Abstract
Purpose: Tumour-cell-derived exosomes (Exo) have been proposed as a new kind of drug carrier, and heat stress can promote release of exosomes from tumour cells. This study investigated the impact of heat stress on the quantity of doxorubicin in exosomes from the same number of doxorubicin-treated MFC-7 tumour cells and their anti-tumour effects.
Materials and methods: Exosomes were isolated from phosphate-buffered saline (Exo), doxorubicin (Exo-Dox) or doxorubicin combined with heat-stress-treated (Exo-Dox-HS) MCF-7 cells. The content of doxorubicin in the exosomes was determined by flow cytometry. The effects of individual types of exosomes on the MCF-7 cell proliferation and apoptosis as well as the tumour growth were determined by MTT assay, flow cytometry and murine xenograft tumour modelling.
Results: We found that the amount of Exo-Dox-HS was higher than that of Exo-Dox from the same number of MCF-7 cells, and Exo-Dox-HS contained higher levels of doxorubicin than Exo-Dox from the same number of cells. Exo-Dox and Exo-Dox-HS, but not Exo or 10 µg/mL doxorubicin, significantly inhibited the MCF-7 cell proliferation and triggered MCF-7 cell apoptosis, associated with increased levels of cleaved caspase-3 and -8 and morphological changes in MCF-7 cells. Treatment with Exo-Dox and Exo-Dox-HS inhibited the growth of implanted breast tumours in mice.
Conclusions: Our study indicated that heat stress increased the quantity of doxorubicin-containing exosomes from tumour cells, and enhanced the anti-tumour effect of exosomes from the doxorubicin-treated tumour cells. Our findings may aid in designing new strategies for cancer therapy by combination of chemotherapy and hyperthermia.
Introduction
Doxorubicin is one of the most effective chemotherapeutic drugs and has potent anti-tumour effects on breast cancer. However, the clinical application of doxorubicin is restricted due to its low bioavailability and serious side effects, including cardiotoxicity and myelosuppression [Citation1]. Recent studies suggest that loading nanoparticles with doxorubicin can effectively deliver doxorubicin, which increases anti-tumour efficacy [Citation2]. However, artificial nanoparticles as an exogenous component may bring some unexpected adverse effects, such as inducing oxidative stress and adverse immune responses [Citation3].
Exosomes are small membrane vesicles of endocytic origin and have a typical bilayer-membrane structure and a diameter of 40–100 nm. They contain microRNAs, mRNAs, and proteins, which shuttle from donor cells to recipient cells to communicate and transport information between different cells. Therefore, exosomes may serve as a specific delivery vehicle for drugs targeted to specific tumours [Citation4].
Curcumin is a drug with anti-inflammatory activity, but its low solubility limits its clinical value. Previous studies have shown that curcumin delivered by exosomes is highly effective [Citation5,Citation6]. Our previous study and those of others have shown that exosomes derived from IL-2 and IL-18 gene-modified tumour cells contain IL-2 and IL-18, suggesting that IL-2 and IL-18 can be incorporated into exosomes [Citation7,Citation8]. It has also been demonstrated that tumour cells can efflux doxorubicin and cisplatin through exosome secretion [Citation9–11]. These findings suggest that exosomes can accumulate cellular contents, including chemotherapeutic drugs. Chemotherapeutic agent-containing exosomes have also been reported to possess potent anti-tumour effects, but have no obvious side effect. Unlike nanoparticles, which are synthesised in vitro, exosomes come from autologous tumour cells, thus probably leading to minimal toxicities when being transferred into target cells [Citation3,Citation12]. Hence, exosomes represent a novel strategy to deliver chemotherapeutic drugs into tumour tissues without obvious side effect. However, it is unclear whether exosomes from the doxorubicin-treated MFC-7 cells contain doxorubicin.
Previous data have reported that heat stress can promote release of exosomes from tumour cells [Citation13–15]. However, the impact of heat stress on the quantity of doxorubicin in exosomes from the same number of doxorubicin-treated MFC-7 tumour cells and their anti-tumour effects have not been clarified. In this study we characterised exosomes from the doxorubicin-treated MFC-7 tumour cells with or without heat stress and investigated their anti-tumour effects. Our novel data indicated that heat stress increased the production of doxorubicin-containing exosomes from the same number of doxorubicin-treated MFC-7 cells and enhanced their anti-tumour activity in vitro and in vivo.
Materials and methods
Animals and cell lines
Female athymic nu/nu mice at 5–6 weeks of age were obtained from Joint Ventures Sipper BK Experimental Animal (Shanghai, China) and housed in a specific pathogen-free facility. Human breast cancer MCF-7 cell line was obtained from ATCC (Manassas, VA, USA) and maintained according to the ATCC instructions.
Isolation of doxorubicin-containing exosomes from heat-stress-treated tumour cells
MCF-7 cells (1 × 107/flask) were treated with phosphate-buffered saline (PBS) or 30 µg/mL doxorubicin in a 37 °C humidified tissue culture incubator for 4 h, and the doxorubicin-treated cells were incubated in a water bath at 42 °C (heat stress) or 37 °C (without heat stress) for 1 h. After 4 h of recovery at 37 °C, exosomes in the supernatants of cultured cells were isolated by sequential centrifugation (4 °C) at 800 × g for 10 min and 1200 × g for 30 min. Subsequently the supernatants were collected and centrifuged at 10,000 × g for 60 min. The pellets were resuspended in 0.25 M sucrose and floated onto a discontinuous density cushion containing 20 mM Tris/30% sucrose/45% sucrose (pH 7.2), then centrifuged at 100,000 × g for 2 h. The liquid phase between the interfaces was collected and mixed with a large volume of 30 ml cold PBS, followed by centrifuging at 100,000 × g for 1 h. The final exosome pellets were resuspended in the same volume of cold PBS and the concentrations of proteins in the prepared exosomes were determined using a BCA protein detection assay (Pierce Biotechnology, Rockford, IL).
Electron microscopy
The prepared exosomes were fixed in 4% paraformaldehyde and loaded onto electron microscopy grids coated with Formvar carbon, followed by contrasting and embedding in a mixture of uranyl acetate and methylcellulose. The ultra-thin sections were examined under a Philips Tecnai-10 transmission electron microscope (TEM) operating at 80 kV (Phillips Electronic Instruments, Mahwah, NJ).
Western blot analysis of doxorubicin-containing exosomes
Western blot assay was performed, as described previously [Citation16]. Briefly, exosomal proteins (30 µg) were first separated by 10% sodium dodecyl sulphate polyacrylamide (SDS-PAGE) gel, and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% fat-free dry milk in Tris-buffered saline with Tween (TBST) and probed with primary antibodies against Hsp70, CD63, TSG101 and β-actin, according to recommended dilutions (Santa Cruz Biotechnology, Santa Cruz, CA). The bound antibodies were detected by horse radish peroxidase (HRP)-conjugated secondary antibodies and the immuno-complex was visualised by enhanced chemiluminescence reagent (Pierce Biotech, Rockford, France). The relative levels of target proteins to the control β-actin were determined by densitometric scanning using a UVP gel documentation system, c-80 (UVP, Upland, CA).
Measurement of doxorubicin in the exosomes
The contents of doxorubicin in the exosomes prepared from doxorubicin-treated MCF-7 cells with or without heat stress, or from the PBS-treated MCF-7 cells were determined by flow cytometry. Briefly, equal amount of Exo, Exo-Dox or Exo-Dox-HS were coupled onto 4 μm aldehyde-sulphate latex beads (Invitrogen, Eugene, OR) and the mean fluorescence intensity of doxorubicin in latex beads was detected with an excitation wavelength of 488 nm and emission wavelength of 575 nm by flow cytometry.
In addition, the contents of doxorubicin in individual exosome samples were also determined by detecting the fluorescent intensity in a DTX 880 multimode detector (Beckman Coulter, Palo Alto, CA), according to a standard curve established using different concentrations of doxorubicin.
Measurement of MCF-7 cell proliferation
MCF-7 cells (3 × 105/well) were cultured in 96-well plates and treated in triplicate with or without Exo-Dox-HS, Exo-Dox and Exo (from 1 × 107 or 2 × 107 cells), 10 μg doxorubicin for 72 h at 37 °C in a humidified atmosphere of 5% CO2. During the last 4-h incubation, MTT assay solution (10 µL/well) was added. The formazan generated in individual wells was solubilised with 10% SDS–5% isobutanol, 0.012 mL/l HCl (100 µL/well). After 12 h development at room temperature, the optical density (OD) values at 450 nm were measured by Bioteck EL-340 microplate reader (Bioteck, Winooski, VT).
Detection of tumour cell apoptosis by FACS
MCF-7 cells (1 × 106/mL) were treated in triplicate with Exo-Dox-HS, Exo-Dox (from 1 × 107 cells) for 24 h. The cells were stained with FITC-Annexin V (BD Pharmingen, San Diego, CA) and propidium iodide (Sigma-Aldrich, Saint Louis, MO) for 5 min at 4 °C in the dark. The apoptotic cells were analysed by flow cytometry. In addition, the cells were subjected to electron microscopy (EM) analysis of apoptotic characteristics.
MCF-7 cells (1 × 106/mL) were treated in triplicate with Exo-Dox-HS, Exo-Dox (from 1 × 107 cells) for 3 h, and the relative levels of cleaved caspase-8 and -3 in each group of cells were detected by Western blot assay.
Anti-tumour effect of doxorubicin-containing exosomes in vivo
Individual BALB/c nude mice were inoculated subcutaneously with 1 × 107 MCF-7 tumour cells. Three days after inoculation the mice were injected subcutaneously near the tumours with Exo-Dox-HS, Exo-Dox (from 1 × 107 or 2 × 107 cells), Exo, Exo-HS (from 2 × 107 cells), 10 μg doxorubicin, or PBS (100 µL), n = 5 per group). The identical treatment was repeated every 3 days for five times. The tumour volumes were monitored with a caliper every 3 days and calculated by the formula volume = length × (width2)/2. The experimental protocol was approved by the Animal Care and Research Committee of Zhejiang Cancer Hospital.
Statistical analysis
Experimental data are expressed as the mean ± SD. The difference among the groups was analysed using one-way ANOVA and SPSS 11.5. A p-value of < 0.05 was considered statistically significant.
Results
Identification of doxorubicin-containing exosomes
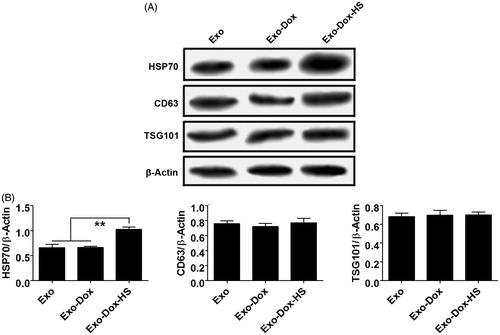
Through serial centrifugation and sucrose gradient ultracentrifugation we isolated and purified the exosomes from MCF-7 cells without doxorubicin treatment, or doxorubicin-treated MCF-7 cells with or without heat-stress treatment. We first characterised the morphology of all kinds of exosomes by EM. As shown in , the Exo, Exo-Dox and Exo-Dox-HS exosomes had similar characteristics of ‘saucer’ or round morphology limited by a bi-layer membrane and had diameters ranging from 40 to 100 nm. In addition, these types of exosomes contained several exosomal characteristic proteins, including Hsp70, CD63 and TSG101 (). Moreover, the relative levels of Hsp70, but not CD63 and TSG101 in the Exo-Dox-HS were significantly higher than that of Exo and Exo-Dox (). These data indicated that heat-stress treatment promoted the production of doxorubicin-containing exosomes from the same number of MCF-7 cells.
Figure 1. Morphological analysis of exosomes by EM. Exosomes from MCF-7 cells without doxorubicin treatment, or doxorubicin-treated MCF-7 cells with or without heat-stress treatment were purified by serial centrifugation and sucrose gradient ultra-centrifugation. The morphology of exosomes was visualised under EM. Bars = 100 nm. Data were representative images of individual exosome samples from three independent experiments.
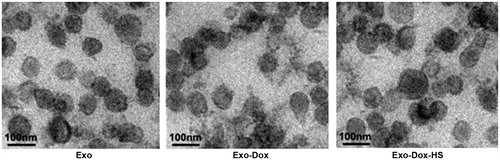
Figure 2. Western blot analysis of exosomes. The relative levels of Hsp70, CD63 and TSG 101 to the control β-actin in the Exo, Exo-Dox and Exo-Dox-HS exosome samples were determined by Western blot assays and quantified by densitometric scanning. (A) Western blot analysis. (B) Quantitative analysis. Data are representative images and expressed as the mean ± SD of the relative values from three independent experiments. **p < 0.01.
Heat stress did not alter the quantity of doxorubicin packed into exosomes
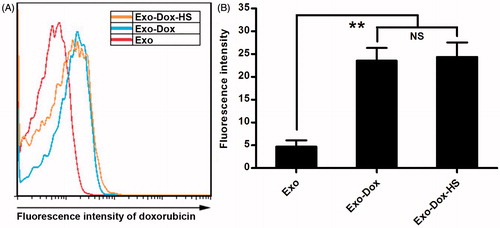
To examine whether exosomes from the doxorubicin-treated tumour cells could contain doxorubicin, the contents of doxorubicin in the Exo, Exo-Dox and Exo-Dox-HS exosomes were determined by flow cytometry. As shown in , the fluorescence intensity of the same amount of Exo-Dox and Exo-Dox-HS from doxorubicin-treated tumour cells was similar and was significantly higher than that of the same amount of Exo from MCF7 cells without doxorubicin and heat stress treatment. These results indicated that doxorubicin-treated MCF-7 cells could produce doxorubicin-containing exosomes and heat-stress treatment did not significantly alter the quantity of doxorubicin packed into exosomes. These results indicate that tumour cells encapsulated doxorubicin into exosomes and heat-stress treatment did not significantly alter the quantity of doxorubicin encapsulated into exosomes produced by the doxorubicin-treated MCF-7 cells.
Figure 3. Quantification of doxorubicin in exosomes. The contents of doxorubicin in the same amount of Exo, Exo-Dox, and Exo-Dox-HS exosomes were determined by flow cytometry and quantified for the fluorescent intensity. (A) Representative histograms. (B) The fluorescence intensity of doxorubicin in the different groups of exosomes. Data are representative histograms and expressed as the mean ± SD of the fluorescent intensity of each group of exosome samples from three separate experiments. **p < 0.01; NS, not significant.
Heat stress enhanced production of doxorubicin-containing exosomes from tumour cells
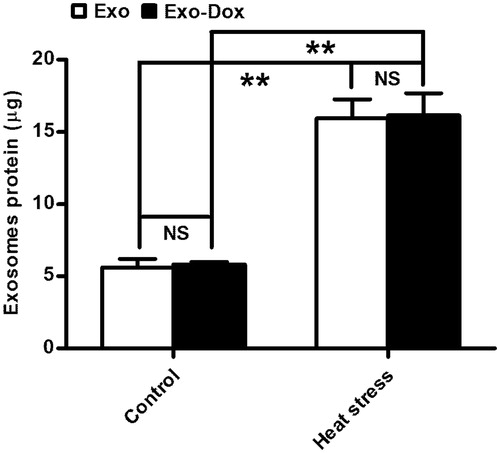
Next, we examined whether heat stress could affect the production of doxorubicin-containing exosomes from MCF-7 tumour cells. MCF-7 cells (1 × 107) were treated with or without doxorubicin. Subsequently, the cells were treated with or without heat stress. The Exo, Exo-HS, Exo-Dox and ExoDox-HS exosomes produced were purified simultaneously by sucrose gradient ultra-centrifugation. As shown in , the levels of total proteins in the Exo-HS and Exo-Dox-HS exosomes were similar (Exo-HS 15.96 ± 2.29 μg versus Exo-Dox-HS 16.16 ± 2.65 μg, p > 0.05, ) and were significantly higher than those of Exo (5.63 ± 1.01 μg, p < 0.01) and Exo-Dox exosomes (5.81 ± 0.35 μg, p < 0.01). These data indicate that treatment with doxorubicin at this dose did not affect the production of exosomes and heat stress significantly enhanced the production of exosomes from MCF-7 cells.
Figure 4. The quantity of exosomes produced by the heat-stress-treated MCF-7 cells. MCF-7 cells (1 × 107/flask) were treated with PBS or doxorubicin and some cells from each group were treated with heat stress. The exosomes produced were purified simultaneously and the total amounts of proteins in each group of exosomes were determined by BCA assay. Data are expressed as the mean ± SD of each group from three independent experiments. **p < 0.01.
Heat stress enhanced the cytotoxicity of doxorubicin-containing exosomes from the same number of tumour cells in vitro
To compare the cytotoxicity of Exo-Dox, Exo-Dox-HS and free doxorubicin we quantified the contents of doxorubicin in exosomes from doxorubicin-treated MCF-7 tumour cells. Firstly, we made the standard curve of doxorubicin concentrations and values of fluorescent intensity (). Furthermore, we detected the values of fluorescent intensity of exosomes from 1 × 107 MCF-7 tumour cells with or without heat-stress treatment. We found that the Exo-Dox and Exo-Dox-HS exosomes from 1 × 107 doxorubicin-treated MCF-7 tumour cells contained doxorubicin of 1.73 ± 0.24 or 4.91 ± 0.97 μg, respectively (p < 0.001, ). Subsequently we tested the effect of Exo, Exo-Dox, Exo-Dox-HS (from 1 × 107 or 2 × 107 cells), 10 μg doxorubicin (similar to the quantity in Exo-Dox-HS from 2 × 107 cells) or PBS on the proliferation of MCF-7 cells by MTT assay. We found that treatment with either Exo-Dox or Exo-Dox-HS (from 1 × 107 or 2 × 107 cells) significantly inhibited the proliferation of MCF-7 cells, related to treatment with vehicle alone (, ). Although the inhibitory effects of Exo-Dox or Exo-Dox-HS tended to be dose-dependent, the inhibitory effects of Exo-Dox-HS on the proliferation of MCF-7 cells were more powerful than those of Exo-Dox from the same number of doxorubicin-treated MCF-7 cells. In addition, treatment with 10 μg doxorubicin did not significantly alter the proliferation of MCF-7 cells in our experimental conditions. Collectively, these data clearly indicated that doxorubicin-encapsulated in the exosomes from tumour cells had more potent cytotoxicity against tumour cells than free drug and that heat stress enhanced the cytotoxicity of exosomes produced from the same number of doxorubicin-treated MCF-7 cells.
Figure 5. Effects of doxorubicin-containing exosomes on tumour cell proliferation in vitro. (A) A standard curve of doxorubicin fluorescent intensity. (B) Quantity of doxorubicin in exosomes from 1 × 107 doxorubicin-treated MCF-7 tumour cells with or without heat-stress treatment. (C) Exosomes from the doxorubicin-treated MCF-7 cells inhibit the proliferation of MCF-7 cells. MCF-7 cells were treated with Exo-Dox-HS, Exo-Dox, Exo (from 1 × 107 or 2 × 107 MCF-7 cells), or 10 μg doxorubicin for the indicated time periods. The cells in medium alone served as negative controls. The proliferation of different groups of MCF-7 cells was measured by MTT assay. Data are expressed as the mean ± SD of individual groups of cells from three separate experiments. ***p < 0.001.
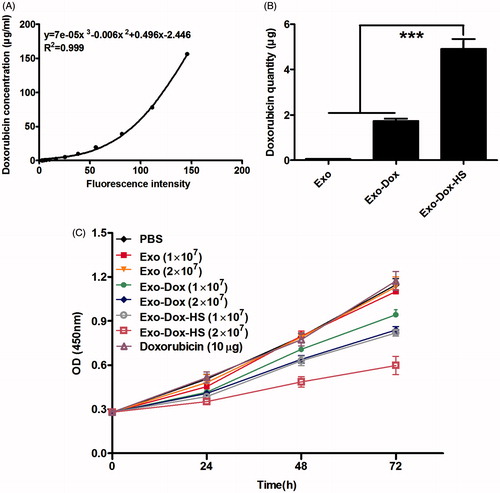
Table 1. Statistical analysis of each group to inhibit MCF-7 proliferation.
Heat stress enhanced the cytotoxicity of doxorubicin-containing exosomes from the same number of tumour cells by inducing tumour cell apoptosis in vitro
Given that doxorubicin can inhibit growth of tumour cells through inducing tumour cell apoptosis [Citation17], we investigated the effect of Exo-Dox and Exo-Dox-HS exosomes form the same number of doxorubicin-treated MCF-7 cells on the apoptosis of MCF-7 cells in vitro. As shown in , treatment with either Exo-Dox or Exo-Dox-HS both slightly decreased the early apoptotic MCF-7 cells (Annexin V+PI- cells) but significantly increased the frequency of late apoptotic MCF-7 cells (Annexin V+PI+ cells), as compared with treatment with Exo. Furthermore, the percentages of apoptotic MCF-7 cells in the Exo-Dox-HS-treated MCF-7 cells were significantly higher than that in the Exo-Dox-treated cells (p < 0.05). Neither treatment increased the necrotic MCF-7 cells (Annexin V−PI+ cells). In addition, TEM revealed the characteristics of apoptosis, such as chromatin condensation, margination and nuclear condensation in the Exo-Dox-treated cells and severe swelling in the Exo-Dox-HS-treated cells ().
Figure 6. Doxorubicin-containing exosomes induce MCF-7 cell apoptosis. MCF-7 cells were treated with Exo-Dox-HS, Exo-Dox or Exo from the same number of MCF-7 cells for 24 h, and stained with FITC-Annexin V and propidium iodide. The frequency of apoptotic cells was analysed by flow cytometry. Some cells from each group were subjected to TEM examination or Western blot analysis of the relative levels of cleaved caspase-3 and -8. Data are representative images, flow cytometry charts, and expressed as the mean ± SD of each group of cells from three separate experiments. (A) Flow cytometry analysis of apoptotic cells. (B) Quantitative analysis of apoptotic cells. (C) TEM analysis of apoptotic cells. (D) Western blot analysis. *p < 0.05, **p < 0.01.
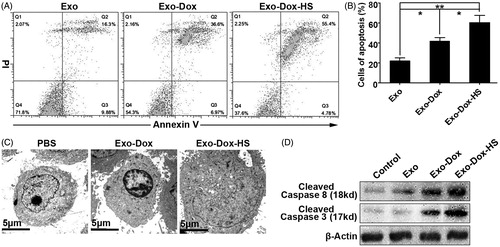
To further confirm the effect of doxorubicin-containing exosomes on the apoptosis of MCF-7 cells, we characterised the relative levels of activated caspase-3 and -8, denoting the activation of cellular apoptotic signalling, by Western blot. As shown in , treatment with either Exo-Dox or Exo-Dox-HS clearly increased the relative levels of activated caspase-3 and -8 and Exo-Dox-HS increased them more than Exo-Dox did in MCF-7 cells. These results indicate that Exo-Dox-HS are more efficient in inducing MCF-7 tumour cell apoptosis than Exo-Dox produced from the same number of doxorubicin-treated MCF-7 cells.
Heat stress enhanced the anti-tumour effect of doxorubicin-containing exosomes from the same number of tumour cells in vivo
Finally, we investigated whether doxorubicin-containing exosomes or a similar quantity of free doxorubicin could inhibit the growth of MCF-7 cells in vivo. MCF-7 cells were inoculated subcutaneously into nude mice. Three days later, the mice were randomised and treated subcutaneously with Exo-Dox-HS, or Exo-Dox from either 1 × 107 or 2 × 107 MCF-7 cells, or exosomes from heat-stress-treated MCF-7 cells (2 × 107) without doxorubicin treatment (Exo-HS), 10 μg doxorubicin or PBS (control) every 3 days for five treatments. In comparison with that in the control group, treatment with Exo-Dox-HS and Exo-Dox significantly inhibited the growth of MCF-7 tumours in a dose-dependent manner (). The anti-tumour effects of Exo-Dox-HS were more powerful than Exo-Dox. However, treatment with doxorubicin at this dose did not significantly inhibit the growth of MCF-7 tumours in mice. Moreover, both Exo and Exo-HS displayed a little anti-tumour effect. The anti-tumour effect of Exo-HS was stronger than that of Exo. The results of statistical analysis of the anti-tumour effect of different therapies are shown in . Together, these results indicate that doxorubicin in the exosomes possesses more potent anti-tumour effect than free doxorubicin does, and heat stress can further improve the anti-tumour effects of doxorubicin-containing exosomes from the same number of tumour cells in vivo.
Figure 7. Doxorubicin-containing exosomes inhibit the growth of MFC-7 cells in vivo. Individual BALB/c nude mice were inoculated subcutaneously with 1 × 107 MCF-7 cells and three days after inoculation, the mice were injected subcutaneously with Exo-Dox-HS, Exo-Dox (from 1 × 107 or 2 × 107 cells), Exo, Exo-HS (from 2 × 107 cells), 10 μg doxorubicin or PBS (100 µL) every 3 days for five times. The growth of implanted breast tumours was monitored. Data are expressed as mean ± SD of tumour volumes from each group (n = 5) from two separate experiments. Arrows indicate the time points for treatment.
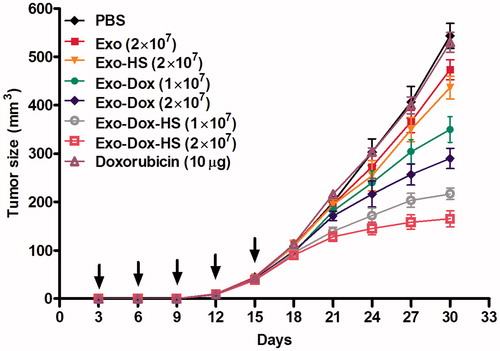
Table 2. Statistical analysis of each group to inhibit MCF-7 tumour growth in vivoa.
Discussion
Currently, the main strategy of nanoparticle drug delivery system is using synthetic nanoparticles to encapsulate therapeutic drugs and deliver them to tumour tissues. However, artificial nanoparticles have many shortcomings. For example, artificial nanoparticles may evoke unexpected immune responses, causing serious adverse effects [Citation3,Citation10]. In addition, artificial nanoparticles are sized between 20 and 50 nm, which may limit a large number of chemotherapeutic molecules entering into them. To address these issues, new carriers are urgently needed to deliver drugs [Citation3,Citation10]. In fact, as a carrier to deliver drugs, exosomes have many advantages. First, exosomes are of cell origin and relatively safe. Furthermore, exosomes with a diameter 40–100 nm can carry many chemotherapeutic drugs. Moreover, the procedure for preparing exosomes is relatively simpler and cost-effective.
Doxorubicin has potent anti-tumour activity but has low bioavailability. Therefore, encapsulating doxorubicin into exosomes may be a promising approach to deliver the drug effectively. In this study we prepared exosomes from the doxorubicin-treated tumour cells. We found that doxorubicin was present within the exosomes, an interesting phenomenon that the tumour cell-derived exosomes ‘express’ doxorubicin. Our data indicated that delivery of doxorubicin by exosomes was an effective approach to improve their anti-tumour effect. We found that doxorubicin in exosomes was a more effective anti-tumour than free doxorubicin. We can rationalise these results from two aspects. Doxorubicin in the exosomes may be a more stable and potent anti-tumour in activity than a free drug, similar to TGF-β1 in the exosomes [Citation18]. Secondly, doxorubicin-containing exosomes probably deliver doxorubicin to tumour tissues more efficiently because of their homology.
Our results revealed that exosomes from the doxorubicin-treated tumour cells contained doxorubicin. However, it is unclear how doxorubicin enters into the exosomal pathway. We speculate that cytoplasmic doxorubicin may be entrapped in exosomes when the limited membranes of the endosomes are invaginated to form multivesicular bodies. Subsequently, doxorubicin is expelled out of tumour cells with the release of exosomes when the multivesicular bodies fuse with the plasma membrane.
In this study we showed the potential application of exosomes as efficient carriers of doxorubicin. Furthermore, we found that heat stress significantly increased the production of exosomes from the doxorubicin-treated tumour cells. As a result, the greater production of exosomes from the same number of doxorubicin-treated tumour cells contained more doxorubicin. Therefore, heat stress enhanced the anti-tumour activities of exosomes from the same number of doxorubicin-treated tumour cells. Indeed, we found that Exo-Dox-HS had more potency to inhibit tumour cell proliferation and induce tumour cell apoptosis than Exo-Dox from the same number of doxorubicin-treated tumour cells. In the present study we also found that Exo-Dox-HS contained more Hsp70, which can induce the activation of natural killer cells [Citation19], and benefit the anti-tumour effect.
In summary, our data demonstrated that doxorubicin was present within the exosomes from doxorubicin-treated tumour cells, and indicated that heat stress increased the production of exosomes from tumour cells. Furthermore, the Exo-Dox-HS exosomes had more potent anti-tumour activity than Exo-Dox from the same number of doxorubicin-treated tumour cells. Accordingly, exosomes carrying various chemotherapy drugs, such as doxorubicin, from the heat-stress-treated cells may serve as an attractive strategy for tumour therapy. Thus, combination of chemotherapy and hyperthermia may improve the anti-tumour effect in patients by producing more chemotherapeutic agent-containing exosomes.
Declaration of interest
This study was supported by a grant from the Natural Science Foundation of Zhejiang Province (LY14H160011). The authors alone are responsible for the content and writing of the paper.
References
- Khiati S, Dalla Rosa I, Sourbier C, Ma X., Rao VA, Neckers LM, et al. Mitochondrial topoisomerase I (top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin Cancer Res 2014;20:4873–81
- Arora HC, Jensen MP, Yuan Y, Wu A, Vogt S, Paunesku T, et al. Nanocarriers enhance doxorubicin uptake in drug-resistant ovarian cancer cells. Cancer Res 2012;72:769–78
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun 2012;3:1282--92
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14:195–208
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 2010;18:1606–14
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 2011;19:1769–79
- Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, et al. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol 2007;133:389–99
- Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, et al. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med (Berl) 2006;84:1067–76
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res 2003;63:4331–7
- Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 2005;4:1595–604
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014;35:2383–90
- Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013;7:7698–710
- Zhong H, Yang Y, Ma S, Xiu F, Cai Z, Zhao H, et al. Induction of a tumour-specific CTL response by exosomes isolated from heat-treated malignant ascites of gastric cancer patients. Int J Hyperthermia 2011;27:604–11
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci 2005;118:3631–8
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 2011;186:2219–28
- Ma S, Yang Y, Wang C, Hui N, Gu L, Zhong H, et al. Endogenous human CaMKII inhibitory protein suppresses tumor growth by inducing cell cycle arrest and apoptosis through down-regulation of the phosphatidylinositide 3-kinase/Akt/HDM2 pathway. J Biol Chem 2009;284:24773–82
- Wang H, Lu C, Li Q, Xie J, Chen T, Tan Y, et al. The role of Kif4A in doxorubicin-induced apoptosis in breast cancer cells. Mol Cells 2014;37:812–18
- Cai Z, Zhang W, Yang F, Yu L, Yu Z, Pan J, et al. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res 2012;22:607–10
- Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012; 287:15874–85

