Abstract
There is now definitive clinical evidence that hyperthermia can successfully improve the response of certain human tumour types to radiation therapy, but, there is still the need for improvement. From a biological standpoint this can be achieved by either targeting the cellular or vascular components of tumours. Strategies include targeting the radiation DNA repair processes, improving drug delivery using nanoparticles, exploiting immunotherapy mechanisms, reducing tumour pH, or modifying the tumour vascular supply. All of these approaches have been combined with either hyperthermia or radiation in preclinical models and clear benefits in tumour response observed. But few of these methods have actually been combined with thermoradiotherapy. Furthermore, very few combinations have been tested in relevant normal tissue studies, despite the fact that it is the normal tissue response that controls the maximal heat or radiation treatment that can be applied. Here we review the most clinically relevant biological approaches that have been shown to enhance thermoradiotherapy, or have the potential to be applied in this context, and suggest how these should be moved forward into the clinic.
Introduction
The general consensus of opinion is that heat has no real potential as a curative treatment unless high thermal ablation temperatures are achieved [Citation1,Citation2]. However, lower temperatures in the hyperthermia range (40–45 °C) can play a significant role in improving tumour response to more conventional therapies such as chemo- and radiotherapy. Indeed, there is now good clinical evidence that when hyperthermia and radiation are combined significant improvements in both local tumour control and even overall survival are possible [Citation2–5]. Despite these positive results there is still the need for improvement both in those tumour sites where hyperthermia has already been shown to be beneficial and more importantly in those sites where as yet no benefit has been reported. While technological advances will clearly result in a better application of hyperthermia and radiation, improvements in the interaction between these two modalities should also be possible using biologically based approaches.
These biological approaches can be divided into two types: those that target the cellular components of tumours and those that target tumour vasculature. The growth and development of virtually all solid tumours requires the formation of a functional vascular supply to meet the oxygen and nutrient demands of the growing tumour cell population [Citation6,Citation7]. This tumour neo-vasculature is formed from the host vascular supply by the process of angiogenesis [Citation8,Citation9], but the system that develops is primitive and chaotic when compared to the normal host vessels [Citation10,Citation11]. As a result, diffusion gradients are established resulting in tumour areas that are oxygen deficient, nutrient deprived, and highly acidic [Citation10,Citation11], as shown in . Cells can survive under these adverse conditions and are a major source of resistance to radiation [Citation12,Citation13]. However, such cells are also sensitive to cell killing by heat [Citation14,Citation15] and targeting these cells with hyperthermia is one mechanism by which heat can improve the radiation response. Transient fluctuations in tumour blood flow also occur and these can result in cells being temporarily starved of oxygen and nutrients (). Such cells are radiation resistant, but their transient nature means they are not heat sensitive [Citation12,Citation13]. However, heat could also be used to improve the radiation sensitivity of these resistant cells as well as those that are in the non-deprived areas and thus radiation sensitive; by combining with hyperthermia these cells could be killed using lower radiation doses. The tumour vascular supply not only controls the microenvironment within tumours, thereby influencing cellular response to therapy, it also affects the ability to heat tumours. The relationship between blood flow and heating has been examined, and generally the lower the blood flow rate the easier it is to heat tissues [Citation16,Citation17]. Thus, by targeting the vascular supply one can both modify the tumour microenvironment and improve the ability to heat the tumour, thereby having a therapeutic benefit.
Figure 1. Schematic illustration of the interrelationship between tumour cells and their vascular supply. On the left, a functional tumour vessel is shown from which the tumour cells obtain their oxygen and nutrient supply. But, due to diffusion gradients from that vessel, areas develop that contain viable cells that are oxygen deficient, nutrient depleted and highly acidic. A similar arrangement is shown on the right, but here blood flow is transiently stopped and this results in cells being temporarily starved of oxygen and nutrients. Modified from Horsman et al. [Citation12].
![Figure 1. Schematic illustration of the interrelationship between tumour cells and their vascular supply. On the left, a functional tumour vessel is shown from which the tumour cells obtain their oxygen and nutrient supply. But, due to diffusion gradients from that vessel, areas develop that contain viable cells that are oxygen deficient, nutrient depleted and highly acidic. A similar arrangement is shown on the right, but here blood flow is transiently stopped and this results in cells being temporarily starved of oxygen and nutrients. Modified from Horsman et al. [Citation12].](/cms/asset/43cc64ab-4a01-44dc-b320-ff6d699d2f6e/ihyt_a_1099169_f0001_c.jpg)
These various cellular, microenvironmental and vascular factors are potential targets for improving the combination of radiation and hyperthermia. Numerous preclinical studies during the last 50 years or so have demonstrated the potential of a range of different approaches to improve tumour response to hyperthermia or radiation by targeting these parameters. Unfortunately, very few studies have investigated the potential of such approaches to enhance a combined radiation and heat treatment, and as a result none have made it into routine clinical use. The aim of this current review is to summarise the more clinically relevant biological approaches that been shown to enhance thermoradiotherapy, or have the potential to be applied in this context, and suggest how these should be moved forward into the clinic.
Improving the response to thermoradiotherapy
Cell-based approaches
The majority of attempts to influence tumour response to hyperthermia have focused on targeting the cellular component of tumours. Such approaches are listed in . Tumour cells distant from blood vessels often experience oxygen and nutrient deficiencies due to gradients that arise following consumption of these products by cells closer to blood vessels [Citation10,Citation11]. Cells under low oxygenation (hypoxic) conditions are resistant to radiation and there have been numerous attempts to eliminate this resistant population [Citation12,Citation13]. Drug-based approaches have involved using agents that are generally inactive under normoxic conditions, but in the presence of hypoxia they are converted to products that can radiosensitise or kill the hypoxic cells [Citation13]. These drugs are generally nitroaromatic compounds, and while most studies have shown that such agents can improve the response to radiation therapy there are a few studies with specific drugs (e.g. misonidazole, tirapazamine, RSU 1069) showing they can also improve the response to heating [Citation18–20], although the mechanism for that effect is not known. An increase in tumour response has also been reported when these drugs, radiation and hyperthermia have been combined [Citation20,Citation21]. However, to date the only bioreductive drug in clinical evaluation is the nitroaromatic compound TH-302 [Citation22], but the preclinical [Citation23] and clinical [Citation22] studies have focused on its combination with chemotherapy rather than radiation, and certainly not hyperthermia. If this drug shows clinical benefit our previous knowledge would suggest it to be a potential agent for testing with thermoradiotherapy.
Table 1. Cell-based approaches with the potential to improve thermoradiotherapy. Agents that have been combined with hyperthermia, radiation or thermoradiation to improve anti-tumour activity are indicated.
While hypoxia increases resistance to radiation, the acidic conditions arising from the nutrient deficiency make the same cells sensitive to killing by heat [Citation14,Citation15], and this complementary effect can in part explain the benefit of combining heat and radiation. Attempts have been made to find agents that can further lower tumour pH to increase the anti-tumour effect of heat. This has been achieved using drugs like quercetin [Citation24,Citation25] and lonidamine [Citation26,Citation27], and by administering glucose [Citation28,Citation29]; the pH reduction can be enhanced by combining meta-iodobenzylguanidine (MIBG) with glucose [Citation30,Citation31], although this latter approach has not been combined with hyperthermia. Such a drop in acidity would also likely increase the heat sensitivity of those cells that before treatment were not acidic, and this would include cells that were not radiobiologically hypoxic as well as those that were acutely hypoxic. Additional studies with lonidamine [Citation32] and MIBG + glucose [Citation33] have also shown these treatments to be capable of improving tumour oxygenation status through effects on decreasing oxygen consumption, thereby increasing the oxygen diffusion distance, and this would explain the ability of these agents to improve radiation response [Citation32–35]. Such results add strong support to the use of these agents in combination with thermoradiotherapy, especially since several of these agents have undergone clinical evaluation, so it is surprising that such studies have not been reported.
Following radiation treatment a certain degree of induced cellular damage is repaired using a variety of processes [Citation36]. It is now well documented that heat has the potential to inhibit DNA repair [Citation37–39]. Moreover, this is likely to occur in any cell regardless of its physiological characteristics, although there may be differences in the level of repair between normoxic, chronically hypoxic, and acutely hypoxic cells. The degree of repair is dependent on the degree of damage induced, so one would expect less repair in hypoxic cells. However, since chronically hypoxic cells are also nutrient-deprived they may have a lower capacity for repair than acutely hypoxic cells that are not nutrient-deprived. The normoxic cell population will likely show the greatest initial damage following irradiation, and the largest repair capacity. While these cells are not radiation resistant, by using heat to inhibit the repair that occurs one may be able to induce the same degree of cell killing using a lower dose of irradiation, and this would certainly be beneficial; applying a low radiation dose would be expected to reduce normal tissue complications. Numerous preclinical studies have demonstrated the potential of applying small molecule-based approaches to inhibit repair and so improve radiation sensitivity, especially using inhibitors of poly (ADP) ribose polymerase (PARP), and a number of these agents are in clinical evaluation [Citation40,Citation41]. Preliminary data now suggests that hyperthermia can actually sensitise cells to PARP inhibitors [Citation42], thus it would seem logical to actually add such inhibitors to a hyperthermia/radiation combination both in preclinical studies to demonstrate the potential of this approach, and hopefully clinically when that has been shown.
Nano technology approaches are also being used in the field of hyperthermia. Magnetic nanoparticles have been developed for actually heating tumours and these have undergone preclinical and clinical evaluation [Citation43,Citation44]. An alternative approach involves using nanoparticle-based drug delivery systems [Citation45,Citation46]. Here, drugs are either physically encapsulated within, or conjugated to, carriers which are then administered to the host in an effort to improve tumour exposure and reduce systemic toxicity. This approach has also undergone clinical evaluation [Citation47,Citation48]. By using a thermosensitive drug delivery system and locally heating tumours one can induce drug accumulation and release within the tumour [Citation49,Citation50]. Such thermosensitive drug delivery systems, especially using thermosensitive liposomes, have undergone extensive preclinical [Citation49,Citation51] and even clinical [Citation52,Citation53] evaluation. Several preclinical studies have also demonstrated that doxorubicin encapsulated into liposomes can be combined with radiation to improve anti-tumour response [Citation54,Citation55] and again this concept has been moved into patients [Citation56,Citation57]. The wealth of preclinical data showing the benefits of combining nano-based drug delivery systems with either heat or radiation clearly support the suggestion of using this approach with hyperthermia and radiation, and it is somewhat surprising that this has not already been undertaken. Despite this lack of preclinical data, a multicentre phase II clinical study of radiotherapy, hyperthermia, and thermosensitive liposomal doxorubicin has already been initiated in patients with loco-regional recurrent breast cancer (Gabriele et al., unpublished observations).
It has been mentioned that hyperthermia and radiation both have direct effects on tumour cells. However, it is now becoming apparent that heat [Citation58–60] and radiation [Citation61,Citation62] can also have indirect, non-targeted effects that are mediated through the immune system. This is probably best illustrated by the abscopal effect where tumour regression is observed at non-treated, distant sites, an effect that has been seen following local treatment with both heat [Citation63,Citation64] and radiation [Citation65,Citation66]. These findings clearly suggest that an immune response would be likely when hyperthermia and radiation are combined, and preclinical studies investigating this aspect are warranted. There is now considerable interest in the application of various immunomodulating agents, especially checkpoint inhibitors (e.g. CLA-4, anti-PD1, and anti-PDL1) and it has been suggested that these inhibitors should be combined with radiation [Citation67,Citation68]. It is interesting to speculate as to the likely benefits of combining these immunomodulators with hyperthermia alone, or even hyperthermia with radiation.
A number of different factors have been implicated as being involved in the activation of an immune response following heat treatment [Citation60]. Principal among these are the heat shock proteins (HSPs), which are molecular chaperones that are up-regulated in response to cellular stress, especially heat [Citation60,Citation69]. The proteins chaperoned by HSPs are those that function as components of cancer cell pathways necessary for controlling tumour growth and resistance to chemo- and radio-therapy [Citation70]. As such, HSPs represent unique molecular targets, the inhibition of which should improve conventional cancer therapy. One of the principal HSPs targeted in this way is Hsp90, and numerous inhibitors exist [Citation71], many of which are in clinical development [Citation72]. Such inhibitors have been shown to sensitise tumours to chemotherapy [Citation73,Citation74], radiation [Citation70,Citation75], and even chemoradiotherapy [Citation76,Citation77]. Since HSP up-regulation is involved in a heat-induced immune response one might expect Hsp90 inhibitors to have negative effect on heat response. However, this is not the case, with Hsp90 inhibitors being reported to increase cellular sensitivity to both heat alone [Citation78,Citation79] and even thermoradiation [Citation79]. Hsp90 inhibitors may also play an important role in combination with other cell-based approaches already described for enhancing heat response. Apoptosis and cell death can be significantly increased if the geldanamycin analogues 17AAG and 17DMAG are combined with quercetin and heat [Citation73]. There is also evidence that Hsp90 inhibitors, like hyperthermia, enhance radiation damage by interfering with DNA damage repair mechanisms [Citation80–82] and that this can be enhanced using the PARP inhibitor olaparib [Citation83], which further supports our earlier suggestion of combining PARP inhibitors with thermoradiation.
Vascular-based approaches
The tumour vascular supply is one of the principal factors controlling the microenvironmental conditions within tumours, and as such the vascular supply indirectly impacts the response to therapy. It also influences the ability to heat a tissue; in a well perfused region the heat applied is rapidly washed out, thus a poorly perfused region becomes easier to heat. Targeting tumour blood flow is, therefore, a potential method for improving thermal radiotherapy either by changing the tumour microenvironment to make the cells more sensitive to treatment or by making it easier to heat. lists the various vascular-based methods that could be, or have been, used in this context.
Table 2. Vascular-based approaches with the potential to improve thermoradiotherapy. Agents that have been combined with hyperthermia, radiation or thermoradiation to improve anti-tumour activity are indicated.
Many of the physiological modifiers listed in actually decrease tumour blood flow, although for some of these agents (e.g. angiotensin II, noradrenaline, and certain anaesthetics) both an increase and decrease have been reported [Citation84]. A decrease in blood flow would certainly not be beneficial for radiation treatment, but could be expected to enhance the effect of hyperthermia. Indeed, such enhancements have been demonstrated with clamping, hydralazine and its analogue cadralazine, sodium nitroprusside, and glucose [Citation84,Citation85]. These improved responses to heating are believed to result from both a better tumour heating and a decrease in tumour pH [Citation84,Citation86]. Decreasing tumour blood flow could potentially be used to improve the response to thermoradiotherapy if the blood flow modifier was administered after irradiating but before heating. However, this has only been demonstrated with hydralazine [Citation84]. Since the effect of hydralazine is both difficult to predict and control [Citation87] the clinical applicability of such a treatment remains questionable. Certain bioreductive drugs and chemical radiosensitisers can also transiently modify tumour blood flow [Citation84] and will enhance the effect of hyperthermia, radiation or thermoradiation [Citation13,Citation18–20]. However, these enhancements are believed to primarily be cell-based effects rather than due to blood flow modifications.
Specifically targeting the tumour vascular supply as a therapeutic approach using inhibitors of angiogenesis or agents that specifically disrupt tumour vasculature has received considerable attention in the last few years, and many agents have now undergone clinical evaluation [Citation88,Citation89]. An extensive list of the angiogenesis inhibitors (AIs) is given in . The effect of these AIs on tumour blood flow is somewhat controversial. It has been proposed that AIs induce vascular normalisation [Citation90] and as such would be expected to increase tumour blood perfusion and thus decrease the level of adverse microenvironmental conditions within tumours. A number of preclinical studies have, in fact, demonstrated this effect [Citation91,Citation92]. However, the window of activity for this improved effect is generally short, and rather more studies have shown that the opposite effect (e.g. a decrease in tumour perfusion and associated increase in tumour hypoxia and pH) can occur [Citation91,Citation92]. Not knowing what effect is happening will make the timing of giving AIs with heat or radiation problematic. Nevertheless, there are studies showing that AIs can be used to improve the response to radiation [Citation91]. An increase in anti-tumour response has also been reported for the combination of AIs and hyperthermia [Citation93–96], but this is not always the case [Citation97]; in that latter study it was suggested that the lack of enhancement of the heat response was because the AI used (batimastat) was itself ineffective [Citation97]. While the combination of AIs with radiation has been taken forward into the clinic [Citation88] the same cannot be said for combining AIs with hyperthermia. Nor are there any studies, preclinical or clinical, that have combined AIs with heat and radiation.
Vascular disrupting agents (VDAs) are more consistent in their effects on tumour perfusion. As a result of the vascular damage, tumour perfusion is decreased [Citation98–101] with a subsequent increase in tumour hypoxia [Citation102] and decrease in tumour pH [Citation103]. These changes make VDAs perfect agents for improving tumour response to hyperthermia, and a number of preclinical studies have demonstrated just such a benefit [Citation98,Citation100,Citation104–109]. VDAs have also been extensively combined with radiation [Citation91,Citation110,Citation111]. In these studies the VDAs were always given after irradiating; administering VDAs prior to irradiation generally decreases radiation response as one would predict from the induction of hypoxia. This is not always the case. The new combretastatin analogue OXi4503 also undergoes oxidative activation to a quinone intermediate and that gives this VDA direct cell killing properties in addition to its ability to induce vascular damage [Citation112]. Thus, it probably kills any cells that are made hypoxic due to the vascular shut-down and as a result is equally effective at enhancing radiation response whether given before or after irradiating [Citation102]. Unlike most of the other treatments we have so far described, VDAs have actually also been combined with thermoradiation, although this has only been shown for a limited number of agents. These include flavonoid compounds like flavone acetic acid [Citation113] and DMXAA [Citation114], tubulin binding agents such as combretastatin [Citation115] and its derivative OXi4503 [Citation116], and anti-cancer drugs like arsenic trioxide [Citation117]. The results show a clear improvement in response with the enhancement obtained using a temperature of 41.5 °C being actually greater than that observed with 43 °C in the absence of a VDA [Citation2]. Furthermore, this enhanced tumour response is not seen in normal tissues [Citation2,Citation118], thus resulting in a clear therapeutic benefit. Although VDAs are currently under evaluation in a number of clinical trials [Citation89], only one study involves their combination with radiation and none focus on the potential application with thermoradiotherapy.
Importance of normal tissue studies
Patients receiving conventional chemotherapy or radiotherapy are generally treated to tolerance. In other words, the maximum dose delivered is dependent on the response of the normal tissues in terms of morbidity. As such, any increase in efficacy of the conventional therapy in tumours that occurs due to the application of an additional treatment must be compared to potential enhanced effects in normal tissues; a similar increase in normal tissue damage as seen in tumours will not result in any therapeutic advantage. Despite the significance of this aspect in the clinic, it is often an overlooked issue in preclinical studies. This was illustrated in a review that summarised all preclinical studies in which radiation was combined with vascular targeting agents [Citation91]. A total of 58 studies involving 25 drugs reported significant enhancements in the radiation response of tumours, but only five of these preclinical studies, using three agents, included normal tissue data; four studies investigated effects on early responding normal skin reactions, while one study involved late responding tissues.
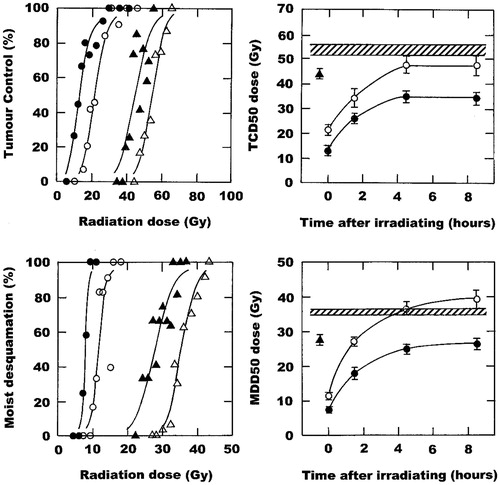
The importance of doing similar studies with biological modifiers of thermoradiation is illustrated in . In this example the well-established relationship between the enhancement of radiation damage by hyperthermia and the timing of the combination is shown. Simultaneously heating and irradiating produces the greatest enhancement of radiation response, but the effect is the same in tumours and normal tissues so no therapeutic benefit is obtained. These enhancements are reduced with the introduction of an interval between the radiation and heat, but while the normal tissue response returns to the level seen with radiation alone with a 4-h interval there is still a small yet persistent effect in tumours, thus resulting in a therapeutic benefit. Nicotinamide is an agent that prevents transient fluctuations in tumour blood flow that lead to acute hypoxia [Citation119,Citation120]. As such it has been shown to not only improve the response to radiation [Citation121,Citation122], but also to enhance the effect of agents that specifically target chronic hypoxia [Citation122,Citation123]. That has led to clinical testing in combination with carbogen (95% O2 + 5% CO2) breathing to combat hypoxia resistance in the BCON (Bladder, CarbOgen, and Nicotinamide) [Citation124] and ARCON (Accelerated Radiation, CarbOgen, and Nicotinamide) [Citation125] trials. The ability of nicotinamide to enhance tumour response to radiation alone and when radiation and heat are combined is illustrated in ; the latter effect appears to be independent of the time interval between irradiation and heating. But, that figure also shows the effects that nicotinamide has on radiation response, with or without heating, in normal skin. Here, nicotinamide has an almost identical effect as seen in tumours, so no matter what combination schedule is applied there would be little or no therapeutic benefit. Clearly, such data strongly supports the suggestion that any biological modifier of thermoradiotherapy in tumours must be tested in appropriate normal tissues.
Figure 2. The effect of a single intraperitoneal injection of nicotinamide (1000 mg/kg) and local water bath heating (42.5 °C; 60 min) on the radiation response of foot-implanted C3H mammary carcinomas and normal foot skin. Left panels: Radiation dose response curves for tumour control (top) or moist desquamation in skin (bottom) when treated with radiation alone (Δ), nicotinamide 30 min before irradiating (▴), radiation administered in the middle of heating (○), or the combination of nicotinamide, radiation and heat (•). Points are for an average of 11 mice with the lines drawn following logit analysis. Right panels: TCD50 (top) or MDD50 (bottom) doses (radiation dose producing 50% tumour control or moist desquamation, respectively) as a function of time after irradiating; data are from the curves shown in the left panels, and for other similar dose response curves. Results show radiation alone (![]()
Conclusions
Clearly, there are numerous different approaches that have been shown to be capable of improving tumour response to either hyperthermia or radiation. Few of these approaches have actually been combined with heat and radiation, and since the most likely clinical application of heat is with more conventional therapies such as radiation this deficiency is hard to understand. Many of these approaches are, or have been, in clinical development; thus it would seem as if it would be easy to transfer any preclinical findings, combining them with radiation and heat, into the clinic. Exactly which approaches have the best clinical potential is not clear, but what is needed to advance feasible strategies is good data demonstrating anti-tumour effects without increasing normal tissue complications/morbidity.
Furthermore, heterogeneity within tumours and between patients is likely to influence response to thermoradiation. Non-invasive functional imaging using positron emission tomography, computed tomography, or magnetic resonance imaging [Citation12,Citation126] of both the microenvironmental and vascular components of tumours will help in elucidating tumour heterogeneity and thus help in selecting the appropriate modifier for combination with hyperthermia. There is also evidence that the expression of specific genes or gene profiles can be used to identify critical processes associated with various cellular and vascular responses of tumours to heat and radiation [Citation127–129] and thus further aid in our ability to predict response and individualise the treatment plan.
Acknowledgements
The author would like to thank Dorthe Grand, Pia Schjerbeck, and Inger Marie Horsman for excellent technical assistance, and Marianne Verner Bjerre and Marianne Kristiansen for animal care. This study was made possible by financial support from the Danish Cancer Society, the Danish Council for Independent Research: Medical Sciences, and the Danish Hyperthermia Fund.
Declaration of interest
The author reports no conflict of interest and was entirely responsible for the content and writing of this paper.
References
- Overgaard J. Rationale and problems in the design of clinical trials. In: Overgaard J, editor. Hyperthermic Oncology, Vol. 2. London: Taylor and Francis; 1985. pp 325–38
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Franckena M. Review of radiotherapy and hyperthermia in primary cervical cancer. Int J Hyperthermia 2012;28:543–8
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev 2015. PMID: 26051911
- Brem S, Brem H, Folkman J, Finkelstein D, Patz A. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res 1976;36:2807–12
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29(Suppl16):15–18
- Hahnfeldt P, Panigrahi D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: A dynamic theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res 1999;59:4770–5
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003;3:401–10
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic micro-environment of human tumors: A review. Cancer Res 1989;49:6449–65
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Sem Radiat Oncol 2004;14:198–206
- Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012;9:674–87
- Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther 2015;153:107–24
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiol 1977;123:511–14
- Gerweck LE, Nygaard TG, Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res 1979;39:966–72
- Jain RK, Grantham FH, Gullino PM. Blood flow and heat transfer in Walker 256 mammary carcinoma. J Natl Cancer Inst 1979;62:927–33
- Patterson J, Strang R. The role of blood flow in hyperthermia. Int J Radiat Oncol Biol Phys 1979;5:235–41
- Bleehen NM, Honess DJ, Morgan JE. Interaction of hyperthermia and the hypoxic cell sensitizer Ro-07-0582 on the EMT6 mouse tumour. Br J Cancer 1977;35:299–306
- Horsman MR, Overgaard J, Chaplin DJ. The interaction between RSU-1069, hydralazine and hyperthermia in a C3H mammary carcinoma as assessed by tumour growth delay. Acta Oncol 1988;27:861–62
- Mao HS, Grau C, Overgaard J. Interaction of misonidazole, hyperthermia, and irradiation in a C3H mammary carcinoma and its surrounding skin in vivo. Int J Radiat Oncol Biol Phys 1992;22:115–22
- Masunaga S-I, Liu Y, Sakurai Y, Tanaka H, Suzuki M, Kondo N, et al. Usefulness of combined treatment with continuous administration of tirapazamine and mild temperature hyperthermia in γ-ray irradiation in terms of local tumour response and lung metastatic potential. Int J Hyperthermia 2012;28:636–44
- Borad MJ, Reddy SG, Bahary N, Uronis HE Sigal D, Cohn AL, et al. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2015;33:1475–81
- Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, et al. TH-30, a hypoxic-activated prodrug with broad in vivo preclinical combination therapy efficacy: Optimization of dosing regimens and schedules. Cancer Chemother Pharmacol 2012;69:1487–98
- Kim JH, Kim SH, Alfieri AA, Young CW. Quercetin, an inhibitor of lactate transport and a hyperthermic sensitizer of HeLa cells. Cancer Res 1984;44:102–6
- Asea A, Ara G, Teicher BA, Stevenson MA, Calderwood SK. Effects of the flavonoid drug Quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int J Hyperthermia 2001;17:347–56
- Kim JH, Kim SH, Alfieri A, Young CW, Silvestrini B. Lonidamine: A hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology 1984;41(Suppl):30–5
- Coss RA, Storck CW, Wells TC, Kulp KA, Wahl M, Leeper DB. Thermal sensitization by lonidamine of human melanoma cells grown at low extracellular pH. Int J Hyperthermia 2014;30:75–8
- Hiraoka M, Hahn GM. Changes in pH and blood flow induced by glucose, and their effects on hyperthermia with or without BCNU in RIF-1 tumours. Int J Hyperthermia 1990;6:97–103
- Urano M. Tumor response to hyperthermia. In: Urano M, Douple EB, editors. Hyperthermia and Oncology, Vol. 1. Utrecht: VSP; 1988. pp 161–200
- Burd R, Lavorgna SN, Daskalakis C, Wachsberger PR, Wahl ML, Biaglow JE, et al. Tumor oxygenation and acidification are increased in melanoma xenografts after exposure to hyperglycemia and meta-iodo-benzylguanidine. Radiat Res 2003;159:328–35
- Kalliomaki T, Hill RP. Effects of tumour acidification with glucose + MIBG on the spontaneous metastatic potential of two murine cell lines. Br J Cancer 2004;90:1842–9
- Stryker JA, Gerweck LE. Lonidamine-induced, pH dependent inhibition of cellular oxygen utilization. Radiat Res 1988;113:356–61
- Lee I, Glickson JD, Dewhirst MW, Leeper DB, Burd R, Poptani H, et al. Effect of mild hyperglycemia ± meta-iodo-benzylguanidine on the radiation response of R3230 Ac tumors. Adv Exp Med Biol 2003;530:177–86
- Kim JH, Alfieri AA, Kim SH, Young CW. Potentiation of radiation effects on two murine tumors by lonidamine. Cancer Res 1988;46:1120–3
- Kim JH, Kim SH, He SQ, Alfieri AA, Young CW. Potentiation of radiation effects on multicellular tumor spheroids (MTS) of HeLa cells by lonidamine. Int J Radiat Oncol Biol Phys 1989;16:1277–80
- Wouters BG, Begg AC. Irradiation-induced damage and the DNA damage response. In: van der Kogel AJ, Joiner M, editors. Basic Clinical Radiobiology for Radiation Oncologists, 4th ed. London: Hodder Arnold; 2009. pp 233–45
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int J Radiat Biol 2001;77:399–408
- Ihara M, Takeshita S, Okaichi K, Okumura Y, Ohnishi T. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia 2014;30:102–9
- Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 2012;28:509–17
- Benafif S, Hall M. An update on PARP inhibitors for the treatment of cancer. Oncol Targets Therapy 2015;8:519–28
- O’Connor MJ, Martin NMB, Smith GCM. Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene 2007;26:7816–24
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. PNAS 2011;108:9851–6
- Thiesen B, Jordan A. Clinical implications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 2008;24:467–74
- Hilger I. In vivo applications of magnetic nanoparticle hyperthermia. Int J Hyperthermia 2013;29:828–34
- Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res 1994;54:3352–6
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yaun F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 2006;98:335–44
- Yardley DA, Burrias HA III, Spigel DR, Clark BL, Vazquez E, Shipley D, et al. A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first-line treatment of women with metastatic breast cancer. Clin Breast Cancer 2009;9:247–52
- Richards DA, Loesch D, Vukelja SV, Wu H, Hyman WJ, Nieves J, et al. Phase I study of pemetrexed and pegylated liposomal doxorubicin in patients with refractory breast, ovarian, primary peritoneal, or fallopian tube cancer. Invest New Drugs 2011;29:963–70
- Landen CD, Park J-Y, Needham D, Dewhirst DW. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed 2011;3:38–64
- McDaniel JR, Dewhirst MW, Chilkoti A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. Int J Hyperthermia 2013;29:501–10
- Manzoor AA, Lindner LH, Landon CD, Park J-Y, Simnick AJ, Needham D, et al. Overcoming limitations in nanoparticle drug delivery: Triggered intravascular release to improve drug penetration into tumors. Cancer Res 2012;72:5566–75
- Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia 2010;26:514–21
- Zagar TM, Vujaskovic Z, Formenti S, Rugo H, Muggia F, O’Connor B, et al. Two phase I dose-escalation/pharmacokinetic studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int J Hyperthermia 2014;30:285–94
- Davies CDL, Lundstrøm LM, Frengen J, Eikenes L, Bruland ØS, Kaalhus O, et al. Radiation improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Cancer Res 2004;64:547–53
- Petznek H, Kleityer M, Tichy A, Fuchs-Baumgartinger A, Hohenadl C. Murine xenograft model demonstrates significant radio-sensitising effect of liposomal doxorubicin in a combination therapy for feline injection site sarcoma. Res Vet Sci 2014;97:386–90
- Koukourakis MI, Koukouraki S, Giatromanolaki A, Kakolyris S, Georgoulias V, Velidaki A, et al. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas-rationale for combination with radiotherapy. Acta Oncol 2000;39:207–11
- Koukourakis MI, Romanidis K, Froudarakis M, Kyrgias G., Koukouraki GV, Retalis G, et al. Concurrent administration of docetaxel and stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: Excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer 2002;87:385–92
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res 2013;1:210–16
- Repasky EA. Progress in development of biomedical applications of heat shock proteins and thermal stress. Int J Hyperthermia 2013;29:359–61
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718–26
- Gaipl US, Multhoff G, Scheithauer H, Lauber K, Hehlgans S, Frey B, et al. Kill and spread the word: Simulation of antitumor immune responses in the context of radiotherapy. Immunotherapy 2014;6:597–610
- Vartak S, George KC, Singh BB. Antitumor effects of local hyperthermia on a mouse fibrosarcoma. Anticancer Res 1993;13:727–29
- Wang H, Zhang L, Shi Y, Javidiparsijani S, Wang G, Li X, et al. Abscopal antitumor immune effects of magnet-mediated hyperthermia at a high therapeutic temperature on Walker-256 carcinosarcomas in rats. Oncol Lett 2014;7:764–70
- Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Frontiers Oncol 2014;4:325
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503–10
- Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: A new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831–8
- Wattenberg MM, Fahim A, Ahmed MM, Hodge JW. Unlocking the combination: Potentiation of radiation-induced antitumor responses with immunotherapy. Radiat Res 2014;182:126–38
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008;44:2546–54
- Kabakov AE, Kudryavtsev VA, Gabai VL. Hsp90 inhibitors as promising agents for radiotherapy. J Mol Med 2010;80:241–7
- Hall JA, Forsberg LK, Blagg BSJ. Alternative approaches to Hsp90 modulation for the treatment of cancer. Future Med Chem 2014;6:1587–605
- Bhat R, Tummalapalli SR, Rotella DP. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J Med Chem 2014;57:8718–28
- McConnell JR, Buckton LK, McAlpine SR. Regulating the master regulator: Controlling heat shock factor 1 as a chemotherapy approach. Biorg Med Chem Letts 2015;25:3409–14
- Pedersen KS, Kim GP, Foster NR, Wang-Gillam A, Erlichman C, McWilliams RR. Phase II of gemcitabine and tanespimycin (17AAG) in metastatic pancreatic cancer: A Mayo Clinic phase II consortium study. Invest New Drugs 2015;33:963–8
- Camphausen K, Tofilon PJ. Inhibition of Hsp90: A multitarget approach to radiosensitization. Clin Cancer Res 2007;13:4326–30
- Yoshida S, Koga F, Tatokoro M, Kawakami S, Fuji Y, Kumagai J, et al. Low-dose Hsp90 inhibitors tumor-selectively sensitize bladder cancer cells to chemotherapy. Cell Cycle 2011;10:4291–9
- Patel K, Wen J, Magliocca K, Muller S, Liu Y, Chen ZG, et al. Heat shock protein (Hsp90) is overexpressed in p16-negative oropharyngeal squamous cell carcinoma, and its inhibition in vitro potentiates the effects of chemoradiation. Cancer Chemother Pharmacol 2014;74:1015–22
- Wolf F, Li W, Li F, Li CY. Non-invasive, quantitative monitoring of hyperthermia-induced EGFR activation in xenograft tumours. Int J Hyperthermia 2011;27:427–34
- Milanovic D, Firat E, Grosu AL, Niedermann G. Increased radiosensitivity and radiothermosensitivity of human pancreatic MIA PaCa-2 and U251 glioblastoma cell lines treated with the novel Hsp90 inhibitor NVP-HSP990. Radiat Oncol 2013;8:42
- Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res 2006;66:9211–20
- Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, et al. Hsp90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther 2008;7:1985–92
- Zaidi S, McLaughlin M, Bhide SA, Eccles SA, Workman P, Nutting CM, et al. The Hsp90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage. PLoS One 2012;7:e35436
- Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radiosensitization of human glioma cells by combining inhibition of poly (ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther 2009;8:2243–54
- Horsman MR. Modifiers of tumor blood supply. In: Urano M, Douple EB, eds. Hyperthermia and Oncology, Vol. 4. Utrecht: VSP, 1994, pp. 259–83
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006;22:197–203
- Horsman MR, Christensen KL, Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia 1989;5:123–36
- Horsman MR, Christensen KL, Overgaard J. Relationship between the hydralazine-induced changes in murine tumour blood supply and mouse blood pressure. Int J Radiat Oncol Biol Phys 1992;22:455–8
- Clinical trials information for patients and caregivers. US National Cancer Institute. Available from http://www.cancer.gov/clinicaltrials
- Clémenson C, Chargari C, Deutsch D. Combination of vascular disrupting agents and ionizing radiation. Crit Rev Oncol/Hematol 2013;86:143–60
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med 2001;7:987–9
- Horsman MR, Siemann DW. Pathophysiological effects of vascular targeting agents and the implications for combination with conventional therapies. Cancer Res 2006;66:11520–39
- Siemann DW, Dai Y, Horsman MR. Hypoxia, metastasis, and antiangiogenic therapies. In: Mellilo G, editor. Hypoxia and Cancer: Biological Implications and Therapeutic Opportunities. New York: Springer; 2014. pp 205–27
- Yano T, Tanase M, Watanabe A, Sawada H, Yamada Y, Shino Y, et al. Enhancement effect of an anti-angiogenic agent, TNP-470, on hyperthermia-induced growth suppression of human esophageal and gastric cancers transplantable to nude mice. Anticancer Res 1995;14:1355–8
- Nishimura, Y., Murata, R., Hiraoka, M. Combined effects of an angiogenesis inhibitor (TNP-470) and hyperthermia. Br J Cancer 1996;73:270–4
- Verhulst J. Effects of bevacizumab and hyperthermia in a rodent model of hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Hyperthermia 2013;29:62–70
- Nie W, Ma XL, Sang YX, Li YL, Gao X, Xu GC, et al. Synergic antitumor effect of SKLB1002 and local hyperthermia in 4T1 and CT26. Clin Exp Med 2014;14:203–13
- Eikesdal, H.P., Bjorkhaug, S.T., Dahl, O. Hyperthermia exhibits anti-vascular activity in the BT4An rat glioma: Lack of interaction with the angiogenesis inhibitor batimastat. Int J Hyperthermia 2002;18:141–52
- Horsman MR, Sampson LE, Chaplin DJ, Overgaard J. The in vivo interaction between flavone acetic acid and hyperthermia. Int J Hyperthermia 1996;12:779–89
- Murata R, Overgaard J, Horsman MR. Comparative effects of combretastatin A-4 disodium phosphate and 5,6-dimethylxanthenone-4-acetic acid on blood perfusion in a murine tumour and normal tissues. Int J Radiat Biol 2001;77:195–204
- Horsman MR, Murata R, Breidahl T, Nielsen FU, Maxwell RJ, Stødkilde-Jørgensen H, Overgaard J. Combretastatins: Novel vascular targeting drugs for improving anti-cancer therapy. Adv Exp Med Biol 2000;476:311–23
- Horsman MR, Murata R. Vascular targeting effects of ZD6126 in a C3H mouse mammary carcinoma and the enhancement of radiation response. Int J Radiat Oncol Biol Phys 2003;57:1047–55
- Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol 2013;52:1320–6
- Breidahl T, Nielsen FU, Stødkilde-Jørgensen H, Maxwell RJ, Horsman MR. The effects of the vascular disrupting agents combretastatin A-4 disodium phosphate, 5,6-dimethylxanthenone-4-acetic acid and ZD6126 in a murine tumour: A comparative assessment using MRI and MRS. Acta Oncol 2006;45:306–16
- Kallinowski F, Moehle R, Vaupel P. Substantial enhancement of tumor hyperthermic response by tumor necrosis factor. In: Sugahara T, Saito M, editors. Hyperthermic Oncology, Vol. 1. London: Taylor and Francis; 1989. pp 258–9
- Lin JC, Park HJ, Song CW. Combined treatment of IL-α and TNF-α potentiates the antitumour effect of hyperthermia. Int J Hyperthermia 1996;12:335–44
- Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, et al. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia 2000;2:555–60
- Griffin RJ, Monzen H, Williams BW, Park H, Lee SH, Song CW. Arsenic trioxide induces vascular damage via oxidative stress and increases thermosensitivity of tumours. Int J Hyperthermia 2003;19:575–89
- Horsman MR, Murata R. Combination of vascular targeting agents with thermal and radiation therapy. Int J Radiat Oncol Biol Phys 2002;54:1518–23
- Horsman MR, Murata R. Vascular targeting therapies and hyperthermia. In: Siemann DW, editor. Vascular-targeted Therapies in Oncology. Chichester: Wiley; 2006. pp 137–57
- Siemann DW, Warrington KH, Horsman MR. Vascular targeting agents: Adjuvants to radiation therapy. Radiother Oncol 2000;57:5–12
- Siemann DW, Horsman MR. Vascular targeted therapies in oncology. Cell Tissue Res 2009;335:241–8
- Folkes LK, Christlieb M, Madej E, Stratford MRL, Wardman P. Oxidative metabolism of combretastatin A-1 produces quinine intermediates with the potential to bind to nucleophiles and to enhance oxidative stress via free radicals. Chem Res Toxicol 2007;20:1885–94
- Horsman MR, Murata R, Overgaard J. Improving local tumor control by combining vascular targeting drugs, mild hyperthermia and radiation. Acta Oncol 2001;40:497–503
- Murata R, Horsman MR. Tumour specific enhancement of thermoradiotherapy at mild temperatures by the vascular targeting agent 5,6-dimethylxanthenone-4-acetic acid. Int J Hyperthermia 2004;20:393–404
- Murata R, Overgaard J, Horsman MR. Combretastatin A-4 disodium phosphate: A vascular targeting agent that improves the anti-tumor effects of hyperthermia, radiation and mild thermoradiotherapy. Int J Radiat Oncol Biol Phys 2001;51:1018–24
- Hokland S, Horsman MR. The new vascular disrupting agent combretastatin A-1 disodium phosphate (OXi4503) enhances tumour response to mild hyperthermia and thermoradiosensitisation. Int J Hyperthermia 2007;23:599–606
- Griffin RJ, Williams BW, Koonce NA, Bischof JC, Song CW, Asur R, et al. Vascular disrupting agent arsenic trioxide enhances thermoradiotherapy of solid tumors. J Oncol 2012;2012:934918
- Horsman MR. The therapeutic potential of using the vascular disrupting agent OXi4503 to enhance mild temperature thermoradiation. Int J Hyperthermia 2015;31:453–9
- Horsman MR, Wood PJ, Chaplin DJ, Brown JM, Overgaard J. The potentiation of radiation damage by nicotinamide in the SCCVII tumour in vivo. Radiother Oncol 1990;18:49–57
- Horsman MR, Nordsmark M, Khalil AA, Hill SA, Chaplin DJ, Siemann DW, Overgaard J. Reducing acute and chronic hypoxia in tumours by combining nicotinamide with carbogen breathing. Acta Oncol 1994;33:371–6
- Horsman MR, Brown DM, Lemmon MJ, Brown JM, Lee WW. Preferential tumour radiosensitization by analogs of nicotinamide and benzamide. Int J Radiat Oncol Biol Phys 1986;12:1307–10
- Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumour cells. Cancer Res 1990;50:7430–6
- Chaplin DJ, Horsman MR, Siemann DW. Further evaluation of nicotinamide and carbogen as a strategy to reoxygenate hypoxic cells in vivo: Importance of nicotinamide dose and pre-irradiation breathing time. Br J Cancer 1993;68:269–73
- Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 2010;28:4912–18
- Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: Results of a phase III randomized trial. J Clin Oncol 2012;30:1777–83
- Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. Assessment of antiangiogenic and antivascular therapeutics using MRI: Recommendations for appropriate methodology for clinical trials. Br J Radiol 2003;76:S87–91
- Harima Y, Togashi A, Horikoshi K, Imamura M, Sougawa M, Sawada S, et al. Prediction of outcome of advanced cervical cancer to thermoradiotherapy according to expression profiles of 35 genes selected by cDNA microarray analysis. Int J Radiat Oncol Biol Phys 2004;60:237–48
- Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. PNAS 2010;107:20477–82
- Chi J-T, Thrall DE, Jiang C, Snyder S, Fels D, Landon C, et al. Comparison of genomics and functional imaging from canine sarcomas treated with thermoradiotherapy predicts therapeutic response and identifies combination therapeutics. Clin Cancer Res 2011;17:2549–60

