fact that the impact on normal thyroid tissue can be minimised with these ablation techniques; only the targeted nodules are affected. Findings in post-ablative scintigraphic imaging support this statement; the 99 mTc-pertechnetate uptake only decreased in the ablation area; surrounding tissue was not affected [Citation27]. As 99 mTc-pertechnetate uptake depends on the sodium iodide symporter in thyroid tissue [Citation28], the regular uptake in surrounding tissue indicates that molecular changes only occurred in the ablation zone. General thyroid function was not affected.
MWA is assumed to affect tissue through so-called thermal effects with temperatures higher than 60 °C, and non-thermal effects. Thermal effects on tissue are coagulation necrosis and protein denaturisation caused by heat [Citation29]; non-thermal effects, for instance electroporation [Citation30], ion acceleration and collision, and also dipole rotation [Citation31] also generate changes in protein structure. In this study thyroid hormones and antibodies as protein structures are not influenced by MWA other than the protein structure in the ablation zone itself. This suggests that MWA does only affect the ablation zone itself, preserving all other remaining thyroid tissue as well as hormones and antibodies.
In the study presented, one patient had increased TRAb levels before and after MWA. It is very unlikely that the MWA treatment influenced the constantly elevated TRAb level in this particular situation. Another patient from this study showed elevated TRAb levels at 3-month follow-up, Graves’ disease was diagnosed. To our knowledge, there is no description of a correlation between MWA and Graves’ disease so far. Concerning RIT of functional autonomy, autoimmune thyroiditis can be found in approximately 1% of all cases; higher incidences are possible with pre-existing TPO antibodies [Citation32]. Nygaard et al. [Citation33] states that the presence of TPO-antibodies before RIT is also a marker for elevated risk of hypothyroidism. This patient showed no elevated anti-TPO levels before treatment, the possible mechanism of antibody development can only be assumed. Carracio et al. [Citation34] found positive antibody levels after treatment of cold nodules with EA, ‘sensitization after massive release of partially denatured thyroid substances along with a rise in serum interleukin-6 concentration’ (p. 702) [Citation34] are supposed to be potential causes. Although very rare, Graves’ disease can also appear after thyroidectomy. Yu et al. [Citation35] ascribe 11 cases to pre-existing antibodies.
In fact, pre-existing thyroid antibodies seem to be a major factor for development of post-ablative thyroid dysfunction. Most patients with a dysfunction had antibodies before treatment [Citation7,Citation15,Citation19], some developed thyroid antibodies after treatment [Citation6,Citation17].
Yu et al. state three different hypotheses for the development of TRAb after thyroidectomy: (1) with demolition of thyroid cells TSH receptors, thyroglobulin antigens and microsomal antigens are secreted from the thyroid, leading to stimulation of helper T cells which generate autoantibodies, (2) the development is caused by an abnormality of antigen presenting cells, and (3) the stress from general anaesthesia and surgery lead to neuroendocrine fluctuations and immunological homeostasis [Citation35].
The first hypothesis for development of autoimmune thyroiditis after ablation is supported by Carracio et al. [Citation34] as mentioned above, as well as Monzani et al., who suggest that EA leads to massive release of partially denatured thyroglobulin, which could be the reason for immunisation [Citation17].
Thyroglobulin represents a tumour marker and is used for measurement of thyroid mass or thyroid injury [Citation25]. In this study Tg was significantly elevated 24 h after MWA and decreased significantly again until the 3-month follow-up. In 21 of 30 patients the levels fell below initial values; still, the difference was not significant. The elevation directly after MWA can most likely be attributed to the release of Tg due to thyroid injury, as mentioned above. As levels decrease respectively in the follow-up period this could be interpreted as a sign of recovery and reduction of the nodule volume. Unfortunately, a correlation between the overall reduction of Tg and the volume reduction could not be found. Presumably, the non-significant change of Tg at 3 months can be attributed to the short follow-up period as there was not enough time for the Tg level to recover completely. Valcavi et al. [Citation25] discovered similar findings after LA of benign thyroid nodules; the marked evaluation of Tg was attributed to release from intraglandular stores.
In this study, a few complications emerged, but none required any treatment; all vanished within the observation period. The development of Horner’s syndrome was the most serious complication emerging in this study. Most likely, the cause was thermal injury to the medial type of the middle sympathetic ganglion which is located on the longus colli muscle on the C6 vertebra level [Citation36]. Injuries to nerval structures due to thermal ablation have been described before; damage happened to the recurrent laryngeal nerve or the brachial plexus [Citation12,Citation14,Citation37]. In all cases the impact of heat was suspected to be the cause; Feng et al. [Citation12] presumed compression caused by perinodular oedema as another possible source. To avoid damage to nerval structures in further studies, pre-ablative ultrasound examinations should be advanced with regard to vital structures such as sympathetic ganglia [Citation36]. Moreover, arrangements to keep the thermal impact on nerves as low as possible should be made: injection of fluid between the nodule and critical structures [Citation20], temperature monitoring of surrounding tissue or even incomplete ablation for nodules too close to nerves [Citation12]. Further studies are necessary to evaluate the safety of MWA technique.
Regarding effectiveness, a volume reduction of 51.4% at 3-month follow-up and 55.8% at 6-month follow-up confirmed the results of previous studies. Feng et al. [Citation12] evidenced a volume reduction of 45.99 ± 29.9% in 11 patients after 6 months; Yue et al. [Citation11] also showed a decrease of 65 ± 65% in 222 patients after 6 months.
Scintigraphic findings confirmed these results [Citation10,Citation38].
The present study has several limitations, such as a small number of patients and nodules and a short-term follow-up period. The actual incidence of pathological thyroid function and antibody development after MWA in the present patient population might not represent the final outcome at a later stage. A larger follow-up study with longer follow-up duration would address these issues.
The fact that five patients who presented with elevated antibody levels before treatment were included in the study constitutes another limitation. Therefore, additional tests concerning thyroid function and antibodies are necessary to confirm the findings of this study.
Conclusion
The data assembled in this study suggest that MWA is an alternative for the treatment of benign thyroid nodules. Due to small sample size, the findings of this study need to be verified by larger studies. Laboratory parameters and especially antibodies need to be monitored in additional follow-up periods.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blank W, Braun B. Sonografie der Schilddrüse. Teil 1 – Untersuchungstechnik, Normalbefund, Struma diffusa und Struma nodosa. [Sonography of the thyroid - Part 1.] Ultraschall Med 2007;28:554–68
- Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: Safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244–50
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2014:1–8. PMID: 25367011
- Korkusuz H, Sennert M, Fehre N, Happel C, Grünwald F. Local thyroid tissue ablation by high-intensity focused ultrasound: Effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia 2014;30:480–5
- Guglielmi R, Pacella CM, Bianchini A, Bizzarri G, Rinaldi R, Graziano FM, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: Role and efficacy. Thyroid 2004;14:125–31
- Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: A randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 2007;17:229–35
- Baek JH, Lee JH, Sung JY, Bae J, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: A multicenter study. Radiology 2012; 262:335–42
- Liu S, Liang P, Yu X, Cheng Z, Han Z, Yu J. Percutaneous microwave ablation for liver tumours adjacent to the marginal angle. Int J Hyperthermia 2014;30:306–11
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol 2009;38:135–43
- Korkusuz H, Happel C, Heck K, Ackermann H, Grünwald F. Percutaneous thermal microwave ablation of thyroid nodules: preparation, feasibility, efficiency. Nuklearmedizin 2014;53:123–30
- Yue W, Wang S, Wang B, Xu Q, Yu S, Yonglin Z, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: Safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11–16
- Feng B, Liang P, Cheng Z, Yu X, Yu J, Han Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 2012;166:1031–7
- Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 2014;30:150–7
- Kim Y, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: Initial clinical experience. Thyroid 2006;16:361–7
- Baek JH, Moon W, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009;33:1971–7
- Di Lelio A, Rivolta M, Casati M, Capra M. Treatment of autonomous thyroid nodules: value of percutaneous ethanol injection. Am J Roentgenol 1995;164:207–13
- Monzani F, Caraccio N, Goletti O, Lippolis PV, Casolaro A, Del Guerra P, et al. Five-year follow-up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: A study of 117 patients. Clin Endocrinol (Oxf) 1997;46:9–15
- Kim DW, Rho MH, Park HJ, Kwag HJ. Ultrasonography-guided ethanol ablation of predominantly solid thyroid nodules: A preliminary study for factors that predict the outcome. Br J Radiol 2012;85:930–6
- Livraghi T, Paracchi A, Ferrari C, Reschini E, Macchi RM, Bonifacino A. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: 4-year experience. Radiology 1994;190:529–33
- Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: Basic principles and clinical application. Int J Endocrinol 2012;2012:919650
- Happel C, Truong PN, Bockisch B, Zaplatnikov K, Kranert WT, Korkusuz H, et al. 99mTc-Szintigraphie versus Farbduplex-Sonographie. Kann zur Diagnose der funktionellen Schilddrüsenautonomie auf die Szintigraphie verzichtet werden? [Colour-coded duplex-sonography versus scintigraphy. Can scintigraphy be replaced by sonography for diagnosis of functional thyroid autonomy?] Nuklearmedizin 2013;52:186–91
- Etzel M, Happel C, Müller von F, Ackermann H, Bojunga J, Grünwald F. Palpation and elastography of thyroid nodules in comparison. Nuklearmedizin 2013;52:97–100
- Christou N, Mathonnet M. Complications after total thyroidectomy. J Visc Surg 2013;150:249–56
- Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 2009;19:219–25
- Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: A 3-year follow-up study in 122 patients. Thyroid 2010;20:1253–61
- Ha EJ, Baek JH, Lee JH, Sung JY, Lee D, Kim JK, et al. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid 2013;23:289–93
- Korkusuz H, Happel C, Heck K, Ackermann H, Grünwald F. Percutaneous thermal microwave ablation of thyroid nodules. Preparation, feasibility, efficiency. Nuklearmedizin 2014;53:123–30
- Kogai T, Brent GA. The sodium iodide symporter (NIS): Regulation and approaches to targeting for cancer therapeutics. Pharmacol Ther 2012;135:355–70
- Vogl TJ, Mack M, Eichler K, Nour-Eldin NE, Mbalisike E, Zangos S, et al. Interventionelle Thermoablation von malignen Lebertumoren und Lebermetastasen: Vergleich von Radiofrequenzablation (RFA), laserinduzierter Thermotherapie (LITT) und Mikrowellenablation (MWA). [Interventional thermoablation of malignant liver tumors and liver metastases: a comparison of radiofrequency ablation (RFA), laser-induced thermotherapy (LITT), and microwave ablation (MWA).] Hessisches Ärzteblatt 2011;72:606–16
- Shamis Y, Taube A, Mitik-Dineva N, Croft R, Crawford RJ, Ivanova EP. Specific electromagnetic effects of microwave radiation on Escherichia coli. Appl Environ Microbiol 2011;77:3017–22
- Banik S, Bandyopadhyay S, Ganguly S. Bioeffects of microwave – a brief review. Bioresour Technol 2003;87:155–9
- Dietlein M, Dressler J, Grünwald F, Leisner B, Moser E, Reiners C, et al. Guideline for radioiodine therapy for benign thyroid diseases (version 4). Nuklearmedizin 2007;46:220–3
- Nygaard B, Faber J, Veje A, Hegedüs L, Hansen JM. Transition of nodular toxic goiter to autoimmune hyperthyroidism triggered by 131I therapy. Thyroid 1999;9:477–81
- Caraccio N, Goletti O, Lippolis PV, Casolaro A, Cavina E, Miccoli P, et al. Is percutaneous ethanol injection a useful alternative for the treatment of the cold benign thyroid nodule? Five years’ experience. Thyroid 1997;7:699–704
- Yu HM, Park SH, Lee JM, Park KS. Graves’ disease that developed shortly after surgery for thyroid cancer. Endocrinol Metab (Seoul) 2013;28:226–30
- Shin JE, Baek JH, Ha EJ, Choi YJ, Choi WJ, Lee JH. Ultrasound features of middle cervical sympathetic ganglion. Clin J Pain 2014. doi: 10.1097/AJP.0000000000000184. [Epub ahead of print]. PMID: 25411859
- Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 2013;23:1044–9
- Korkusuz H, Happel C, Grünwald F. Ultrasound guided percutaneous microwave ablation of hypofunctional thyroid nodules: Evaluation by scintigraphic 99mTc-MIBI imaging. Nuklearmedizin 2013;52:N68