Abstract
Objectives: This study sought to assess the mid-term efficacy of magnetic resonance-guided high-intensity focused ultrasound (MRgFUS) (Sonalleve system) for uterine fibroids.
Methods: We retrospectively included patients treated by MRgFUS controlled by real-time MR-thermometry. Clinical efficacy was defined as the minimum reduction of ten points in the Transformed Symptom Severity Score (tSSS) without additional treatment. Fibroid volumes were assessed at 6 months, and patients were contacted to assess mid-term efficacy using tSSS.
Results: Thirty-six patients were included; 22 patients (61.1%) exhibited clinical efficacy with a mean follow-up duration of 21.4 (95%CI: 16.3–26.5) months. In addition, the tSSS mean decreased significantly from 42.8 ± 16 to 25.4 ± 18 (p < 0.0001). MRgFUS exhibited a preferential effect on menorrhagia (p = 0.001) and symptoms related to pelvic heaviness and swelling (p = 0.004). The volume reduction was 27% (p < 0.001) and was correlated with the non-perfused volume (NPV) after treatment (r = 0.373; p = 0.028). Cumulative re-intervention rates (surgery or uterine artery embolisation) at 12 months, 18 months and 24 months were 2.8%, 8.5% and 21.6%, respectively. No serious adverse events were reported. Two pregnancies occurred during the follow-up period.
Conclusions: Treatment of uterine fibroids by MRgFUS is efficient and results in low morbidity and satisfactory clinical efficacy with a mean follow-up of 21.4 months.
Introduction
Uterine fibroids are the most common type of benign female pelvic tumour. They are clinically apparent in up to 25% of women of childbearing age [Citation1] and are three times more common among women of African ethnicity. Disparity in this disease is evidenced by an earlier age of onset, greater severity of symptoms, and different responses to treatment [Citation2].
Fibroids are associated with clinically significant symptoms such as menorrhagia, urinary or intestinal discomfort, abdominal pressure and pelvic pain, leading to a decrease in overall quality of life [Citation3]. Treatment options for symptomatic uterine fibroids include conservative medication treatment (non-hormonal and hormonal treatments), surgery (myomectomy or hysterectomy) and less invasive methods such as uterine artery embolisation (UAE). The choice of treatment depends on the type, the location and the number of fibroid-related symptoms as well as on the patient’s wishes [Citation4].
Traditionally, hysterectomy was the gold standard therapy for uterine fibroids. However, patient demands for less invasive and more conservative alternative treatment have increased over the last decade, encouraging the development of less-invasive methods such as uterine artery embolisation. This technique has proven effective with comparable results to surgery but with faster recovery and better tolerance [Citation5,Citation6]. More recently, a novel non-invasive ablative technique termed magnetic resonance-guided high-intensity focused ultrasound (MRgFUS) was developed [Citation7–9].
This new technology uses thermal energy deposited by focused ultrasound, causing tissue necrosis and thermo-coagulation in the focal zone. MRgFUS has proven to be safe and effective and achieves a good success rate while preserving the surrounding tissue [Citation10–12]. Thus, MRgFUS represents an alternative treatment for fibroids, especially for patients with few symptomatic fibroids who wish to avoid surgery.
Currently, two MRgFUS devices are used, the ExAblate 2000 (InSightec, Haifa, Israel) and the Sonalleve MRgFUS fibroid therapy system (Philips Healthcare, Vantaa, Finland). The main difference between these two devices is the ablative process; the ExAblate 2000 performs point-by-point sonication, whereas the Sonalleve system performs volumetric heating. Mid- and long-term efficacy of the ExAblate 2000 device is well documented [Citation11,Citation13–16], while only studies reporting on short-term follow-up have been published using the Sonalleve system [Citation17–19].
The purpose of this study was to assess the mid-term therapeutic outcomes of uterine fibroid treatment using a Sonalleve device to perform MRgFUS.
Patients and methods
Patients
From October 2008 to November 2012 we retrospectively and consecutively included women referred to our centre and treated by MRgFUS. Institutional Review Board approval was obtained and written informed consent was waived by the Institutional Review Board.
Before treatment, patients underwent magnetic resonance imaging (MRI) and ultrasound examination to assess the technical feasibility of MRgFUS treatment. We considered MRgFUS treatment for the patient’s fibroids in the following cases: 1) Age ≥18 years old without menopause, 2) one or two fibroids larger than 3 cm and less than 15 cm, 3) a Transformed Symptom Severity Score (tSSS) of at least 10 points.
Exclusion criteria for considering MRgFUS were: 1) sub-mucosal fibroids, 2) type 3 fibroids based on Funaki et al. [Citation20], 3) more than 8 cm between the skin and the fibroid, 4) intra-fibroid calcification, 5) evidence of degeneration, 6) other pelvic disease (endometriosis, adenomyosis and inflammatory disease), 7) bowel interposition between the ultrasound beam and the fibroid, 8) large abdominal scar, and 9) more than five fibroids [Citation21].
We excluded patients with MRgFUS failure from the efficacy and outcome analyses. MRgFUS failures included both technical and heating failures. Technical failure was defined as the impossibility of placing a treatment cell in the fibroid due to the long distance between the ultrasound beam and the fibroid (>8 cm) (uterine retroversion the day of treatment) or bowel interposition between the ultrasound beam and the fibroid. Heating failure was defined as the repeated premature cessation of the heating process by the patient (due to pain) or as the inability to increase the temperature on the treatment cell due to the amount of power needed being greater than the device was able to deliver.
MRgFUS system
Treatments were performed on a clinical MRgFUS platform (Sonalleve Philips Vantaa, Finland) integrated into a 1.5-Tesla clinical MRI (Achieva 1.5 T, Philips Healthcare, Best, Netherlands), as described in a previous publication [Citation18].
Using the volumetric ablation technique of the Sonalleve system, the high-intensity focused ultrasound (HIFU) focus was electronically steered along a trajectory composed of several outward-moving concentric circles. This approach resulted in enhanced energy efficiency because the heat already deposited during the sonication of the inner part of the trajectory was used to preheat the outer parts of the trajectory instead of being allowed to dissipate out of the target area [Citation7,Citation22–24].
Volumetric ablations were performed using ellipsoid treatment cells 4, 8, 10 and 12 mm in axial diameter. These ablations included feedback of the temperature and of the deposited thermal dose, which regulated the time of the ablation according to the measured temperature and resulting thermal dose in real time, and stopped the heating system when the thermal dose was achieved according to the ‘treatment cell’ sizes [Citation25,Citation26].
Treatment procedure
After the introduction of an intravenous line and a Foley catheter in the bladder of the patient, the patient was positioned in the prone position on the MRI table, with the probe placed over the fibroid. A 20-mm-thick gel pad (Parker laboratories, Fairfield, NJ) was positioned on the window of the transducer to ensure the absence of gas interposed between the ultrasound beam and the depilated skin. All patients received a non-opioid analgesic infusion (infusion solution 100 mg/100 mL paracetamol over 20 min), a non-steroidal oral analgesic (ketoprofen, 400 mg) and an antispasmodic (phloroglucinol, 80 mg) before and during the treatment for pain prophylaxis. The radiologist used a software program to plan treatment and position the target cells. The radiologist chose the size, location and number of treatment cells used to perform the ablathermy.
Real-time temperature mapping and measurement of the thermal dose helped the radiologist monitor heating of the targeted fibroid tissue. Utilising real-time temperature mapping, the radiologist could control the temperature elevation of the surrounding tissue and stop the heating if the temperature became too high in the non-targeted tissues. At the end of the treatment, a contrast MRI agent (gadoteric acid, DOTAREM®, Guerbet, Aulnay-Sous-Bois, France, 0.1 mmol/kg) was injected to evaluate the non-perfused volume (NPV).
Data collection
Baseline
Clinical data collected the day of treatment included: age, symptoms assessed by the tSSS before treatment, and adverse events after treatment. The tSSS is based on the first 8 items of the disease-specific Uterine Fibroid Symptom and Health-related Quality Of Life (UFS-QOL) questionnaire [Citation21]. The tSSS utilises the following formula: tSSS = 100 × (SSS − 8)/32. MRI data collected at baseline included fibroid volume, T2-weighted signal [Citation27], and the NPV ratio at the end of the treatment (NPV%) (NPV / volume of the fibroid targeted by the treatment). Serious adverse events were defined according to the Society of Interventional Radiology guidelines [Citation28]. They were evaluated immediately and at 3 and 6 months after the treatment.
Follow-up
Imaging follow-up consisted of an MRI examination 6 months after treatment to calculate the degree of change in fibroid volume. The volumes of the treated fibroids were computed using the ellipsoid method from the measurement of the three largest orthogonal diameters.
A phone interview using a standard questionnaire recording tSSS, pregnancy and any additional treatments including the type and the date performed at 6 months follow-up and at the end of 2013. If a patient was lost to follow-up, we used the data recorded at 6 months follow-up.
Outcome definitions
Clinical efficacy was defined as a decrease of at least 10 points on the tSSS without additional treatment (surgery, uterine artery embolisation or MRgFUS) during the follow-up period [Citation12,Citation17,Citation29]. If patients underwent additional treatments for recurrence of the initial symptomatology it was considered a treatment failure at the date of additional treatment.
Statistical analysis
Data are expressed as means (95% confidence intervals (CIs). Quantitative data were compared using either the two-sample t-test, Mann-Whitney test, or rank test, according to the data distribution. Correlations were assessed using Pearson or Spearman coefficients. p < 0.05 was considered indicative of a significant difference. Statistical analyses were performed with SPSS software (version 20, SPSS, Chicago, IL).
Results
Patients
A total of 45 patients met the inclusion criteria for MRgFUS treatment. Eight patients were excluded from the analysis of treatment outcomes: 1) four due to bowel interposition or uterine retroversion on the day of treatment; 2) three due to heating failure (premature heating cessation, n = 2; and inability to increase temperature, n = 1); and 3) one was lost to follow-up after treatment. The baseline characteristics of the 36 patients included are summarised in . The mean follow-up time was 21.4 (95%CI: 16.3–26.5) months with a range of 6–59 months. Four patients were lost to follow-up after six months without phone call. As illustrated in , 44.4% of patients had a follow-up duration between 12 and 23 months, and 41.7% had a follow-up duration longer than 23 months. One (2.8%) patient had a total of two fibroids, six patients (16.7%) had a total of three fibroids, 13 patients (36.1%) had a total of four fibroids, and three patients (8.3%) had a total of five fibroids. As only fibroids greater than 3 cm were treated, a single patient had two fibroids treated while the remaining 35 patients had one fibroid per patient treated.
Figure 1. Timescale for patient follow-up. In cases in which a surgical intervention or embolisation was performed, the follow-up period represents the time from the MRgFUS treatment to the surgical/embolisation procedure.
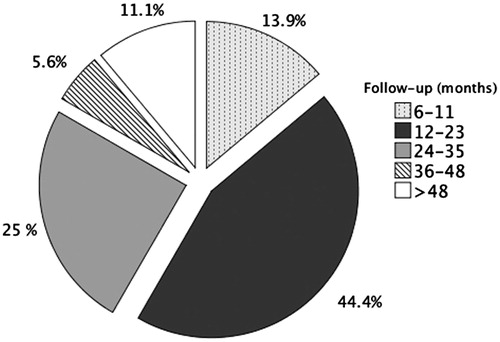
Table 1. Patient characteristics (n = 36).
Clinical efficacy
Clinical efficacy was demonstrated in 22/36 patients (61.1%) at a mean follow-up of 21.4 (95%CI: 16.3–26.5) months. In addition, the tSSS mean decreased significantly from 42.8 (95%CI: 37–48) to 25.4 (95%CI: 19–31.8) in treated patients (p < 0.0001). MRgFUS also showed a positive effect on menorrhagia (p = 0.001) and symptoms related to pelvic heaviness and swelling (p = 0.004) (). The correlation between fibroid volume decrease and clinical efficacy (p = 0.692) or tSSS decrease (%) after treatment (p = 0.235) was not significant. The cumulative treatment failure rates at 12 and 24 months were 26.9% and 45.9%, respectively.
Table 2. Details of the Transformed Symptom Severity scores (8 items). All data are presented as medians (interquartile ranges in brackets).
The clinical efficacy rates in patients with a follow-up period > 12 months and ≥ 18 months were, respectively, 61.3% (19/31) and 70.6% (12/17). For patients with follow-up >18 months, the five-treatments failure was due to re-intervention; the other 19 women have not reached the 18 months follow-up yet (n = 14), have already had re-intervention (n = 1), or were lost to follow-up (n = 4). Among the 14 treatment failures, six patients (16.7%) had undergone additional treatment (recorded as treatment failure) at a mean time of 20.2 (95%CI: 12.4–28.2) months after MRgFUS. Cumulative re-intervention rates at 12 months, 18 months and 24 months were 2.8%, 8.5% and 21.6% respectively. The additional treatments included two hysterectomies, one uterine artery embolisation and three myomectomies. The uterine arterial embolisation was performed six months after treatment because of insufficient symptomatology resolution (under 10 points on the tSSS) and fibroid volume increase despite treatment. The eight other patients with treatment failures did not undergo additional treatments; these patients chose medical treatment or elected to wait for menopause (four were older than 45). No difference was noted with respect to the baseline characteristics between patients undergoing additional treatment relative to those not undergoing additional interventions (all characteristics p > 0.307).
Volume shrinkage and NPV
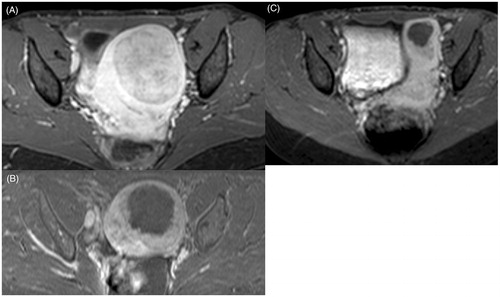
The mean fibroid volume decreased by 27% (95%CI: 19–35), from a mean fibroid volume of 255 cc (95%CI: 190–319) to 207 cc in treated patients (95%CI: 141–273) (p < 0.0001), at six months. The mean NPV after treatment was 27% (95%CI: 21–33). shows an example of fibroid volume reduction compared to post-treatment imaging. In our study, fibroid volume reduction was significantly correlated with NPV (r = 0.373; p = 0.028).
Pregnancies and treatment complications
Two patients reported natural pregnancies that led to live births by vaginal delivery. One occurred during the second year post-treatment, and the other occurred 3 years after treatment. Both refused invasive or minimally invasive treatment such as myomectomy and uterine artery embolisation and chose MRgFUS treatment.
No serious adverse events were reported during the treatment or follow-up. However, three minor events (grade B of the Society of Interventional Radiology classification) were recorded [Citation28]: one patient had a superficial skin burn (first degree) that was treated conservatively and resolved within a week; a second patient experienced pyelonephritis and was home-treated with oral antibiotics (ciprofloxacin) for 10 days; and the third patient suffered an infection of the necrotic treated fibroid several days after treatment, which was treated by oral antibiotic therapy for 14 days (ofloxacin 800 mg/day and metronidazole 1 g/day). All of these patients recovered completely with appropriate therapy.
Discussion
Herein, we present the results of our study assessing the clinical outcomes of volumetric MRgFUS ablation of uterine fibroids with a Sonalleve device with a mean follow-up duration of 21.4 months. This study reviews the longest clinical follow-up to date of patients treated with the Sonalleve device.
We observed clinical efficacy (reduction in the tSSS of at least 10 points without additional treatment) in 61.1% of cases (after a mean follow-up of 21.4 months) with a median fibroid volume decrease of 27% at six-months. However, we were unable to identify predictive factors of successful mid-term outcomes.
It is difficult to compare clinical efficacy with previous studies, as definitions of clinical efficacy are disparate across studies. We considered the treatment effective if the tSSS decreased at least 10 points without additional treatment (embolisation, MRgFUS or surgery) [Citation12,Citation29]. Our treatment efficacy rate was higher than that reported by Ikink et al. [Citation17] using the same device (Sonalleve) (54% at 6 months) and the same definition of treatment efficacy. Stewart et al. (35) also reported on reduction in symptom severity with 51% of patients in their study achieving at least 10 points in improvement at 12 months using the ExAblate device with a mean decrease of 22.3 points. Froeling et al. noted a 16-point improvement at month 61, although these authors evaluated patients without defining efficacy as a 10-point decrease in the tSSS [Citation30]. We observed a 17-point mean improvement in the tSSS at 21.4 months of follow-up. Using the ExAblate 2000 device, Machtinger et al. [Citation15] reported that 24% of patients required additional treatment for their fibroids at a mean of 33 months following treatment. Gorny et al. [Citation13] using the same ExAblate device reported 19% of patients at 36 months and 23% of patients at 48 months required additional treatments in their study. This compares to our re-intervention rate of 16.7% at 21.4 months and a cumulative re-intervention rates of 21.6% at 24 months. Other studies reported additional treatment incidences ranging from 8.8% to 66.7% beyond 12 months follow-up [Citation13,Citation17,Citation30–32]. The transient effectiveness of HIFU could be explained by its action on fibroid angiogenic factors [Citation17,Citation33,Citation34]. Beyond the area of induced necrosis, the effect of ultrasound could extend to cells in the periphery, leading to blockade of cellular interactions and alterations in angiogenic factors in the residual peripheral fibroid tissue. This effect would likely result in an early outcome, particularly with respect to bleeding symptoms, which could be secondarily followed by a progressive recovery of fibroids with increasing size and revascularisation.
One interesting point is that MRgFUS seemed to exhibit a positive effect on menorrhagia and bulk-related symptoms in our cohort, with a significant decrease in several items, including ‘heavy bleeding during menstrual period’ (p = 0.001); ‘feeling pelvic tightness or pressure’ (p = 0.004); ‘urination frequency during daytime’ (p = 0.005); and ‘frequent night-time urination’ (p = 0.029).
In our study, the mean fibroid shrinkage was approximately 27% (p < 0.0001). This result is similar to those reported by most previous studies, which ranged from 14% to 36% [Citation4,Citation35–37]. Moreover, the NPV was (weakly) correlated with the decrease in fibroid volume (r = 0.373; p = 0.028). Our NPV ratio (27%) was smaller than those reported in most previous studies (approximately 40%) [Citation17,Citation30,Citation38–40]. Occasionally, the fibroid periphery is difficult to treat with MRgFUS due to poor accessibility. Indeed, because we used the first Sonalleve system, and the large ultrasound beam could not be moved to reach the periphery of the fibroid, and we could not shut down a part of the ultrasound beam in cases of partial bowel interposition. All of these characteristics have been addressed in the latest MRgFUS system and should offer a larger NPV ratio. Furthermore, another explanation for our small NPV ratio could be the learning curve for this technique, as explained by Okada et al. [Citation32]. It should also be recognised that the most recent studies of MRgFUS in the treatment of fibroids reported higher NPV values (approximately 60–80%) [Citation41–43]. Most authors have focused on the baseline NPV; some have demonstrated that for a greater baseline NPV, fibroid shrinkage may be superior. These previous authors suggested that an NPV of 60% should be the aim of MRgFUS treatment [Citation4,Citation44]. Leblang et al. [Citation29] reported that for an NPV greater than 75%, the fibroid reduction may approach 51%. NPV was not associated with clinical outcomes in our cohort. Furthermore, baseline NPV does not seem to be the best parameter to predict clinical outcomes, as most studies have not observed any correlation between baseline NPV and clinical efficacy. Similar to other studies, we did not observe a correlation between a decrease in fibroid size and clinical efficacy. Park et al. reported significantly greater fibroid shrinkage for an NPV >80%, although this shrinkage was associated only with a trend towards clinical improvement (not statistically significant) [Citation42].
Other studies have reported larger baseline NPVs in fibroids with low T2-weighted signals. In our study, we did not observe this correlation because we deliberately excluded fibroids with high-intensity signals on T2-weighted images [Citation27,Citation45].
The main side effects of MRgFUS reported in the literature include moderate skin burns, nausea, back or lower limb pain, transient sciatica or reversible transient paresis and sciatic nerve injury [Citation12,Citation46]. No serious adverse events were observed during or following the procedure in our study. Moreover, following the treatment of minor events, as noted earlier, no long-term sequelae were observed.
Certain limitations of our study should be discussed. First, this was a single-centre retrospective case series study with no comparison group. Moreover, only a small patient population was included, and we did not examine symptom evolution at 3, 6 and 12 months, which limited the analysis of the distribution of relapses over time. Furthermore, our NPV ratio was lower than that expected using the latest MRgFUS devices.
In summary, this study demonstrates that MRgFUS using the Sonalleve system exhibits low morbidity with a clinical efficacy of 61% at a mean of 21.4 months, which is similar to the results reported using the ExAblate 2000 device. The cumulative re-intervention rate at 24 months was 21.6%.
Declaration of interest
The authors of this manuscript are permanent employees of Bordeaux University Hospital. This study has received funding from Philips Healthcare Netherlands in the form of a free MRgFUS treatment platform and treatment material. Institutional Review Board approval was obtained and written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, were all performed at one institution. The authors alone are responsible for the content and writing of the paper.
References
- Stewart EA. Uterine fibroids. Lancet 2001;357(9252):293–98
- Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med 2013;31:370–9
- Chavez NF, Stewart EA. Medical treatment of uterine fibroids. Clin Obstet Gynecol 2001;44:372–84
- Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids – early experience. Eur J Obstet Gynecol Reprod Biol 2008;139:199–203
- Hehenkamp WJK, Volkers NA, Birnie E, Reekers JA, Ankum WM. Symptomatic uterine fibroids: Treatment with uterine artery embolization or hysterectomy–results from the randomized clinical Embolisation versus Hysterectomy (EMMY) Trial. Radiology 2008;246:823–32
- Pinto I, Chimeno P, Romo A, Paúl L, Haya J, de la Cal MA, et al. Uterine fibroids: Uterine artery embolization versus abdominal hysterectomy for treatment – a prospective, randomized, and controlled clinical trial. Radiology 2003;226:425–31
- Kim Y, Trillaud H, Rhim H, Lim HK, Mali W, Voogt M, et al. MR thermometry analysis of sonication accuracy and safety margin of volumetric MR imaging-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Radiology 2012;265:627–37
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2015;31:302–9
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9
- Stewart EA, Gedroyc WMW, Tempany CMC, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 2003;189:48–54
- Kim HS, Baik J-H, Pham LD, Jacobs MA. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: Long-term outcomes. Acad Radiol 2011;18:970–6
- Fennessy FM, Tempany CM, McDannold NJ, So MJ, Hesley G, Gostout B, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – results of different treatment protocols. Radiology 2007;243:885–93
- Gorny KR, Borah BJ, Brown DL, Woodrum DA, Stewart EA, Hesley GK. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: Review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol 2014;25:1506–12
- Gorny KR, Woodrum DA, Brown DL, Henrichsen TL, Weaver AL, Amrami KK, et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: Review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol JVIR 2011;22:857–64
- Machtinger R, Inbar Y, Cohen-Eylon S, Admon D, Alagem-Mizrachi A, Rabinovici J. MR-guided focus ultrasound (MRgFUS) for symptomatic uterine fibroids: Predictors of treatment success. Hum Reprod Oxf Engl 2012;27:3425–31
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584–9
- Ikink ME, Voogt MJ, Verkooijen HM, Lohle PNM, Schweitzer KJ, Franx A, et al. Mid-term clinical efficacy of a volumetric magnetic resonance-guided high-intensity focused ultrasound technique for treatment of symptomatic uterine fibroids. Eur Radiol 2013;23:3054–61
- Voogt MJ, Trillaud H, Kim YS, Mali WPTM, Barkhausen J, Bartels LW, et al. Volumetric feedback ablation of uterine fibroids using magnetic resonance-guided high intensity focused ultrasound therapy. Eur Radiol 2012;22:411–17
- Kim Y-S, Kim J-H, Rhim H, Lim HK, Keserci B, Bae D-S, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: Initial clinical outcomes. Radiology 2012;263:600–9
- Funaki K, Fukunishi H, Funaki T, Kawakami C. Mid-term outcome of magnetic resonance-guided focused ultrasound surgery for uterine myomas: From six to twelve months after volume reduction. J Minim Invasive Gynecol 2007;14:616–21
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300
- Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009;36:3521–35
- Hijnen NM, Heijman E, Köhler MO, Ylihautala M, Ehnholm GJ, Simonetti AW, et al. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. Int J Hyperthermia 2012;28:141–55
- Venkatesan AM, Partanen A, Pulanic TK, Dreher MR, Fischer J, Zurawin RK, et al. Magnetic resonance imaging-guided volumetric ablation of symptomatic leiomyomata: Correlation of imaging with histology. J Vasc Interv Radiol 2012;23:786–94 e4
- Mougenot C, Quesson B, de Senneville BD, de Oliveira PL, Sprinkhuizen S, Palussière J, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU). Magn Reson Med 2009;61:603–14
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584–9
- Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199–202
- LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: Report of 80 patients. Am J Roentgenol 2010;194:274–80
- Froeling V, Meckelburg K, Schreiter NF, Scheurig-Muenkler C, Kamp J, Maurer MH, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: Long-term results. Eur J Radiol 2013;82:2265–9
- Ruhnke H, Eckey T, Bohlmann MK, Beldoch MP, Neumann A, Agic A, et al. MR-guided HIFU treatment of symptomatic uterine fibroids using novel feedback-regulated volumetric ablation: Effectiveness and clinical practice. RöFo Fortschritte Auf Dem Geb Röntgenstrahlen Nukl 2013;184:983–91
- Okada A, Morita Y, Fukunishi H, Takeichi K, Murakami T. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: Impact of the learning curve on patient outcome. Ultrasound Obstet Gynecol 2009;34:579–83
- Stewart EA, Nowak RA. Leiomyoma-related bleeding: A classic hypothesis updated for the molecular era. Hum Reprod Update 1996;2:295–306
- Pagan J, Przybyla B, Jamshidi-Parsian A, Gupta K, Griffin RJ. Blood outgrowth endothelial cells increase tumor growth rates and modify tumor physiology: Relevance for therapeutic targeting. Cancers 2013;5:205–17
- Stewart EA, Rabinovici J, Tempany CMC, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006;85:22–9
- Marret H, Bleuzen A, Guérin A, Lauvin-Gaillard M-A, Herbreteau D, Patat F, et al. French first results using magnetic resonance-guided focused ultrasound for myoma treatment. Gynécologie Obstétrique Fertil 2011;39:12–20
- Lénárd ZM, McDannold NJ, Fennessy FM, Stewart EA, Jolesz FA, Hynynen K, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – imaging predictors of success. Radiology 2008;249:187–94
- Gorny KR, Borah BJ, Weaver AL, Brown D, Woodrum DA, Stewart EA, et al. Clinical predictors of successful magnetic resonance-guided focused ultrasound (MRgFUS) for uterine leiomyoma. J Ther Ultrasound 2013;1:15
- Froeling V, Meckelburg K, Scheurig-Muenkler C, Schreiter NF, Kamp J, Maurer MH, et al. Midterm results after uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Cardiovasc Intervent Radiol 2013;36:1508–13
- Yoon S-W, Cha SH, Ji YG, Kim HC, Lee MH, Cho JH. Magnetic resonance imaging-guided focused ultrasound surgery for symptomatic uterine fibroids: Estimation of treatment efficacy using thermal dose calculations. Eur J Obstet Gynecol Reprod Biol 2013;169:304–8
- Mindjuk I, Trumm CG, Herzog P, Stahl R, Matzko M. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: Results from a single centre. Eur Radiol 2015;25:1317–28.
- Park MJ, Kim Y, Rhim H, Lim HK. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol 2014;25:231–9
- Park MJ, Kim Y-S, Keserci B, Rhim H, Lim HK. Volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids: Treatment speed and factors influencing speed. Eur Radiol 2013;23:943–50
- Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: Early clinical experience. Radiat Med 2008;26:198–205
- Lénárd ZM, McDannold NJ, Fennessy FM, Stewart EA, Jolesz FA, Hynynen K, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – imaging predictors of success. Radiology 2008;249:187–94
- Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CMC. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 2007;110:279–87