Abstract
Purpose: Polysaccharide peptide (PSP) extracted from the Coriolus versicolor mushroom is frequently suggested as an adjunct to the chemo- or radiotherapy in cancer patients. In a previous study we showed that PSP induced a tumour necrosis factor-α (TNF-α)-dependent anapyrexia-like response in rats. Thus, PSP appears to be a factor which modifies a number of pathophysiological responses. Because of this, PSP is suggested as a potential adjuvant for cancer therapy during which cancer patients frequently contract microbial infections accompanied by fever. The aim of the present study was to investigate whether or not PSP can modulate the course of the fever in response to an antigen such as lipopolysaccharide (LPS). Materials and methods: Body temperature (Tb) of male Wistar rats was measured by biotelemetry. PSP was injected intraperitoneally (i.p.) at a dose of 100 mg kg−1, 2 h before LPS administration (50 µg kg−1, i.p.). The levels of interleukin (IL)-6 and TNF-α in the plasma of rats were estimated 3 h and 14 h post-injection of PSP using a standard sandwich ELISA kit. Results: We report that i.p. pre-injection of PSP 2 h before LPS administration expanded the duration of endotoxin fever in rats. This phenomenon was accompanied by a significant elevation of the blood IL-6 level of rats both 3 h and 14 h post-injection of PSP. Pre-treatment i.p. of the rats with anti-IL-6 antibody (30 µg/rat) prevented the PSP-induced prolongation of endotoxin fever. Conclusions: Based on these data, we conclude that PSP modifies the LPS-induced fever in IL-6-related fashion.
Introduction
Polysaccharide peptide (PSP) isolated from Coriolus versicolor strain COV-1, has been widely used as adjunct therapy in cancer patients undergoing chemo- or radio-therapy [Citation1] and its non-toxic properties under acute and chronic conditions have been confirmed [Citation2]. Clinical trials have shown that PSP improved the quality of life of patients by decreasing cancer treatment-related symptoms such as fatigue, loss of appetite, nausea, vomiting, and pain [Citation3]. This mushroom-derived polysaccharide exerts its activities primarily via immunomodulation [Citation4]. Therefore, it can be classified as a biological response modifier, defined as an agent capable of modifying the host’s biological response by stimulating the immune system and thereby eliciting various therapeutic effects [Citation5]. The immunostimulatory effect of PSP (in vitro and in vivo) includes elevation of pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor α (TNF-α) as well as prostaglandin E2 (PGE2) and histamine [Citation3], increase in the production of reactive oxygen and nitrogen intermediates [Citation6], natural killer cells (NK) activity, activation of complement-3, T-cell proliferation [Citation7], and many other effects.
The above-mentioned cytokines and PGE2 secreted by PSP-stimulated cells are important components of the physiological mechanism of fever. This phenomenon is regarded as a part of the acute-phase response to infection, inflammation, injury and trauma [Citation8]. The increase of body temperature (Tb) during fever has several advantages over infections: inhibition of bacterial growth, increased bactericidal activities of neutrophils and macrophages, T cell proliferation and differentiation, B cell proliferation and the production of antibodies or stimulation of acute-phase protein synthesis [Citation9,Citation10]. The initial step in the cascade of events leading to fever is considered to be the stimulation of a large number of various types of immune cells, including monocytes, macrophages and neutrophils by exogenous stimuli, exogenous pyrogens [Citation11]. These stimuli are represented by bacteria wall components such as lipopolysaccharide (LPS), viral components such as double-stranded RNA, and bacterial DNA (CpG-DNA) [Citation12,Citation13]. Stimulation of the immune cells by the various exogenous pyrogens leads to the synthesis of the pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and interferon-γ (IFN-γ), collectively ascribed as endogenous pyrogens [Citation11,Citation14–16]. These cytokines trigger liberation of arachidonic acid from membrane phospholipids, activation of cyclooxygenase (COX), and subsequent production of prostanoids. It is thought that induction of the expression of COX-2 and generation of PGE2 play a critical role in affecting the thermoregulatory centres to start the fever [Citation17].
As we described previously, PSP provoked an anapyrexia-like response rather than fever in rats, and the response was TNF-α-dependent [Citation18]. Thus, PSP appears to be a factor which modifies a number of pathophysiological responses. Because PSP is suggested as a potential adjuvant for cancer therapy when cancer patients frequently contract microbial infections accompanied by fever, the aim of the present study was to investigate whether or not PSP can modulate the course of the fever. To the best of our knowledge, this phenomenon has not yet been studied. Moreover, our studies are aimed to explore the role of PSP as a modulator of endotoxin fever in response to an antigen such as LPS.
Materials and methods
Experimental animals and body temperature measurement
Eighty eight male Wistar rats weighing from 250–300 g were obtained from the Mossakowski Medical Research Centre, Polish Academy of Sciences in Warsaw, Poland. Animals were housed in individual plastic cages and maintained in a temperature/humidity/light-controlled chamber set at 23° ± 1 °C, 12:12 h light:dark cycle, with lights on at 07:00 a.m. Rodent laboratory food and drinking water were provided ad libitum. A week after the shipment, 48 rats (6 animals per group) under sterile conditions the rats were implanted with battery-operated miniature biotelemeters (PhysioTel® model TA10TA-F40, Dataquest A.R.T. System, New Brighton, MN) to monitor deep Tb with accuracy ±0.1 °C as described previously [Citation19]. The experiments described were started 10 days after surgery. All procedures were approved by the local Bioethical Committee for Animal Care in Bydgoszcz (Poland, permission no. 17/2013).
Polysaccharide peptide and lipopolysaccharide preparation and administration
PSP (extract from the Cov 1 strain of C. versicolor, MycoMedica, Czech Republic) was dissolved in sterile 0.9% sodium chloride (saline) and injected intraperitoneally (i.p.) at a dose of 100 mg kg−1. As we described previously, this was the dose of PSP which modulated normal Tb in male Wistar rats [Citation18]. In our studies we also tested a lower dose of PSP (50 mg kg−1), causing a smaller Tb decrease in rats. However, since the lower dose of PSP did not provoke any significant effect on the LPS-induced febrile response in rats (data not shown), the dose of 100 mg kg−1 of PSP was selected for further experiments.
LPS extracted from Escherichia coli (0111:B4, Sigma Chemicals, St Louis, MO) was dissolved in sterile 0.9% sodium chloride. Before injection the stock solution of LPS (2.5 mg mL−1) was diluted in a warm sterile saline to the desired concentration, and injected i.p. at a dose of 50 μg kg−1, as described previously [Citation19]. All injection solutions were warmed to 37 °C before administration. PSP was injected at 7:00 a.m., 2 h prior to the LPS administration (9:00 a.m.). The control rats were administered an equivalent volume of pyrogen-free saline i.p. The rats were briefly restrained and not anaesthetised during the injections. Immediately after the injections, the animals were placed in their home cages.
IL-6 and TNF-α assays
Blood samples were collected 40 rats (4 animals per group) via cardiac puncture onto a solution of ethylenediamine tetra-acetic acid disodium salt (Na2EDTA, Sigma-Aldrich, cat. no. E 5134) at 3 h (10:00) and 14 h (21:00) post-injection of PSP or pyrogen-free saline from rats anaesthetised with a mixture of ketamine/xylazine (87 mg kg−1 and 13 mg kg−1, respectively, intramuscular injection). After centrifugation (20 min, 1500 × g), the resulting plasma was stored at −20 °C until assay. Levels of IL-6 and TNF-α were determined by standard sandwich ELISA kits from R&D Systems (Minneapolis, MN, cat. no. R6000B and RTA00, with a detection limit of 21 pg mL−1 and 5 pg mL−1, respectively) according to the manufacturer’s instructions. Colorimetric changes in the assays were detected using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT).
Interleukin 6 antibody injection
Interleukin 6 (IL-6) antibody (rabbit polyclonal IgG antirat IL-6 (ARC0062, Invitrogen, Carlsbad, CA) was injected i.p. at a dose of 30 µg/rat in a volume of 500 µL of phosphate buffered saline (PBS, pH 7.4). This injection was performed 2 h (17:00) prior to the earlier observed significant difference in Tb between the examined group of rats (PSP/LPS) and the positive control (saline/LPS). Rabbit IgG (10500C, Invitrogen) at a dose of 30 µg/rat was used as control injection. Rats were restrained and not anaesthetised during i.p. injections.
Statistical analysis
All values are reported as means ± standard error mean (SEM) and were analysed by analysis of variance (ANOVA) followed by the Student’s t-test with the level of significance set at p < 0.05. For the Tb measures, the data were recorded and computed at 5-min intervals using a data acquisition program (Dataquest A.R.T. System). For data presentation these 5-min temperature recordings were pooled into 30-min averages. Statistical analyses were performed with GraphPad Prism 5 (La Jolla, CA).
Results
Pre-treatment with PSP expanded the duration of endotoxin fever in rats
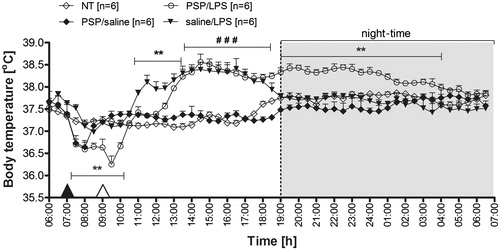
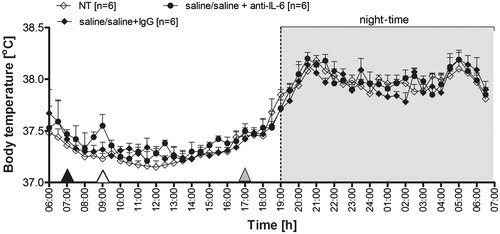
The effect of PSP on changes in Tb in male Wistar rats during endotoxin fever is illustrated in . The rats were injected i.p. with PSP at a dose of 100 mg kg−1 at 7:00 a.m., 2 h prior to the LPS administration. Pre-treatment of the animals with PSP resulted in significant alterations of the post-LPS Tb that can be regarded as a protraction of the time-course of fever response to the administration of endotoxin. As can be seen in , the rats treated with PSP followed by LPS responded with fever, which started 3.5 h post-injection of LPS (12:30 p.m.), whereas this phenomenon in the saline/LPS-injected animals was observed 1.5 h post-injection of LPS (10:30 a.m.). Moreover, as we described previously [Citation18], PSP administration caused a drop in Tb. However, the Tb of PSP/LPS-treated rats (38.2° ± 0.2 °C) was comparable to the Tb of saline/LPS-injected rats (38.3° ± 0.1 °C) measured from 13:30 to 18:00 p.m. (p = 0.25). On the other hand, the rats pre-treated with saline 2 h prior to LPS administration returned to Tb observed in the non-treated group of animals (NT) 12 h post-injection of PSP (19:00 p.m.), whereas this phenomenon was observed in the PSP/LPS-treated rats only after 21 h from injection (04:00). The average Tb of the rats from 19:00 to 04:00 for the PSP/LPS-treated animals was 38.3° ± 0.1 °C versus 37.8° ± 0.2 °C in the saline/LPS treated rats (p < 0.01). I.p. injection of sterile 0.9% sodium chloride (solvent for PSP) 2 h prior to the i.p. saline administration (solvent for LPS) did not induce alterations in Tb of rats (data not shown).
Figure 1. Changes of body temperature (°C) over time (h) of rats treated i.p. with PSP (100 mg kg−1) or 0.9% sterile saline at 7:00 a.m. (black arrowhead) and then injected i.p. with LPS (50 µg kg−1) or 0.9% sterile saline at 9:00 a.m. (white arrowhead) in comparison to non-treated animals (NT). Values are means ± SEM at 30-min averages. n, sample size in a respective group, *significant differences between PSP/LPS and saline/LPS groups; #significant differences between examined groups (PSP/LPS and saline/LPS) and control groups (NT and PSP/saline) at defined time intervals (**p < 0.01; ###p < 0.001, respectively).
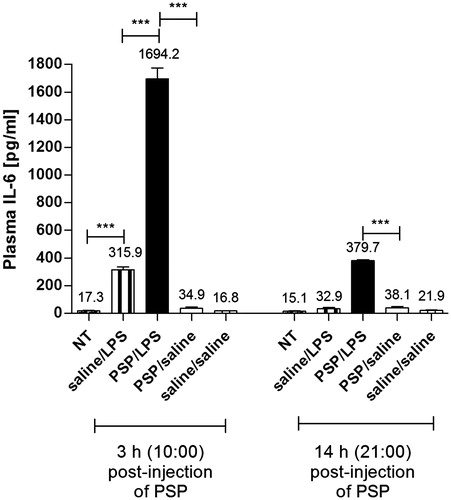
PSP increased the level of plasma IL-6 during endotoxin fever in rats
The blood was collected at 10:00 a.m. and 21:00 p.m. when the changes in Tb of rats in experimental group (PSP/LPS) reached its minimum and maximum values. The levels of plasma IL-6 were determined at 3 h (10:00) and at 14 h (21:00) post-injection of PSP or pyrogen-free saline in all groups of animals. Non-treated rats (NT), like the PSP/saline and saline/saline injected animals, did not show any significant elevation of IL-6 either at 3 h or at 14 h post-injection of PSP or saline (). Moreover, the concentrations of this cytokine in these three groups of rats were below the lowest standard of the ELISA kit, which was 62.5 pg mL−1 (respectively 17.3 ± 3 pg mL−1, 16.8 ± 2 pg mL−1 and 34.8 ± 1 pg mL−1 for the plasma concentration measured 3 h post-injection; 15.1 ± 3 pg mL−1, 21.9 ± 2 pg mL−1 and 38.1 ± 2 pg mL−1 for the level of IL-6 estimated 14 h post-injection). In contrast, the levels of IL-6 in the plasma of rats treated with PSP followed by LPS were significantly higher in comparison to the animal’s injected i.p. with pyrogen-free saline 2 h prior the LPS administration. This phenomenon was observed in both at 10:00 (1694.2 ± 80 pg mL−1 versus 315.9 ± 20 pg mL−1, p < 0.001) and at 21:00 (379.7 ± 7 pg mL−1 versus 32.9 ± 9 pg mL−1, p < 0.001).
Figure 2. Plasma levels of IL-6 (pg mL−1) estimated at 3 h and 14 h post-injection of PSP or saline in the rats injected i.p. with PSP (100 mg kg−1) or saline 2 h prior to the LPS administration (50 µg kg−1) in comparison to non-treated animals (NT) and rats pre-treated with PSP followed by sterile saline. Values are expressed as means ± SEM. Assays were performed on four individuals in each group. *significant difference (***p < 0.001).
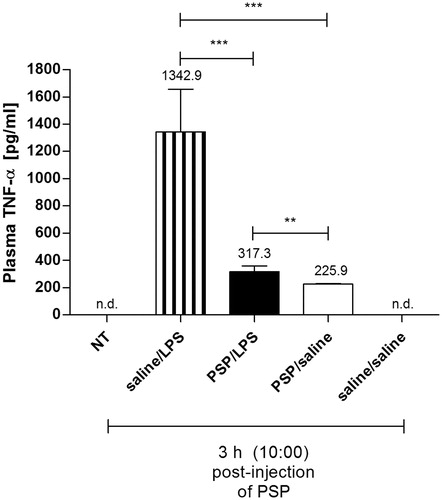
PSP decreased the level of plasma TNF-α during endotoxin fever in rats
The plasma levels of TNF-α as well as IL-6 were also determined at 3 h (10:00) and at 14 h (21:00) post-injection of PSP or pyrogen-free saline. As can be seen in , the concentration of this cytokine in the plasma of rats pre-treated with PSP followed by LPS (317.3 ± 40 pg mL−1) was significantly lower in comparison to the animals injected i.p. with pyrogen-free saline 2 h prior to the LPS injection (1342.9 ± 310 pg mL−1, p < 0.001). Moreover, the concentration of TNF-α measured in rats pre-treated with PSP and then injected with LPS (317.3 ± 40 pg mL−1) were significantly higher compared to PSP/saline-treated animals (225.9 ± 4 pg mL−1, p < 0.01). The plasma levels of this cytokine measured at 21:00 in all the groups of rats tested were below the minimum detectable dose of rat TNF-α in the ELISA kit used, which was 5 pg mL−1 (data not shown).
Figure 3. Plasma levels of TNF-α (pg mL−1) estimated at 3 h post-injection of PSP or saline in the rats injected i.p. with PSP (100 mg kg−1) or saline 2 h prior to the LPS administration (50 µg kg−1) in comparison to non-treated animals (NT) and rats pre-treated with PSP followed by sterile saline. Values are expressed as means ± SEM. Assays were performed on four individuals in each group. *significant difference (**p < 0.01 and ***p < 0.001, respectively).
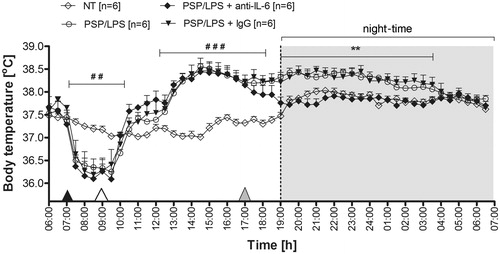
Anti-IL-6 antibody prevented the extension of endotoxin fever in rats
As can be seen in , injection of IL-6 antibody prevented the extension of endotoxin fever in rats pre-treated with LPS. The Tb of rats injected with PSP followed by LPS was similar to that observed at 17:00 in the PSP/LPS-treated rats injected with rabbit IgG (38.3° ± 0.1 °C versus 38.2° ± 0.1 °C, counting from 19:00 to 4:00, p = 0.39). On the other hand, the PSP/LPS-injected animals treated i.p. with IL-6 antibody responded with a decrease in Tb to the value observed in the non-treated rats (NT) at 12 h post-injection of PSP (19:00). The Tb in these two groups of animals (37.8° ± 0.1 °C and 37.8° ± 0.1 °C respectively, p = 0.35) was significantly lower compared to PSP/LPS-treated rats (38.3° ± 0.1 °C) and PSP/LPS-treated animals injected with rabbit IgG (38.2° ± 0.1 °C) counting from 12 h (19:00) to 21 h (4:00) post-injection of PSP (p < 0.01).
Figure 4. Changes of body temperature (°C) over time (h) of rats treated i.p. with PSP (100 mg kg−1) or 0.9% sterile saline at 7:00 a.m. (black arrowhead), then injected i.p. with LPS (50 µg kg−1) or 0.9% sterile saline at 9:00 a.m. (white arrowhead) and finally administered i.p. with rabbit polyclonal IgG anti rat IL-6 or rabbit IgG at 17:00 p.m. (30 µg/rat, grey arrowhead). Values are means ± SEM at 30-min averages. n, sample size in a respective group; *significant differences between PSP/LPS + IgG and PSP/LPS + anti-IL-6 groups; #significant differences between examined groups of rats and non-treated animals (NT) at defined time intervals (**p < 0.01; ##p < 0.01; ###p < 0.001, respectively).
To determine whether the dose of an anti-IL-6 antibody used in the experiments affects the course of Tb in rats, a separate group of animals was treated i.p. with sterile 0.9% saline at 7:00 and 9:00 (control vehicle for PSP and LPS). Afterwards the rats were injected i.p. with rabbit polyclonal IgG anti-rat IL-6 antibody at a dose of 30 µg/rat or with rabbit IgG (control injection at the same dose) at 10 h (17:00) after the first injection of sterile saline. As can be seen in , administration of IL-6 antibody did not affect Tb in rats. The average Tb of rats treated i.p. with IL-6 antibody, injected i.p. with rabbit IgG and non-treated (control) animals was similar (37.9° ± 0.1 °C), counting from 17:00 to 06:00.
Figure 5. Changes of body temperature (°C) over time (h) of rats injected i.p. with sterile 0.9% saline at 7:00 a.m. (control vehicle for PSP injection, black arrowhead) and at 9:00 a.m. (control vehicle for LPS administration, white arrowhead), and finally treated i.p. with rabbit polyclonal IgG anti-rat IL-6 or rabbit IgG at 17:00 (30 µg/rat, grey arrowhead) in comparison to non-treated animals (NT). Values are means ± SEM at 30-min averages. n, sample size in a respective group.
Discussion
In the present report we demonstrate for the first time the effect of polysaccharide peptide (PSP) on endotoxin fever in rats. Pre-treatment with PSP provoked significant alterations of the Tb in LPS-injected rats that can be regarded as a prolongation of fever response to the administration of endotoxin (). This effect was accompanied by a significant elevation of the LPS-induced blood IL-6 level at both 3 h and 14 h (). Plasma levels of TNF-α () and IL-6 suggest that PSP-induced extension of endotoxin fever in rats is related rather to IL-6 concentration than TNF-α. The extension of fever was prevented by an i.p. injection of anti-IL-6 antibody (). The dose of this antibody (30 µg/rat) used in the experiments affected neither normal Tb nor circadian rhythm of Tb (). In our studies we also examined the plasma concentration of IL-1β (one of the key cytokines that contributes to induction of fever). In all the groups of rats tested, this was, however, below the minimum detectable concentration of IL-1β in the ELISA kit used (less than 5 pg mL−1) both 3 h and 14 h post-injection of PSP (data not shown).
Immunostimulatory effects of PSP (in vitro and in vivo) include elevation of pro-inflammatory cytokines, such as IL-6 and TNF-α [Citation3]. Similarly, it is well known that stimulation of immune cells by exogenous stimuli such as LPS leads to synthesis of pro-inflammatory mediators, among which the most important are cytokines such as IL-6 and TNF-α [Citation11,Citation16,Citation20]. Experimental data strongly suggest the important role of IL-6 as an endogenous mediator in LPS-induced fever. The presence of IL-6 is critical for fever, as seen by the absence of the febrile response to peripheral immune challenge in IL-6 knockout mice as well as in animals treated with IL-6 antiserum [Citation20,Citation21]. In the present data we showed that the pre-treatment of the rats with PSP expanded the duration of LPS-induced fever, and the response was IL-6-related. Therefore, we suppose that PSP may intensify the production of IL-6 by the immune cells such as monocytes, macrophages and neutrophils. However, further in vitro studies are needed to investigate the reactivity of peripheral blood mononuclear cells (PBMCs) isolated from the rats pre-treated with PSP and then injected with LPS. This reactivity can be measured as the production of pro-inflammatory cytokines (IL-6, TNF-α) by PBMCs.
As we described previously, PSP derived from the mushroom C. versicolor induced a TNF-α-dependent drop of Tb in rats [Citation18]. In the present studies, the results of measurement of the plasma concentration of TNF-α showed that pre-injection of PSP prevented the LPS-induced elevation of plasma TNF-α. In contrast, the concentration of this cytokine in rats pre-treated with PSP and then injected with LPS was significantly higher compared to PSP/saline-treated animals (). Moreover, PSP demonstrates an additive effect on the synthesis of IL-6 during the LPS-induced fever (). Potential explanation of this phenomenon may be related to the Toll-like receptor 4 (TLR4) signal transduction pathway. It is well known that LPS constitutes a pathogen-associated molecular pattern (PAMP) recognised by TLR4 [Citation22–24]. In contrast, there are only a few reports showing that PSP acts via TLR4. Li et al. [Citation25] showed that PSP up-regulated expression of 22 genes, including five members of TLR family: LY64, TLR5, TLR6, TLR7 and finally TLR4 in PBMCs stimulated with PSP. Moreover, these authors also observed the increase in an expression of genes related to nuclear factor-κB (NF-κB) pathway – one of the most important transcription factors, which is necessary for the induction of the synthesis of pro-inflammatory cytokines, including IL-6 and TNF-α [Citation26]. It is well known that a common downstream pathway operates in the signal transduction via TLRs involving the myeloid differentiation factor 88 (MyD88)-dependent and MAPK-dependent up-regulation of the NF-κB [Citation27]. Similarly, Wang et al. [Citation28] demonstrated that PSP has an immunoregulatory effect through regulation of the TLR4-TIRAP/MAL-MyD88 signalling pathway in PBMCs from breast cancer patients. There are also reports indicating that the compounds derived from C. versicolor and having a similar structure as PSP are recognised by TLR4. Yang et al. [Citation29,Citation30] showed that C. versicolor mushroom polysaccharides (CVP) which, like PSP, exert a broad range of biological effects including anti-tumour and immunoregulatory activities, and can bind and induce B cell activation using membrane Ig (B cell antigen-receptor; BCR) and TLR4 as potential immune receptors. Consequently, CVP activates mouse B cells through the MAPK and NF-κB signalling pathway [Citation31]. Based on these results we presume that PSP may constitute the PAMP recognised by TLR4.
It has been accepted that the TLR4 signal transduction pathway could be divided into two sub-pathways including MyD88-dependent and TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent (MyD88-independent) according to the different adaptors. MyD88 adaptor-like protein (Mal) is an essential adapter protein together with the MyD88. Activated MyD88/Mal activates, i. a. (inter alia) transforming growth factor-β-activated protein kinase 1 (TAK1), which also activates members of the mitogen-activated protein kinases (MAPK) to activate an alternative closely related pathway that phosphorylates, e.g. p38 MAPK. The p38 MAPK is regarded as the essential regulator of pro-inflammatory molecules in the cellular responses that occur following induction of inflammatory gene transcription [Citation32,Citation33].
In addition to the above-mentioned signal transduction pathways, among the many inflammatory mediators induced by LPS which signals via TLR4, IL-6 trans-signalling via STAT3 is a critical modulator of LPS-driven pro-inflammatory responses through cross-talk regulation of the TLR4/Mal signalling pathway [Citation34]. IL-6 mediates its biological activities through a receptor complex composed of the specific signal-transducing receptor subunit gp130. After ligand binding, the gp130 recruits transcription factors of the STAT family (i.e. STAT3). Activated STATs translocate to the nucleus and bind to enhancer elements of target genes [Citation35].
The hyper-responsiveness of gp130F/F mice to LPS involved the specific up-regulation of IL-6 in a gp130/STAT3- and TLR4/Mal-dependent manner, suggesting both pathways synergise to promote the production of IL-6 in response to LPS. Moreover, there is the preferential up-regulation of IL-6 after LPS stimulation compared with TNF-α in an in vivo disease model (i.e. gp130F/F mice) [Citation36]. Although the mechanism of this phenomenon remains unclear, it is likely to reflect subtle differences in the transcriptional regulation of specific pro-inflammatory genes produced via TLR4 signalling cascades. For instance, activation of p38 MAPK is required for the LPS/TLR4-induced expression of TNF-α, but not IL-6 [Citation37,Citation38]. Moreover, in vitro studies have shown that blocking STAT3 activity preferentially inhibits LPS-mediated IL-6 production, but not TNF-α in RAW264.7 cells [Citation39], and STAT3 activation does not directly regulate LPS-induced TNF-α production in human monocytes [Citation40]. Based on these results, it can be concluded that the LPS/TLR4-induced production of TNF-α, but not IL-6, requires the activity of p38 MAPK. On the other hand, the signalling pathway via STAT3 is critical for increasing the expression of IL-6, but not TNF-α. In the present study we have shown that PSP alone (without LPS) induces TNF-α, but not IL-6 expression in rats. Therefore, we suppose that PSP may act via the TLR4/p38 MAPK signalling pathway. Our assumptions are consistent with the observations of Yang et al. [Citation31], who demonstrated that C. versicolor mushroom polysaccharides induced, in a time-dependent manner, the increase of phosphorylation of p38 MAPK.
Our results also demonstrated that PSP and LPS showed the additive effect on IL-6 expression, whereas the injection of PSP alone (without LPS) did not induce the secretion of IL-6 (plasma level measured 3 h post-injection of PSP). Based on these results we presume that PSP alone is not able to activate both TLR4-induced signal transduction pathways involving p38 MAPK and STAT3. On the other hand, the simultaneous activation of the TLR4 signalling pathway by LPS and PSP causes the additive effect on IL-6 production. This phenomenon may result from the fact that PSP as well as LPS induces the TLR4 signalling pathway, which leads to the activation of NF-κB [Citation25,Citation31,Citation32]. Moreover, both inducers may also activate the signalling pathway via STAT3. The other potential explanation of this phenomenon may result from the fact that in our experiment PSP was injected in rats 2 h prior to the administering of LPS. As we described previously, PSP induced a significant elevation of the blood TNF-α level 2 h post-injection [Citation18]. It can be assumed that raised concentration of TNF-α caused the increase of LPS-induced IL-6 production. Ghezzi et al. [Citation41] showed that the anti-TNF-α antibodies inhibited LPS-induced IL-6 production in three different models: IL-6 production by mouse peritoneal macrophages in vitro, serum IL-6 levels induced by an i.p. injection of LPS, and brain IL-6 concentration induced by an intra-cerebroventricular (i.c.v.) administration of LPS. Similarly, Benigni et al. [Citation42] demonstrated that i.c.v. injection of LPS into TNF receptor-deficient mice produces lower brain IL-6 levels than in wild-type mice. To the best of our knowledge this phenomenon has not yet been examined. Therefore detailed studies on the TLR4 signal transduction pathway involving p38 MAPK and STAT3 in PSP/LPS-treated rats are required.
PSP is considered as a useful adjuvant especially combined with chemotherapy in clinical treatment of cancer patients [Citation1,Citation2]. For this reason it is important to examine the effect of PSP in these patients who may experience fever during microbial infections. Moreover, there are clinical reports suggesting a decreased frequency of fever, or even the lack of capability of generating fever within certain groups of patients, especially amongst cancer patients [Citation43]. It is also well documented that fever directly activates defence against various dangers, including cancer cells [Citation44,Citation45] and the endogenous mediators of fever play a significant role in defence against tumour cells [Citation46,Citation47]. The observation that cancer patients who experienced a feverish period after surgery survived significantly longer than patients without fever, and the fact that spontaneous tumour remission was observed mostly after a fever confirms the significant meaning of this mechanism for a patient’s recovery [Citation48]. A large fraction of spontaneous regressions and remissions of tumours described in the literature was preceded by acute infections especially when accompanied by high fever [Citation49–51]. Based on recent observations in the clinic together with the improved understanding of tumour immunology, it is believed that fever, being a part of innate response, can induce and facilitate an efficient anti-tumour response, and may improve anti-tumour efficacy of immunotherapy [Citation52,Citation53]. However, the mechanism of this phenomenon has not yet been fully elucidated. It is well known that following fever, especially in relation to an acute infection, an increase in pro-inflammatory cytokine levels, stimulation of the differentiation of T cells, and enhancement of cytotoxic potential of neutrophils, NK cells and dendritic cells are observed [Citation9,Citation11]. In addition to the immunological effects of fever, there is also the thermal aspect. Tumour cells are more fragile and vulnerable to heat, with apoptosis taking place at lower temperatures compared to normal cells [Citation49,Citation54].
Although there is lack of research focused on the direct effect of fever on the various aspects of the immune system in cancer patients or/and tumour-bearing animals, the results of studies using a fever-range whole-body hyperthermia (FR-WBH) demonstrate a beneficial activity of temperature in the range of 39.5–40.5 °C, lasting for 4–6 h (physiological status similar to fever). Fever-range temperature is associated with enhancement of the innate and adaptive arms of the immune response through augmentation of T-cell proliferation and cytotoxicity, bioactivity of inflammatory cytokines and neutrophil motility and chemotaxis [Citation11,Citation55,Citation56]. It also promotes the egress of blood-borne lymphocytes across high endothelial venules (HEV) in lymph nodes and Peyer’s patches [Citation57]. Moreover, FR-WBH regulates adhesion molecule expression on select vascular endothelial sites. It increases the expression of intercellular adhesion molecules 1 (ICAM-1) and strongly increases the intravascular display of CCL21, a key homeostatic chemokine which mediates lymphocyte trafficking across high endothelial venules. FR-WBH also enhances L-selectin/α4β7 integrin affinity and/or avidity for endothelial adhesion molecules, ultimately leading to improved homing to lymphoid tissues [Citation58,Citation59]. The studies using tumour-bearing animals revealed that the FR-WBH resulted in significant lymphoid infiltrate and tumour cell apoptosis due to the activity of NK cells. Moreover, Burd et al. [Citation60] showed also that a single treatment of BALB/c mice bearing human breast tumour xenografts with a low-temperature, long-duration, and whole-body hyperthermia for 6–8 h caused a temporary reduction of tumour volume and/or a growth delay. This inhibition was correlated with the appearance of large numbers of apoptotic tumour cells. The authors also suggested that this type of mild heat exposure, comparable to a common fever, is not itself directly cytotoxic, but that it stimulates some component(s) of the immune response, which results in increased anti-tumour activity. In support of this hypothesis, Burd et al. observed an increase in numbers of lymphocyte-like cells, macrophages, and granulocytes in the tumour vasculature and in the tumour stroma immediately following this mild hyperthermia exposure [Citation60]. Similarly, Matsuda et al. [Citation61] demonstrated that the FR-WBH procedure applied alone using a rat tumour model, without any other additional therapy, delayed tumour growth together with a significantly (50%) reduced incidence of lymph node metastases [Citation61].
In addition, fever-range thermal stress can also activate processes involved in the killing of tumour cells. FR-WBH enhances antigen presentation by dendritic cells and promotes dendritic cell maturation, activates immune effector cells (making the tumour cells more sensitive to lysis by NK and lymphocyte CD8 + T cells), and switches the activities of the IL-6 to a predominantly anti-tumorigenic function that promotes anti-tumour immunity by mobilising T cell trafficking in the recalcitrant tumour microenvironment [Citation53,Citation62–66].
Based on these results it seems to be worthwhile to use the immunomodulatory properties of PSP as a factor stimulating the organisms of cancer patients to feverish response.
Conclusion
We conclude that PSP isolated from C. versicolor, which is a bioactive component exhibiting anti-tumour and immunomodulatory properties, expands the duration of LPS-induced fever, and the effect is IL-6-related. Moreover, our results also suggest the compensatory effect of PSP-induced hypothermia on LPS-induced fever during this early stage of the febrile response. Finally, it seems to be worthwhile to use the immunomodulatory properties of PSP as a factor stimulating the organisms of cancer patients to feverish response.
Declaration of interest
This study was supported by the Nicolaus Copernicus University Intramural Grants 1527-B and 1925-B to Tomasz Jedrzejewski (Torun, Poland). The authors alone are responsible for the content and writing of the paper.
References
- Zaidman BZ, Yassin M, Mahajna J, Wasser SP. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol 2005;67:453–68
- Cheng KF, Leung PC. General review of polysaccharopeptides (PSP) from C. versicolor: Pharmacological and clinical studies. Cancer Ther 2008;6:117–30
- Chan SL, Yeung JH. Polysaccharide peptides from COV-1 strain of Coriolus versicolor induce hyperalgesia via inflammatory mediator release in the mouse. Life Sci 2006;78:2463–70
- Dong Y, Kwan CY, Chen ZN, Yang MM. Antitumor effects of a refined polysaccharide peptide fraction isolated from Coriolus versicolor: in vitro and in vivo studies. Res Commun Mol Pathol Pharmacol 1996;92:140–8
- Ng TB. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae). Gen Pharmacol 1998;30:1–4
- Schepetkin IA, Quinn MT. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int Immunopharmacol 2006;6:317–33
- Sekhon BK, Sze DM, Chan WK, Fan K, Li GQ, Moore DE, et al. PSP activates monocytes in resting human peripheral blood mononuclear cells: Immunomodulatory implications for cancer treatment. Food Chem 2013;138:2201–9
- IUPS Thermal Commission. Glossary of terms for thermal physiology, 3rd ed. Jpn J Physiol 2001;51:245–80
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infect Dis Clin North Am 1996;10:1–21
- Roberts Jr NJ. The immunological consequences of fever. In: Mackowiak PA, editors Fever: Basic Mechanisms and Management. New York: Raven Press, 1991. p 125--42
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 1991;71:93–127
- DalNagore AR, Sharma S. Exogenous pyrogens. In: Mackowiak PA, editors Fever: Basic Mechanism and Management, 2nd ed. Philadelphia: Raven-Lippincott, 1997. pp 87–116
- Kozak W, Wrotek S, Kozak A. Pyrogenicity of CpG-DNA in mice: Role of interleukin-6, cyclooxygenases, and nuclear factor-κB. Am J Physiol Regul Integr Comp Physiol 2006;290:R871–80
- Kozak W, Kluger MJ, Tesfaigzi J, Wachulec M, Kozak A, Dokladny K. Molecular mechanisms of fever and endogenous antipyresis. Ann N Y Acad Sci 2000;917:121–34
- Kluger MJ, Leon LR, Kozak W, Soszynski D, Conn CA. Cytokine actions on fever. In: Rothwell NJ, editors Cytokines in the Nervous System. Austin, TX: Landes, 1996. pp 73–92
- Netea MG, Kullberg BJ, van der Meer JWM. Circulating cytokines as mediators of fever. Clin Infect Dis 2000;31:S178–84
- Blatteis CM, Li S, Li Z, Feledr C, Perlik V. Cytokines, PGE2 and endotoxic fever: A reassessment. Prostagl Lipid Mediat 2005;76:1–18
- Jedrzejewski T, Piotrowski J, Wrotek S, Kozak W. Polysaccharide peptide induces a tumor necrosis factor-α-dependent drop of body temperature in rats. J Therm Biol 2014;44:1–4
- Wrotek S, Jedrzejewski T, Potera-Kram E, Kozak W. Antipyretic activity of N-acetylcysteine. J Physiol Pharmacol 2011;62:669–75
- Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR, et al. IL-6 and IL-1 beta in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci 1998;856:33–47
- Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1β: A study on IL-6-deficient mice. J Exp Med 1996;183:311–16
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4(TLR4). J Exp Med 1999;189:615–25
- Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leuk Biol 1988;64:25–32
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–33
- Li W, Liu M, Lai S, Xu C, Lu F, Xia X, et al. Immunomodulatory effects of polysaccharopeptide (PSP) in human PBMC through regulation of TRAF6/TLR immunosignal-transduction pathways. Immunopharmacol Immunotoxicol 2010;32:576–84
- Barnes PJ, Karin M. Nuclear factor-κB, a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–71
- O’Neill LA, Bowie A. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat Rev Immunol 2007;7:353–64
- Wang J, Dong B, Tan Y, Yu S, Bao YX. A study on the immunomodulation of polysaccharopeptide through the TLR4-TIRAP/MAL-MyD88 signaling pathway in PBMCs from breast cancer patients. Immunopharmacol Immunotoxicol 2013;35:497–504
- Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): Implications of cancer immunotherapy. Anticancer Res 2002;22:1737–54
- Kim BC, Kim YS, Lee JW, Seo JH, Ji ES, Lee H, et al. Protective effect of Coriolus versicolor cultivated in citrus extract against nitric oxide-induced apoptosis in human neuroblastoma SK-N-MC cells. Exp Neurobiol 2011;20:100–9
- Yang SF, Zhuang TF, Si YM, Qi KY, Zhao J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol Immunol 2015;64:144–51
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science 2003;300:1524–5
- Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol 1999;162:5367–73
- Greenhill CJ, Gould J, Ernst M, Jarnicki A, Hertzog PJ, Mansell A, et al. LPS hypersensitivity of gp130 mutant mice is independent of elevated haemopoietic TLR4 signaling. Immunol Cell Biol 2012;90:559–63
- Bode JG, Schweigart J, Kehrmann J, Ehlting C, Schaper F, Heinrich PC, et al. TNF–alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol 2003;171:257–66
- Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol 2011;186:1199–208
- Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol 2006;176:3635–41
- Chen Y, Kam CS, Liu FQ, Liu Y, Lui VC, Lamb JR, et al. LPS-induced up-regulation of TGF-beta receptor 1 is associated with TNF-alpha expression in human monocyte-derived macrophages. J Leukoc Biol 2008;83:1165–73
- Samavati L, Rastogi R, Du W, Hüttemann M, Fite A, Franchi L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 2009;46:1867–77
- Prêle CM, Keith-Magee AL, Murcha M, Hart PH. Activated signal transducer and activator of transcription-3 (STAT3) is a poor regulator of tumour necrosis factor-alpha production by human monocytes. Clin Exp Immunol 2007;147:564–72
- Ghezzi P, Sacco S, Agnello D, Marullo A, Caselli G, Bertini R. LPS induces IL-6 in the brain and in serum largely through TNF production. Cytokine 2000;12:1205–10
- Benigni F, Faggioni R, Sironi M, Fantuzzi G, Vandenabeele P, Takahashi N, et al. TNF receptor p55 plays a major role in centrally mediated increases of serum IL-6 and corticosterone after intracerebroventricular injection of TNF. J Immunol 1996;157:5563–8
- Wrotek S, Kamecki K, Kwiatkowski S, Kozak W. Cancer patients report a history of fewer fevers during infections than healthy controls. J Pre-Clin Clin Res 2009;3:31–5
- O’Regan B, Hirshberg C. Spontaneous Remission: An Annotated Bibliography. Petaluma, CA: Institute of Noetic Sciences, 1993
- Maurer S, Koelmel K. Spontaneous regression of advanced malignant melanoma. Onkologie 1998;21:14–18
- Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 1992;10:52–60
- Yang JC, Topalian SL, Parkinson D, Schwartzentruber DJ, Weber JS, Ettinghausen SE, et al. Randomized comparison of high-dose and low-dose intravenous interleukin-2 for the therapy of metastatic renal cell carcinoma: An interim report. J Clin Oncol 1994;12:1572–6
- Baronzio GF, Hager DE. Hyperthermia in Cancer Treatment: A Primer. New York: Springer, 2006
- Hobohm U. Fever therapy revisited. Br J Cancer 2005;92:421–5
- Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother 2001;50:391–6
- Kleef R, Hager ED. Fever, Pyrogens and Cancer. Madame Curie Bioscience Database. Austin, TX: Landes Bioscience, 2000, pp. 276–337
- Køstner AH, Johansen RF, Schmidt H, Mølle I. Regression in cancer following fever and acute infection. Acta Oncol 2013;52:455–7
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9
- Hobohm U, Stanford JL, Grange J M. Pathogen-associated molecular pattern in cancer immunotherapy. Crit Rev Immunol 2008;28:95–107
- Roberts NJ Jr. Impact of temperature elevation on immunologic defenses. Rev Infect Dis 1991;13:462–72
- Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 2001;97:2727–33
- Fisher DT, Vardam TD, Muhitch JB, Evans SS. Fine-tuning immune surveillance by fever-range thermal stress. Immunol Res 2010;46:177–88
- Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs 2009;10:550–8
- Kalamida D, Karagounis IV, Mitrakas A, Kalamida S, Giatromanolaki A, Koukourakis MI. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PloS One 2015;10:1–12
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998;177:137–47
- Matsuda H, Strebel FR, Kaneko T, Danhauser LL, Jenkins GN, Toyota N, et al. Long duration-mild whole body hyperthermia of up to 12 hours in rats: Feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: Implications for adjuvant therapy. Int J Hyperthermia 1997;13:89–98
- Rowe RW, Strebel FR, Proett JM, Deng W, Chan D, He G, et al. Fever-range whole body thermotherapy combined with oxaliplatin: A curative regimen in a pre-clinical breast cancer model. Int J Hyperthermia 2010;26:565–76
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: A temperature’s story. Cancer Lett 2008;271:191–204
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother 2005;55:292–8
- Mikucki ME, Fisher DT, Ku AW, Appenheimer MM, Muhitch JB, Evans SS. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int J Hyperthermia 2013;29:464–73
- Dayanc BE, Bansal S, Gure AO, Gollnick S, Repasky EA. Enhanced sensitivity of colon tumor cells to natural killer cell cytotoxicity after mild thermal stress is regulated through HSF-1 mediated expression of MICA. Int J Hyperthermia 2013;29:480–90

