Dear Sir,
We herein present a case of a soft tissue sarcoma where, due to technical limitations of hyperthermia (HT) applicator size, half of the tumour could be treated with both HT and radiotherapy (RT), and the remaining half by RT alone. This gave us an opportunity to examine the differential impact of RT and HTRT on the gross and microscopic changes in the same tumour of a patient.
Case study
A 78-year-old male patient presented with a swelling in his left arm for the past 2 months. On MRI, the swelling measured 20 × 7 × 5 cm, and was extending down from the left coracobrachialis muscle and axilla to the arm with evidence of fatty degeneration of the adjacent muscles. On an ultrasound-guided biopsy, a diagnosis of malignant myxoid liposarcoma was confirmed. The patient was staged as T2bN0M0Gx.
In view of the large size and its proximity to the brachial neurovascular bundle, he was considered for tumour downstaging with preoperative RT with HT. He received a dose of 50 Gy in 25 fractions delivered over 5 weeks with 3D conformal RT. Since the maximum size of the HT applicator available was an eight-antenna circular applicator with a 12 cm diameter, the upper half (≈10 cm) of the tumour was treated with RT alone, while the lower half (≈10 cm) received HT along with RT (). Local HT, at 915 Mhz for 60 min using a BSD 500 (Salt Lake City, UT) hyperthermia unit, was delivered twice a week and temperature was maintained around 41–43 °C. With 915 Mhz, it would be expected to achieve a maximum temperature of around 43 °C at a depth of 3 cm. However, the tumour with a maximum initial thickness of 5 cm had shown gradual reduction during the course of treatment, enabling it to be effectively heated during the course of 5 weeks. HT was carried out around 30 min before each RT session. Skin surface was cooled with circulating water bolus, and local temperature was monitored using online thermometry. Invasive thermometry was not used as a departmental policy. Overlying real time skin temperatures were monitored using multiple probes at a minimum of four points during each treatment session. The average temperatures recorded over the 60-min session ranged from 40 to 41.4 °C (mean ± SD: 40.7 ± 0.3 °C).
Figure 1. (A) Pretreatment MRI of the tumour having a dimension of 20 × 7 × 5 cm. (B) The cranial part treated with radiotherapy alone had a regression of 65% compared to 85% on the caudal part treated with thermoradiotherapy.
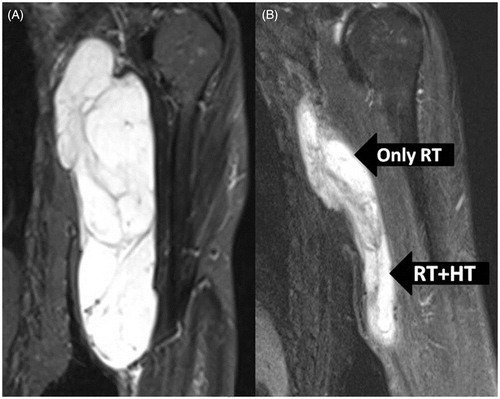
The patient tolerated the treatment fairly well and except for mild local erythema, there were no other acute morbidities that could be attributed to either RT or HTRT. He underwent a repeat MRI 6 weeks following the completion of preoperative treatment. It showed significant reduction in the tumour size and volume; nearly 85% regression in the caudal part treated with HTRT and around 65% regression in the cranial part treated with RT alone ().
The patient underwent complete excision of the tumour at 7 weeks following RT/HTRT and is currently on follow-up at 32 months following treatment. He has no significant functional impairment of his treated arm and is free of any loco-regional or distant disease and continues to lead a normal life.
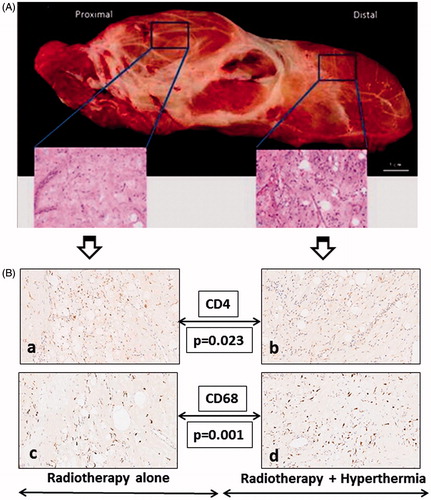
Grossly, the resected tumour specimen was well encapsulated. On histopathology, the entire specimen was devoid of any significant tumour cells and the parts treated with RT or HTRT exhibited a similar post-irradiated morphology with evidence of fibrosis. On a closer perusal of the histopathology, the part treated with HTRT appeared to exhibit a relatively increased cellularity than that exposed to RT alone (). This prompted us to look for the immune cell population in these two parts.
Figure 2. (A) Resected tumour with insets showing the histopathology of the proximal part treated with radiotherapy alone and the distal part treated with thermotherapy. A relatively increased cellular infiltration in the part treated with hyperthermia could be noted. (B) Immunohistochemistry photomicrographs for CD4 (a, b), CD68 (c, d). The immunohistochemistry from the part treated with radiotherapy alone is depicted in panels a and c, while b and d are from the parts treated with thermoradiotherapy.
Immunohistochemistry of the RT- and HTRT-treated parts
Immunohistochemistry (IHC) studies were performed separately from the two segments of the resected specimen, representing regions treated with RT or HTRT. The cell types studied were CD3 (pan T cells), CD4 (T-helper cells), CD8 (cytotoxic T cells), CD20 (pan B cells), CD25 (regulatory T cells), CD56 (natural killer cells), CD68 (macrophages), CD79a (pan B cells), CD138 (plasma cells) and CD34 (progenitor cells of blood vessels). For both parts, five regions of interests (ROIs) each representing an area of 1 mm2 were marked in the sections from each of the two parts (representing RT- and HTRT-treated regions) and the number of cells/vessel were counted within the same five ROIs for each of the above IHC markers. Thus, a total of 50 ROIs, 25 in each RT and HTRT regions were scored for all these IHC studies.
CD4 + T cells were found to be significantly more frequent in the part treated with RT (mean ± SD, RT 8.4 ± 5.3 versus HTRT 1.6 ± 0.9, p = 0.023) while CD68 + cells were predominantly found in the HTRT-treated part (mean ± SD, RT 53.2 ± 3.9 versus HTRT 72.2 ± 6.8, p = 0.001). The CD8 + /CD4 + values were higher with HTRT (mean ± SD 3.6 ± 2.9) than RT (mean ± SD 1.5 ± 1.2, p = 0.159). There was also a trend towards higher CD34 + counts in the HTRT parts compared to RT alone (mean ± SD, HTRT 72.2 ± 6.8 versus RT 53.2 ± 3.9, p = 0.06).
IHC studies showed CD34 expression, a marker for progenitor cells of blood vessels, to be relatively higher in the part treated with HTRT than RT alone. This raised the possibility that HT might increase angiogenesis through the up-regulation of the hypoxia-inducible transcription factor (HIF-1) [Citation1,Citation2]. One may tend to attribute the changes in the immune cell distribution in RT versus HTRT, in part due to an increase in vascularity with HT. However, the vessel counts as evidenced by CD34 + did not show a significant increase in the part treated with HT (p = 0.068). Thus, the observations of a significant increase in CD4 + cells (p = 0.023) in the RT-treated part and significant amplification of CD68 + cells in the HT-treated part might not be solely attributed to the marginal increase in vascularity with HT.
Possible explanation for the differential immune cell population in RT- and HTRT-treated parts
Ionising radiation has been reported to contribute to the development of adaptive anti-tumour immunity through the activation of tumour-associated dendritic cells (DCs), which in turn support the tumour-specific effector CD8 + T cells [Citation3,Citation4]. The latter are presumed to be stimulated by cross-presentation of tumour-derived antigens by the DCs. Besides the killing of tumour cells directly, CD8 + T cells may also trigger various immunomodulatory mechanisms that indirectly favour anti-tumour responses.
Hyperthermia is also known to modulate the innate and adaptive immune system by activating DCs and thereby facilitating the uptake of the tumour antigen derived from necrotic and apoptotic tumour cells, a phenomenon mimicking ‘in situ tumour vaccination’ [Citation5]. Recently, it has been reported that HT alone led to a significant increase in DNA fragmentation and apoptotic bodies between 24 and 72 h following HT in 29 colorectal cancer cell lines [Citation6]. These could further promote an anti-tumour immune response [Citation5,Citation7]. In addition, HT also triggers immune responses through the induction of heat shock proteins (HSPs), especially HSP70. These HSPs and tumour antigen-containing exosomes, released from apoptotic and necrotic cells, could form complexes to attract the resulting DCs, further enhancing their capacity to activate CD8 + T cells. This chain of events would culminate in enhanced tumour cell death, producing cell debris that is taken up for scavenging by CD68 + macrophages. The interaction of CD4 + Th1 (T helper) cells with DCs fosters the activation of naïve CD8 + T cells. However, CD4 + T cells might be also regulatory ones that induce immune suppression. Recent studies are increasingly indicating that HT could play an important role as an immunotherapy through the complex interplay of various immune cells and HSPs [Citation8,Citation9].
The IHC findings in this patient are in the resected tumour specimen that had completed RT or HTRT, 7 weeks earlier. Thus, they are a snap shot of the immune cell components distributed in these regions. RT alone resulted in a significant increase in CD4 + cells, while a slight increase of the CD8 + /CD4 + T-cell ratio was observed in the HTRT part. In addition, the CD68 + macrophages were significantly higher in the HTRT-treated part. Considering the sequence of events that trigger downstream immunostimulatory pathways following RT or HT, the predominance of CD4 + cells and CD68 + cells in the RT and HTRT parts respectively, may reflect the two separate time points in the sequence of immunomodulatory events stimulated by RT and HT. The presence of CD4 + cells in the RT part are likely to represent the initial set of events of RT-induced immunomodulation, while the higher conglomeration of CD68 + cells in the HTRT part could reflect the terminal part of the same process. Thus, HT might be accelerating the entire process of RT-induced immunomodulation, which could have facilitated a greater quantum of tumour regression in the HTRT part.
The above observations are based on the unique opportunity that we got to observe the differential gross and microscopic changes in the same tumour treated with either RT or HTRT. Nonetheless, the significantly differential immune cell populations seen in RT- and HTRT-treated parts hint towards a possible dynamic immunomodulation by HT and needs further evaluation.
Acknowledgements
The authors gratefully acknowledge the valuable inputs from Prof. D.E. Speiser of the Clinical Tumour Biology and Immunotherapy Group, Department of Oncology and Ludwig Cancer Research Centre, University of Lausanne, Switzerland.
Declaration of interest
This study was supported by a partial grant from the Research Council, Kantonsspital Aarau (Forschungsrat KSA). The work of Udo Gaipl was supported by the German Research Foundation (DFG-Graduiertenkolleg 1660: Key signals of the adaptive immune response) and the German Federal Ministry of Education and Research (BMBF, m4 Cluster, 16EX1021R). The authors alone are responsible for the content and writing of the paper.
References
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004;5:429–41
- Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci USA 2010;107:20477–82
- Rubner Y, Wunderlich R, Rühle PF, Kulzer L, Werthmöller N, Frey B, et al. How does ionizing radiation contribute to the induction of anti-tumor immunity? Front Oncol 2012;2:75. doi: 10.3389/fonc.2012.00075
- Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8 + T cells via dendritic cell activation. J Immunol 2012;189:558–66
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42
- Meggyeshazi N, Andocs G, Balogh L, Balla P, Kiszner G, Teleki I, et al. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol 2014;190:815–22
- Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, et al. Combination of ionizing radiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther Onkol 2010;186:587–99
- Repasky EA. Progress in development of biomedical applications of heat shock proteins and thermal stress. Int J Hyperthermia 2013;29:359–61
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9

