Abstract
CEM43 has its roots in the direct cytotoxic effect of heat, whereby the amount of cell death depends on the temperature and exposure time. CEM43 is a normalising method to convert the various time–temperature exposures applied into an equivalent exposure time expressed as minutes at the reference temperature of 43 °C. The CEM43 concept also holds a number of weaknesses, however. When used to predict treatment efficacy of combined radiotherapy plus hyperthermia, CEM43 does not include the effect of sensitisation by enhanced oxygenation, variation in interval time and the effect of multiple fractions. Further, it does not include the effect of increased perfusion at the lower thermal dose – and the occurrence of stasis at the higher thermal dose. Overall, studies towards a thermal dose–effect relationship in radiotherapy plus hyperthermia present a diffuse message without a definitive conclusion. However, prospective studies and studies with large patient numbers did report significant thermal dose–effect relationships and provide a good reason to continue research in the CEM43 model. Such research would be best performed in homogeneous patient groups with a single pathology and a low variation in tumour size and heterogeneity. Further, high quality thermometry, strict treatment schedules with fixed intervals and preferably homogenous heating are important requirements to enhance the probability of detecting a thermal dose–effect relationship. The slowly growing availability of hybrid MR hyperthermia systems should be a strong stimulus to expand these studies with the inclusion of measuring thermal dose-dependent blood flow and oxygen changes in the tumour and normal tissues.
Introduction
An editorial by Overgaard [Citation1] and a letter to Lancet Oncology by Januszewski and Stebbing [Citation2] perfectly summarise why hyperthermia should be explored. Hyperthermia is still the most potent biological sensitiser for radiotherapy and chemotherapy. The exact mechanisms by which hyperthermia drives the improved clinical outcome are slowly revealed. The most recent identification is that HT affects double-strand break DNA repair by degrading BRCA2. More elaborative discussions are available in excellent reviews [Citation3–5]. The beneficial effect of adding hyperthermia to radiotherapy has been demonstrated for a large variety of tumour pathologies. Recently Issels et al. [Citation6,Citation7] showed for the first time in a randomised trial that hyperthermia added to chemotherapy improves the overall 5 years survival in patients with soft tissue sarcoma by 12%. Overall Januszewski & Stebbing [Citation2] state that the results of ‘the few, but impressive trials are evidence that HT is not just an anecdotal technology, but one that warrants continued investment and investigation. The studies’ improvements in tumour response would generally be hailed a success had they been a pharmaceutical product.’
When comparing the results of, or when designing clinical trials, the need for an appropriate thermal dose parameter is evident. To decide what is the most appropriate thermal dose parameter is less obvious. Whenever there is a dispute on the outcome of a clinical trial [Citation8–10], the recognition of uncommon hyperthermia schedules or mechanisms (once per week or daily hyperthermia fractions [Citation11]), the (im)possibility of the requirement to measure the tumour temperature or indicative tumour temperature [Citation12,Citation13], biological mechanisms [Citation4,Citation5] or publication of studies demonstrating the existence of indisputable thermal dose–effect relationships [Citation14–21] or demonstrating the lack of a thermal dose–effect relationship [Citation22], discussion flares up. A single widely accepted thermal dose parameter has not been defined yet and the discussion of what is the most appropriate thermal dose parameter is still ongoing. It illustrates that defining a thermal dose parameter that adequately predicts the plethora of biological effects caused by a hyperthermia treatment is a very difficult task, requiring the input of all the disciplines involved in hyperthermia. In this process biologists are responsible for identifying the most appropriate biological mechanisms related to realistic clinical temperatures. Physicists and engineers are responsible for providing the high quality equipment that is able to deliver and monitor the application of the proposed heat treatment. Subsequently, clinicians and hyperthermia operators should be able to apply the prescribed treatment to the patient within certain margins. Ultimately, the outcome of the clinical studies should confirm or refute the premises of the starting points of the biologists. Not surprisingly, this is a slow iterative process with a long cycle time (at least 10 years per cycle) especially in a small field such as hyperthermia. The question is whether sufficient cycles have passed since the early days of hyperthermia to adapt now to a single dose parameter that can be used uniformly by all international hyperthermia centres to monitor the application of, and to report on the quality of the applied treatment.
This review provides a brief overview of the various aspects that play a role when answering the question whether CEM43 still is a relevant thermal dose parameter for hyperthermia treatment monitoring. The discussion focusses on the use of CEM43 in the application of mild hyperthermia, i.e. heating tissue in the temperature range of 40–44 °C. Hence, the findings reported are not applicable for thermal ablation where dose is commonly measured in damage and concerns more the effectiveness of the treatment to cause cell death by coagulation/necrosis of the tumour tissue. This review starts with defining the requirements of a dose parameter in general. Hereafter, it reviews current biological, physiological, clinical and technological knowledge that should be considered when assessing the limitation and potential of CEM43 as thermal dose parameter in the application.
General requirements of a dose parameter
As stated by Field and Raaphorst [Citation23], ‘the purpose of a dose is to provide a number which relates to a specific biological response.’ In their vision, the thermal dose parameter should provide a means to predict treatment outcome as a function of the applied hyperthermia treatment and should allow the comparison of response for treatments given at different places and times. In this definition it is not required that the thermal dose parameter is a direct reflection of the underlying biological mechanisms causing the biological response. It is sufficient if the dose parameter is related to the biological mechanisms involved. Also, the dose parameter should be a well-defined and measurable physical quantity and as such be a proper means of inter-comparison. These are important requirements and it makes sense to (re)consider whether the CEM43 thermal dose parameter based upon current insights and practice of hyperthermia application still fulfils these basic requirements. This evaluation should be performed from a biological, physiological and clinical perspective focusing on the direct and indirect mechanistic connections between dose and effect. It must also be performed from a technological perspective focusing on the measurability of the value and whether it is representative for the dose administered.
Biological aspects of thermal dosimetry
Heat alone
Excellent reviews on the biological rationale of hyperthermia, and more specifically, the concept of CEM43 as thermal dose parameters have been published by Dewey [Citation24], Dewhirst et al. [Citation5] and most recently by Pearce [Citation25]. The use of CEM43 as a thermal dose parameter finds its roots in the use of Arrhenius plots to study the kinetics of cell killing by hyperthermia [Citation26,Citation27] and the need to compare the results of all in vitro experiments performed at time–temperature exposures during the early years of hyperthermia [Citation28,Citation29]. Although extensive variation in cellular sensitivity for heat was found, even for cells cultured under identical conditions, the overall response showed a similar pattern. The amount of cell death is always dependent on time and temperature. At low temperatures, i.e. < 43 °C and short exposure times, the survival curve shows a shoulder (indicative of accumulation of sub-lethal heat damage), which changes into a constant rate of cell death (the straight line in the Arrhenius or logarithmic cell survival plot) that after several hours is followed by a low rate of cell death due to the development of thermotolerance. For heat exposure at temperatures above 43 °C, cell death is at a constant rate and no shoulder or thermo-tolerance is noted. With the ambition to compare the ‘cell killing’ effectiveness of hyperthermia applied at different temperature–time exposure conditions, Sapareto et al. [Citation29] pointed to the analogy with the Arrhenius model to describe protein inactivation rate at different temperature and time intervals. Based upon this analogy they derived the thermal iso-effect dose (TID) concept which is in principle a normalising method to convert various time–temperature exposures to an equivalent exposure time in minutes at a reference temperature, commonly 43 °C, using the formula:
In this formula T represents the actual applied temperature of the target tissue and R the factor to compensate for a 1 °C temperature change. R is experimentally determined and has been set at a value of 0.5 for T > 43 °C, i.e. the equivalent time doubles per degree temperature increase, and 0.25 for T ≤ 43 °C, i.e. the equivalent time decrease by a factor of four per degree temperature decrease.
Although the model is widely used in studies searching for thermal dose–effect relationships, the ability of the CEM43 concept to predict thermal initiated cell death has a serious number of limitations [Citation24,Citation25]. As elucidated by Pearce [Citation25], the intrinsic mathematical limitations of both the Arrhenius and CEM43 model are that they describe only a single constant irreversible reaction of the total thermal damage process leading to loss of clonogenicity as a surrogate for cell death. The process leading to cell death is, however, a complex cascade of multiple pathways involving thermal effects on proteins, cell membrane, nucleus, cytoskeleton, proliferation, for example [Citation30,Citation31]. Other well-known factors that have been identified to modify the cytotoxic effect of heat are thermal tolerance, step-down heating and difference in thermal sensitivity of cells’ pH, for example [Citation24,Citation32]. In addition, neither model includes prediction of the shoulder region at the beginning of the cell survival curve and predicts cell death at low temperatures for very long exposure duration, whereas these temperatures are known not to be thermally hazardous. Based on these considerations, Pearce [Citation25] considered that only the use of a multi-parameter mathematical model would be able to provide a good prediction of cell death due to heat. Still, it can be questioned whether even a multi-parameter model developed from in vitro experiments can be translated to the clinical hyperthermia environment. In experimental research the cell culture is growing rather homogeneous in Petri dishes, with all cells living in a constant, tumour-cell friendly environment and possessing a more or less uniform sensitivity for the hyperthermia treatment. Certainly, this ideal condition does not exist in human tumours that are selected for hyperthermia. In general, human tumours treated with hyperthermia are large and have a heterogeneous constitution of well and poorly perfused regions resulting in wide variation of local conditions. Hence, a substantial variation in the thermal sensitivity of the tumour cells will be present. Currently, it is still unclear how to incorporate this intrinsic sensitivity variation in a model to predict thermal damage.
Hyperthermia as a direct sensitiser to radiotherapy
As explained, both the Arrhenius and CEM43 model are designed to predict tumour cell death based on the cytotoxic effect of hyperthermia only. In clinical practice, however, hyperthermia is always applied as an adjuvant to radiotherapy. In such a situation sensitisation of tumour cells for radiotherapy will also contribute to the final tumour cell death. Already in 1971 Westra [Citation33] showed in his PhD thesis that applying hyperthermia at 45.5 °C for 10 min prior to radiation resulted in the expected hyperthermia-induced cytotoxic cell death plus a steeper slope of the radiation cell survival curve. Other studies as mentioned in Dewey et al. [Citation28] confirmed the sensitisation effect of heat on the radiation-induced tumour cell death. At temperatures above 43 °C the value for R in heat radiosensitisation experiments was found to be similar to that for heat cytotoxicity (∼2) whereas for temperatures below 43 °C the value of R as found for heat radiosensitisation was 2–3, i.e. lower than the 4–8 for heat cytotoxicity. Another important factor determining the extent of radiosensitisation is the precise scheduling and timing of the combined radiotherapy and hyperthermia treatment. Maximum radiosensitisation is obtained when radiation and heat are applied simultaneously. Less clear is how long radiosensitisation remains present when radiation and heat are applied sequentially. Based on the results obtained from in vitro studies, Dewey et al. [Citation28] concluded that hyperthermia should be applied simultaneously or within 5–10 min after or before radiation in order to benefit maximally from the heat radiosensitisation effect. Based on this observation and the fact that the time interval between radiotherapy and hyperthermia was reported to be between 30–60 min in clinical treatments, they concluded that in the early clinical trials investigating the benefit of adding hyperthermia to radiation, only heat cytotoxicity was responsible for enhanced tumour cell death. From this they advised increasing the thermal dose applied during a hyperthermia treatment to a minimum CEM43T90 for 25 min (CEM43T90 calculated for T90, T90 being the temperature which 90% of the tumour temperatures exceed). In their review on the role of hyperthermia as a potent enhancer of radiotherapy, Horsman and Overgaard [Citation34] also firmly concluded that simultaneous application of heat and radiation provided maximum radiosensitisation. For sequential application they recognise that evidence exists that radiosensitisation can rapidly reduce even with short intervals between radiotherapy and hyperthermia but also report data showing prolonged existence of radiosensitisation observed in in vivo studies. They conclude that when the time between irradiation and subsequent heating is increased the sensitisation effect decreases, but it can take up to 4 h before radiosensitisation completely disappears. When heat is applied prior to radiation thermal radiosensitisation can be present for an interval of 4–6 h. Outside these time intervals, the enhancement effect of hyperthermia is only due to the heat-induced killing of the radiation-resistant hypoxic tumour cells [Citation34]. For normal tissue they report a similar dependence on the interval and timing between the application of heat and radiation, with a maximum radiosensitisation for simultaneous application and a reduced sensitisation for sequential application. Importantly, the radiosensitisation effect of heat in normal tissue decreases more rapidly when hyperthermia is given after radiotherapy compared with hyperthermia prior to radiotherapy. Also the radiosensitisation effect of heat disappears more rapidly in normal tissue than in most tumours, i.e. creating a therapeutic window and thus the required beneficial gain for enhanced tumour control. When radiotherapy and hyperthermia are applied simultaneously the maximal thermal enhancement is obtained. For simultaneous application the thermal enhancement ratio can be 2–3 times higher than for sequential hyperthermia, i.e. 4–5 for simultaneous versus 1.5–2 for sequential radiotherapy plus hyperthermia respectively. Simultaneous application of radiotherapy and hyperthermia, however, requires selective tumour heating, which with the current hyperthermia equipment is not yet realistic.
The inability to achieve selective tumour heating explains why all clinical studies used sequential heating whereby for mostly logistic reasons hyperthermia was applied after radiotherapy. At the same time the clinical study demonstrated that the early defined aims on a minimum tumour thermal dose, defined as T90 of 43 °C during 30–60 min of therapy, could not be achieved. Instead, the reported T90 ranged from 39–42 °C [Citation5]. Clearly at these milder tumour temperatures, the contribution to the overall tumour cell death by heat-induced cytotoxicity will be less than at tumour temperatures >43 °C. However, as mentioned by Dewhirst et al. in their paper ‘Resetting the biological rationale for thermal therapy’ [Citation5], a compelling range of effects is induced by a mild temperature hyperthermia treatment. For the current evaluation of the representative value of CEM43 as dose parameter, only the effects of heat on inhibition of DNA repair and changes in perfusion are further discussed.
It is commonly believed that radiosensitisation by hyperthermia is due to its ability to interact with the mechanisms for DNA damage repair. The heat-initiated obstruction of DNA damage repair affects all repair pathways, though assessment of the exact mechanisms involved is still the subject of ongoing research [Citation35,Citation36]. Early studies focused at temperatures above 43 °C and showed a progressive diminishing of DNA repair capacity with higher temperatures and increasing exposure times. The kinetics of the decreasing DNA repair capacity followed the Arrhenius model [Citation37]. In recent studies it was also demonstrated that mild hyperthermia temperatures of 39–42 °C inhibit homologous recombination (HR) repair of double strand breaks by inducing degradation of the recombination mediator BRCA2 [Citation3,Citation38]. Although the BRCA2 degradation was measured at relative low thermal dose, i.e. 41 °C for 4 h, degradation was more pronounced at a temperature of 42.5 °C and a shorter duration of 75 min. This observation is in line with the earlier studies and may confirm that inhibition of DNA repair is time and temperature dependent, conforming with the Arrhenius model. The latter indicates that the CEM43 model could serve as a valid thermal dose–effect parameter. Adding, the Hsp90 inhibitor 17-DMAG prolongs the temperature-mediated reduction of BRCA2 levels from 2 to 4 h, which is relevant for optimal scheduling when hyperthermia is combined with radiotherapy or chemotherapy.
Physiological aspects of thermal dosimetry
Hyperthermia as indirect sensitiser to radiotherapy via tumour perfusion and oxygenation
Tumour perfusion and microcirculation are known to be affected by tissue temperature and the duration of the elevated temperature. The interaction, however, is very complex, with opposite effects on tumour perfusion depending on the exposure time and elevation of the temperature. In general the changes in perfusion are paralleled by the changes in tumour oxygenation. Heat-induced perfusion effects, and presumably also the proportionality between perfusion and oxygenation changes, vary between species, tumour types, thermal dose applied, the time of measurement (directly after heat or 24 h later), number of heat fractions, and rate of heating, for example [Citation39–45]. Besides the ability of heat to change tumour blood flow, its effect on normal tissue perfusion should also be taken into account, as changing the ratio in tumour–normal tissue perfusion may support the ability to obtain higher temperatures in the tumour [Citation41,Citation42,Citation46].
Perfusion as a function of thermal dose
Increase of tumour perfusion and oxygenation has been reported to occur already at temperatures as low 39–40 °C [Citation39]. In general a mild increase of tumour temperature is expected to result only in a small increase of tumour blood flow and improved microcirculation. When tumour temperature or exposure time is increased (i.e. higher thermal dose) the initial increase in tumour blood flow will then decrease and eventually vascular stasis will occur. The first part of this transient process has been described as the reactive phase and the second part as the destructive phase [Citation46]. Due to the absence of perfusion, a lack of oxygen will develop with subsequent advantages and disadvantages on cell death by radiotherapy and/or hyperthermia. There are indications that the development of vascular shutdown shows an iso-effect relationship according the Arrhenius equation [Citation23]. Using the ‘sandwich’ observation chambers for perfusion measurement Van den Berg-Blok and Reinhold [Citation47] found that the time required for development of vascular stasis in half of the exposed animals followed an exponential relation with temperature according to the CEM43 model with R = 0.4551. In general vascular stasis in rodent tumour is induced at CEM43 ranging from 60–120 min. In an early conceptual model it was hypothesised that the increased perfusion at a mild temperature increase remains even with very long exposure times [Citation40].
Duration of increased perfusion/oxygenation
Increased perfusion at mild heating temperature has been shown to correspond with improved tumour oxygenation in animal [Citation48–50] and clinical [Citation51,Citation52] studies. The duration of the improvement in oxygenation is still a point of controversy [Citation45,Citation53]. In rodent studies improved median pO2 values (increase from 4–10 mmHg) have been found immediately and 24 h after heating. Song et al. [Citation54] refer in their review to studies showing that heat increases tumour perfusion from temperatures as low as 39–40 °C for 30 min, but when heated for 60 min tumour perfusion deteriorated. In the R3230 AC tumour model grown in the hind legs of Fischer rats, tumour blood flow and median pO2 increased continuously immediately and 24 h after heat exposure for 30 min at temperatures between 40.5–43.5 °C. Increasing the exposure time to 60 min resulted in a decrease of tumour blood flow and median pO2 for 42.5 and 43.5 °C immediately after heating, while 24 h later blood flow decreased for 42.5 and 43.5 °C but median pO2 decreased only at 43.5 °C [Citation55]. A similar effect was reported in spontaneous soft tissue sarcomas in dogs [Citation56]. However, Song et al. [Citation54] also mentioned that for Yoshida sarcoma and DS carcinoma doubling the exposure time from 30 to 60 min at the low temperatures of 39–40 °C results in opposite effects on tumour pO2. Finally, Griffin et al. [Citation57] confirmed in a more recent study that fractionated radiotherapy combined with repeated multiple heating at 48-h intervals resulted in a strong oxygen improvement, which was associated with the longest tumour growth delay in mice. These findings confirm the earlier mentioned complex dependence on thermal dose. However, discussion on the cause and relevance of the biological and physiological mechanisms, as far as they are known, falls outside the scope of the current topic. It is sufficient to note that a hyperthermia treatment modulates tumour perfusion and oxygenation, the extent of which is dependent on time and temperature and whereby the onset of vascular stasis has been found to obey an exponential relation of time and temperature analogous to CEM43. Whether the duration (16, 24 or 48 h) of prolonged improvement of perfusion and oxygenation follows an exponential relation is unknown.
Hyperthermia mediated tumour oxygen consumption
Several studies indicate that the improved tumour oxygenation after hyperthermia is a combined effect of the increase in tumour blood flow and also a decrease in oxygen consumption. Explanations given for the decrease in oxygen consumption are the heat-induced tumour cell death [Citation39,Citation50,Citation53], but also alteration of the tumour metabolism processes as suggested by Moon et al. [Citation58]. These authors reported that hyperthermia causes cells to up-regulate the transcription factor, hypoxia-inducible factor-1 (HIF-1) through the ERK pathway. Of the factors that influence oxygen transport, oxygen consumption rate is the most dynamic [Citation59]. As the extent of reoxygenation after hyperthermia is likely a complex product of effects on both perfusion and oxygen consumption rate, future research in the reoxygenation effect of hyperthermia should use measuring techniques that are able to discriminate perfusion and consumption effects. Cu-ATSM PET imaging has been demonstrated to be able to measure the hyperthermia effect on tumour hypoxia independent of the increase of perfusion [Citation60].
Normal tissue to tumour blood flow ratio: impact on ability to heat
Perfusion changes after a single heat exposure
Current knowledge on the response of normal tissue perfusion during hyperthermia is predominantly based on studies performed before 1990. In contrast to the fragile and chaotic vascular structure in tumours, normal tissue has a well-organised vascular bed. Normal tissue is therefore more tolerant to heat exposure than tumour tissue and it reacts primarily by a reactive hyperaemia to heat exposure. For a moderate temperature rise (42–43 °C, i.e. higher than those for tumour tissue) and exposure time, blood flow and vascular volume will increase. Vascular stasis for normal tissue have been mentioned to occur at exposure of 60 min at 45–47 °C for muscle and skin, though a much lower value (41°C) was found for blood vessels of the mouse gut [Citation46]. Further, Dewhirst et al. [Citation61] reported that at a high rate of heating (1 °C/min) vascular stasis occurs at a lower temperature than at slower heating rates (≤ 0.7 °C/min), with normal tissue being more heat resistant than tumour tissue. More recently, extensive overviews have been published on the thermal threshold for normal tissue damage [Citation62–64]. For muscle and fat tissue these studies report a CEM43 threshold for thermal damage of 80 min, while for skin minor damage was seen at 600 min. These values are in line with the values reported by Reinhold and Endrich [Citation46], though large variations in CEM43 thermal threshold values exist as functions of species, tissue and damage end point [Citation62–64].
In addition to the higher thermal threshold for vascular stasis, the potential of a healthy normal tissue vascular network to respond to thermal stress by a perfusion increase is much stronger than that of tumour tissue. Muscle and skin blood flow can increase by a factor of 10–15 compared to only a factor of two for tumour tissue [Citation65]. On the other hand it must be realised that resting blood flow of muscle and skin at normal temperature is substantially lower than that of most tumours [Citation42,Citation65,Citation66] and that a temperature-modulated increase in blood flow requires higher temperatures than for tumour tissue. Again, substantial variations between animal species have been reported. In mice, a single exposure of skin and muscle for 30–90 min at a temperature of 42 °C showed a significant increase in blood flow [Citation42,Citation67], while the same group found for Fischer and Sprague–Dawley rats only a small increase in skin and muscle blood flow after a single exposure at the same temperature and time [Citation41,Citation68,Citation69].
Perfusion changes after multiple heat exposures
In this respect it is interesting to note that the previous results changes completely when multiple hyperthermia treatments are administered separated by 1 or 2 days. Nah et al. [Citation66] report for Fischer rats an increase in skin and muscle blood flow after a single heat treatment at 42.5 °C of 60 min by a factor of about two compared to normal temperature. After a single heat exposure of 60 min at 44.5 °C blood flow in normal tissue increased by a factor of three and four for skin and muscle respectively, while tumour blood flow remained unchanged. When however, the first heating of 60 min at 42.5 °C was followed by a second heating at 44.5 °C for 60 min with an interval of 16, 24 or 48 h an additional increase in blood flow was noted up to a factor 2, 1.4 and 1.1 for skin, muscle and tumour, respectively (derived from to 4 in Nah et al. [Citation66]). Song et al. [Citation42] noticed a similar effect of multiple heating with intervals of 24 or 72 h (1 or 3 days) in C3H mice. After the first heat treatment of 43.5 °C for 1 h maximum blood flow increase was noted in tumour, skin and muscle tissue. At the start of the second heat treatment 1 or 3 days later it was noted that the ‘resting’ of the blood flow in tumour tissue was about half the value of the initial blood flow prior to the first heat treatment. After application of the second heat treatment the tumour blood flow did not respond and remained unchanged at the lower value (50% of the initial value). The same was noted for the third to fifth treatments irrespective of a 1- or 3-day interval between heat fractions. In contrast to the tumour, 24 and 72 h after the first heating blood flow in both skin and muscle normal tissue was 1.5–3 times the earlier resting value at the start of the second to fifth heating sessions and in all sessions the blood flow responded to the heat load with an increase. As a consequence the initial 4–5 times higher blood flow of the tumour tissue compared to normal tissue disappeared after the first heat treatment and changed in favour of normal tissue, with the blood flow in the tumour being slightly lower than that of skin and muscle. It needs no explanation that in the latter situation heating of the tumour is more easily accomplished.
Figure 1. Illustration of the association between number of (A) interstitial measurement points and thermal dose CEM43°CT90tumour (Spearman’s rank correlation −0.64, p < 1e–10) and (B) tumour maximum diameter and thermal dose as total CEM43°CT90tumour (Spearman’s rank correlation −0.70, p < 1e–6) in 72 patients with loco-regional breast cancer treated with superficial hyperthermia applied using 434 MHz microwaves. Picture from De Bruijne et al. [Citation22], with permission.
![Figure 1. Illustration of the association between number of (A) interstitial measurement points and thermal dose CEM43°CT90tumour (Spearman’s rank correlation −0.64, p < 1e–10) and (B) tumour maximum diameter and thermal dose as total CEM43°CT90tumour (Spearman’s rank correlation −0.70, p < 1e–6) in 72 patients with loco-regional breast cancer treated with superficial hyperthermia applied using 434 MHz microwaves. Picture from De Bruijne et al. [Citation22], with permission.](/cms/asset/019d7560-1e6f-4595-b0b1-04834bea3035/ihyt_a_1114153_f0001_b.jpg)
Fast growing animal versus slow growing human tumours
Whether the effects noticed in fast growing rodent tumours will also occur during and after mild hyperthermia (with the observed lower average temperature of 40–42 °C) in human tumours is still the topic of an ongoing debate. In an excellent review on the effects of hyperthermia on tumour pathophysiology Vaupel and Kelleher [Citation53] state, that the observations in animal studies on how blood perfusion changes during and after heat treatment have not been reported in the literature to occur in human tumours. They find evidence that besides microcirculation other factors such as intracellular pH and bioenergetics status could be decisive factors for the thermosensitivity of cancer cells.
Together, some normal tissue effects (stasis and increase of blood flow) show an exponential relation in animals but due to the higher thermal thresholds for these effects in normal tissue it is likely that these effects are of minor relevance for the dose–effect relationship with treatment outcome in patients. It is at present unclear whether the changes in blood flow induced by repeated heat applications also occur in patients, but if they would occur an exponential relation to the thermal dose delivered might be expected from the animal data.
Clinically demonstrated thermal dose–effect relationships
The benefit of adding hyperthermia to radiotherapy or chemotherapy has been demonstrated in a large number of randomised trials for a wide variety of tumour pathologies [Citation7,70–73]. In an extensive review Datta et al. [Citation74] analysed 38 randomised and non-randomised two-armed clinical studies and found that adding hyperthermia to conventional cancer treatment improves the odds ratio by a factor of 2–3 for six common cancer sites: breast, cervix, head and neck, rectum, urinary bladder, oesophagus. Illustrative of the continued interest in the clinical application of hyperthermia is that in 2014 a total of 36 new clinical trials investigating the value of adding hyperthermia to the standard treatment were registered within ClinicalTrials.gov [Citation75].
Although nearly all clinical trials indicate that the intention was to apply hyperthermia at 43 °C in the tumour, the reality is that in most treatments the measured temperatures were much lower, with the T90 temperature ranging in general between 39.5–41 °C for 60–90 min. As explained above, at these temperatures direct tumour cell death by hyperthermia will be negligible, though still many heat-modulated biological and physiological effects will occur that contribute to the improved clinical results seen in the randomised trials [Citation5]. Nevertheless, general consensus exists that tumour response is related to the quality of the hyperthermia treatment and various methods of reasoning can be applied to demonstrate the existence of a thermal dose–effect relationship.
Quality of hyperthermia: indirect proof of thermal dose–effect relationship
The first method is to look at the arguments used to explain the results of inconclusive phase III trials. In these trials especially, quality of the hyperthermia treatment is often identified as the cause of failure. Emami et al. [Citation9] mentioned the inability to deliver adequate hyperthermia (only one patient met the defined basic minimum adequacy criteria for hyperthermia) for the lack of a positive difference in their study of interstitial radiotherapy ± interstitial hyperthermia. In the RTOG study comparing radiation ± hyperthermia in superficial measurable tumours, patients with small chest wall tumours (max 3 cm) responded better than those with large chest wall tumours. In the opinion of the authors, this reflects the fact that small tumours were easier to heat adequately [Citation76,Citation77]. The latter is in remarkably good agreement with the conclusion of van der Zee et al. [Citation78] in their retrospective study on the effectiveness of re-irradiation plus hyperthermia for recurrent breast cancer. That study showed an increase of the complete response rate for tumours > 3 cm from 31% to 65% when using a better heating technique. The more recently performed randomised study for radiotherapy ± hyperthermia in patients with cervical cancer by Vasanthan et al. [Citation10] was internationally criticised for the use of inadequate heating. Besides the standard capacitive radiofrequency heating technique, an additional intracavitary applicator inserted in the posterior vaginal fornix was used in the majority of patients [Citation79,Citation80], resulting in high energy and hence high temperature gradients associated with an expected under dosage in the tissue at the tumour margin. It is interesting to note that only Emami et al. [Citation9] used an objective temperature-based criterion, while the other arguments had a more subjective character.
Retrospective data analyses demonstrate thermal dose–effect relationships
The second method of reasoning applied has been to search retrospectively for dose–effect relationships in patients treated with radiotherapy and hyperthermia using various kinds of technical parameters and many different types of measured temperature parameters to quantify the quality of the thermal dose delivered to the treatment field. Dose–effect relationships have been reported for frequency as a surrogate for penetration depth [Citation78], coverage by the 25% iso-SAR contour [Citation81] and mostly for thermal dose expressed as minimum, average, maximum, T90, T50, T20 temperatures (or a mixture of these values) or sometimes as time above a defined threshold temperature. Further, in nearly all analyses CEM43 was one of the parameters included, being CEM43T90 or CEM43T50 [Citation14–22,82–85]. Despite the biological rationale for the CEM43 model, a correlation between CEM43 and treatment outcome was not found in all studies. The latter should not surprise us, as many other tumour-related parameters will contribute to the final outcome of the treatment. Tumour size clearly matters, as was seen by Perez et al. [Citation86] when treating RIF-1 tumours in mice with radiotherapy (40 Gy) with hyperthermia at 43° or 45 °C. Tumours of 0.5 cm diameter showed the same complete response rate when heated at 43° or 45 °C respectively. For tumours with 1.0 cm diameter a significant higher complete response rate (82% versus 33%, p = 0.036) was obtained at 45° than at 43 °C, respectively. Rau et al. [Citation21] reported that high temperatures were easily reached in the group with non-resectable recurrent rectum tumours, whereas this group of patients did not respond well to the treatment. Tumour features such as necrosis will make them easy to heat but on the other hand will make them less sensitive for radiation. Similar, recurrent tumours may have different heating characteristics from locally advanced or small primary tumours [Citation21,Citation22,Citation87] which may mask potential dose–effect relationships. Franckena et al. [Citation15] showed that thermal dose–effect expressed as CEM43T90 was significantly correlated with CR, pelvic tumour control and disease-specific survival in a large retrospective study of 420 patients with locally advanced cervical cancer. Even after adjusting for other factors in multivariate analysis (radiotherapy dose, tumour stage and size, performance status), intra-luminal measured thermal dose parameter remained significantly correlated with response and survival. At the same time the study of Franckena et al. [Citation15] demonstrated a thermal dose–effect relationship for TRISE (TRISE is defined as the summation of the product of the average temperature increase above 37 °C with the real applied treatment time for all treatments applied and subsequently divided by the total intended time of all anticipated treatments. The latter should be seen as normalization for the variation in time per treatment and number of treatments applied. In formula,
indicating also that other thermal parameters that include time and temperature might provide predictive information on treatment outcome related to the quality of the hyperthermia treatment.
Prospective studies investigating thermal dose–effect relationships
Demonstrating a thermal dose-effect relationship in prospective trials has been shown to be difficult. In a first attempt Maguire et al. [Citation88] tested the CEM43 °CT90 concept to predict response in high-grade soft tissue sarcomas in patients. Despite the innovative approach, including a preheating to include only heatable patients in the study analysis, no correlation of thermal dose with histological response was observed, and prospective control of CEM43T90 failed to achieve the projected > 75% pathological complete response rate following pre-operative thermoradiotherapy. Possible explanations suggested by the authors are, among others, the critical threshold for CEM43 °CT90 might be higher or lower, the limited quality of thermal data acquisition, and questions whether CEM43 °CT90 is the best thermal dose descriptor. Thrall et al. [Citation89] were more successful and they showed that thermal dose is related to duration of local control in canine sarcomas treated by radiotherapy and superficial hyperthermia. In contrast to the earlier Maguire et al. study [Citation88], the prospective testing of the CEM43T90 model in the clinical study by Jones et al. [Citation14] resulted in a positive outcome. They compared the effect of a prospectively prescribed thermal dose of more than 10 CEM43T90 with that of less than 1 CEM43T90 and showed a considerable local control benefit from the higher dose.
Technological opportunities and limitations to demonstrate dose–effect relationships
Limitations of temperature measurement in clinical practice
Good clinical practice procedures for proper assessment of the temperature distribution in the tumour have been extensively addressed in all quality assurance guidelines of the European Society of Hyperthermia Oncology and the Society of Thermal Medicine, USA [Citation90–93]. Unfortunately, neither the patients nor the medical doctors show great enthusiasm for interstitial placement of thermometry catheters irrespective whether it concerns superficial or deep hyperthermia [Citation12,Citation13,Citation94]. As a consequence, placement of catheters during clinical hyperthermia was and still is very rarely performed, and when performed it is with restrictions to placement site, path, and insertion length. In general, for superficial hyperthermia surrogate thermometry is performed at the surface where temperature measurements are highly influenced by the water bolus temperature and thus have poor predictive value for the tissue temperature [Citation95–98]. For deep hyperthermia thermometry is mostly limited to minimally invasive procedures, i.e. intraluminal placement of thermal catheters. Several studies have indicated that for tumours located in the pelvis intraluminal temperature measurements follow intratumoural temperatures [Citation83,Citation99]. The correspondence between intratumoural and intraluminal measured temperatures is, however, subject to exact tumour location and position of temperature probes. Obviously, the above limitations affect the quality of thermometry and the representability of the measured temperature distribution of the real temperature distribution will vary from tumour to tumour.
Representability of measured T90 values
In principle, a good statistical calculation and thus representative T90 values requires at least 10 independent interstitial temperature measuring points within the target volume. Depending on the target definition as macroscopic tumour (GTV) or the tumour including margins (PTV), i.e. the whole radiation field, it will be more or less easy to fulfil the requirement on the total number of interstitial temperature sites. In superficial hyperthermia this will almost automatically result in a lower number of temperature sensors in the smaller tumours, simply because there is insufficient tumour volume. For large tumour volumes fulfilment of the required number of minimal temperature measuring points will depend on the tolerance of the patient, the estimation on the risk of complications by the medical doctor, and the number of temperature sensors available. In clinical practice this translates in to a negative correlation between CEM43T90 with number of interstitial temperature measuring points and tumour maximum diameter, as was found by De Bruijne et al. [Citation22] and shown in . As already mentioned, in deep hyperthermia the majority of hyperthermia centres are hesitant to apply deep interstitial thermometry catheters, and limit themselves to intraluminal thermometry. In this respect the number of catheters is limited to maximal three in female and two in male patients if no contraindications exist. Unknown, is whether and how much the location of the intraluminal thermometry in the central plane and the collection of a series of linear temperature data points affects the representability of the measured versus the real temperature distribution. A tempting solution for the criterion of at least 10 temperature measuring locations for the T90 assessment is to select a small distance between the locations, although one can doubt whether points separated by less than 1.0 cm should be regarded as independent.
Impact of different temperature measuring systems and protocols
Other considerations have a more technological character and consider the equality of temperature measurements using a RF immune multi-sensor fibre-optic thermometry system which provides semi-continuous temperature information versus a RF immune thermal mapping system using Bowman probes with a 5–15-min temperature refreshment rate, versus a RF-filtered multi-sensor thermocouple system with a power off/power on protocol to minimise RF interference. Although all three thermometry systems are applied in clinical hyperthermia it is still unclear whether these systems provide the same information during heating. Differences between institutes in reported T50 values reached 1.9 °C within a single clinical study on the application of radiotherapy plus hyperthermia and chemotherapy in patients with locally advanced cervical carcinoma (LACC) [Citation100]. In an attempt to understand the cause of these differences Fatehi et al. [Citation101] investigated the impact of different data sets on the resulting T50 values. In a group of 22 patients with LACC and treated with triple therapy, they found T50s of 40.4 ± 0.6 °C, 40.0 ± 0.8 °C and 39.8 ± 0.9 °C when averaging over all intraluminal sensors, only all vaginal intraluminal sensors or only the deepest five vaginal intraluminal sensors. It shows that even within rather homogenous patient groups the thermal dose various greatly with the selected temperature measuring points. Clearly, a variation in T50 of this temperature range might affect our ability to find a thermal dose–effect relationship. The latter will be even more pronounced in multi-institutional trials. A way to minimise the risk of large variation in reported temperature and thermal dose values is to implement an unambiguous and well-thought-out temperature measuring and reporting protocols in clinical trials.
Discussion
Dose prescription in oncology varies widely
In radiotherapy the radiation dose is highly accurately prescribed and delivered to the PTV (gross tumour volume plus margins). Under normal conditions 100% of the PTV receives at least 95% of the prescribed radiation dose, with 107% as the maximum dose in the PTV acceptable [Citation102]. In medical oncology dose prescription is in milligrams of drugs per kilogram of body weight or per metre squared of body surface of the patient. Although this type of aggregated dose prescription is accurate, the dose delivered is highly inaccurate and most of the drugs will remain in healthy tissue causing side effects. Not only is the amount of the drug reaching the tumour often unknown, often only less than 1% of the total amount of drugs is delivered to the tumour [Citation103]. For hyperthermia the situation lies between these two extremes. In general, the electromagnetic energy is locally applied with the aim of preferentially heating the target volume. The energy absorption is highly dependent on the tissue type (fat, bone, muscle, skin, tumour) resulting in a heterogeneous distribution with little or no ability to homogenise the absorption pattern. Of even larger impact on the resulting temperature increase is the intrinsic heterogeneous blood flow in the tumour and the normal tissues and its time dynamic behaviour. As a consequence, the temperature distribution over the target volume has a wide variation ranging from 39 °C minimum value in well perfused regions to 44 °C or higher in necrotic parts of the tumour and on top, still varying over time. The challenge for the hyperthermia community is to aggregate the time-varying three dimensional temperature data set into a single number, preferable one that correlates with the biological mechanism(s) involved in hyperthermia. For this purpose the CEM43 model was introduced.
In recent decades the use of CEM43, more specifically CEM43T90, has been widely used for thermal dose–effect relationship studies. An amenity of the CEM43 model is that it provides a very elegant method to convert the history of the temperature profile in a single number of minutes at the selected reference temperature, i.e. 43 °C. The concept works well in cell cultures as long as thermotolerance is avoided [Citation28–31] and has also been shown effective to define CEM43 threshold for thermal damage of normal tissue in larger animals [Citation64]. The CEM43 concept, however, also holds a number of weaknesses when it is used to predict treatment efficacy of the combination of radiotherapy plus hyperthermia. As is explained in the previous sections and summarised in , a number of thermally induced biological effects do not have an exponential response with thermal dose, i.e. time–temperature dependence, or even show opposite effects when a certain thermal dose is exceeded. Moreover, the CEM43 model has no predictive value for the radiation sensitivity of the tumour. A further complicating factor is that due to the heterogeneous blood flow in the tumour, wide temperature differences will exists over the tumour volume, resulting in the simultaneous occurrence of different and sometimes opposing biological effects (see ).
Figure 2. Graphical representation of biological and physiological mechanisms involved in hyperthermia and the range in which they occur. The colour indicates the strength of the effect: blue is low, red is high. Blood flow and oxygenation start to increase at low temperatures, reach a maximum and decrease again when the temperature passes the thermal threshold for stasis (blue→ red →blue). White means unknown.
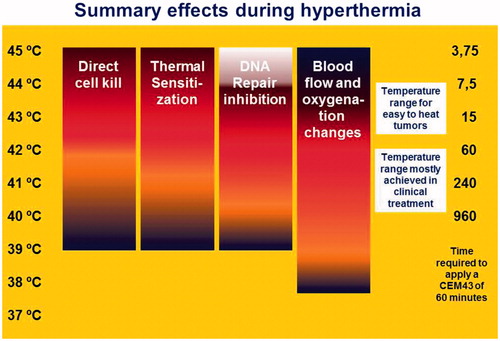
Table 1. Relation of CEM43 with response.
Way forward in clinically practical thermal dosimetry
The question of how to continue in our quest towards the clinically practical thermal dose–effect parameter is not easy to answer as the shortcomings in the CEM43 model will not be easy to solve. Vaupel and Kelleher [Citation53] provide strong arguments to doubt that the changes in blood flow or oxygenation (direct and delayed) as seen in rodents will also occur in human tumours, especially when taking into consideration the relatively low average temperatures commonly achieved in clinical hyperthermia. Pearce [Citation25] proposes the use of multi-parameter models. Although he too provides a number of good arguments, the principal shortcomings of the heterogeneous temperature distribution, i.e. the lack of accurate knowledge of 3D temperature distribution, will involve the same obstacles as are present for the CEM43 model. The approach of Kok et al. [Citation104] deserves special attention as they use the predicted 3D temperature distribution of hyperthermia treatment modelling [Citation105,Citation106] to calculate a biological equivalent thermal–radiotherapy dose. Clinical verification of this approach still needs to be done, but, as indicated by Kok et al. [Citation104], this model also depends heavily on the reliability of the predicted temperature distribution.
To make progress in temperature data collection high expectations exists on combining radiofrequency heating with non-invasive thermometry by MRI in a single hybrid system. Two groups have reported very encouraging correlations between MR temperatures and invasive temperature measurements in patients with soft tissue sarcoma [Citation107,Citation108]. Further confirmation of these findings for different tumour pathologies and locations is required before final conclusions can be drawn on the potential to use the 3D MR temperature data for dose–effect relationship studies. In principal, 3D MR thermometry could solve the problem of insufficient temperature data.
From a mathematical point of view, the challenges on the technical quality of the temperature measurement (e.g. accuracy, good spatial coverage, sampling rate) and many other factors also exist for other temperature-based thermal dose parameters such as minutes above an index temperature: T90, T50, T20, for example. Hence, there is no reason to favour any one of these thermal dose parameters in particular as they also lack the integrated information over time. Besides, as shown in , not all, but still many of the mechanisms related to temperature show an exponential behaviour.
As elucidated earlier, the overall results of the clinical studies towards a thermal dose–effect relationship in the combined treatment of radiotherapy plus hyperthermia show diffuse outcomes, with no definitive conclusion. It is, however, tempting to give higher relevance to the study performed by Franckena et al. [Citation15] based upon the number of patients involved in the analyses (420) and to the studies of Jones et al. [Citation14] and Thrall et al. [Citation89], based on their prospective study design. These studies provide a clear indication that researching thermal dose–effect relationships using the CEM43 model should continue and would be best performed in patient groups with a single pathology and a low variation in tumour size and heterogeneity.
Combined efforts needed for progress in thermal dose–effect studies
Strict treatment schedules with fixed intervals and extensive four-dimensional temperature registration are important requirements to enhance the probability of detecting a thermal dose–effect relationship. As availability of MR hybrid hyperthermia systems is still low, a combined effort of centres with hybrid systems is strongly recommended in order to reach the study size required to fulfil all previously mentioned demands for comparable tumour pathology, size, heatability and quality of the temperature data set. However, the challenge of how to convert the heterogeneous thermal dose into a single number remains. The answer to this problem might be found when additional clinical thermal dose–effect studies are performed under the strict regulations as mentioned earlier.
In addition, strong additional efforts are needed to investigate the biological and physiological changes induced by hyperthermia in tumour and normal tissue [Citation53,Citation57]. Here too, the growing availability of the hybrid MR hyperthermia system should be a strong stimulus to enhance our understanding of which hyperthermia-induced biological and physiological mechanisms are key for a good treatment outcome and to indicate the direction and aims of the future research in hyperthermia. If we do not make this effort in thermal dose–effect studies, combined with assessment of the temperature modulated biological and physiological responses, our knowledge on the clinical application of hyperthermia will stall at the level we already achieved in 1990 [Citation23].
Conclusion
In the current clinical practice of hyperthermia tumour temperatures are heterogeneous and show substantial variation over the treatment time. Hyperthermia applied in the mild temperature range causes a plethora of biological and physiological effects, of which the majority have an exponential correlation with temperature and time. Even though the effect can be the opposite once a thermal threshold has been passed, the exponential relationship still exists. CEM43 provides a practical solution to integrate the temperature–time history in an exponential manner and therefore a thermal dose parameter that is related to the biological and physiological mechanisms involved in hyperthermia. The challenge in the application of CEM43 is in accurate and representative measurement of the temperature distribution. This is, however, a generic challenge for all temperature-based dose parameters and will certainly also apply to non-temperature-based parameters. In future, thermal dose–effect studies should be performed in homogeneous patient populations with respect to tumour pathology, size and heatability, following strict treatment schedules with fixed intervals and applying high quality thermometry aiming at collecting an extensive four-dimensional temperature data set.
Declaration of interest
This study is supported by the Dutch Cancer Society grants EMCR 2012-5472 and DDHK 2013-6072. The authors alone are responsible for the content and writing of the paper.
References
- Overgaard J. Editorial. The heat is (still) on – the past and future of hyperthermic radiation oncology. Radiother Oncol 2013;109:185–7
- Januszewski A, Stebbing J. Letter. Hyperthermia in cancer: is it coming of age? Lancet Oncol 2014;15:565–6
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond HM, Odijk H, et al. Temperature-controlled induction of BRCA2 degradation and homologous recombination deficiency sensitizes cancer cells to PARP-1 inhibition. Proc Natl Acad Sci 2011;108:9851–6
- Kampinga HH. Cell biological effects on hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia 2006;22:191–6
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biological rationale for thermal therapy. Int J Hyperthermia 2005;21:779–90
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Issels RD, Lindner LH, Ghadjar P, Reichardt P, Hohenberger P, Verweij J, et al. Improved overall survival by adding regional hyperthermia to neoadjuvant chemotherapy in patients with localized high-risk soft tissue sarcoma (HR-STS): long-term outcomes of the EORTC 62961/ESHO randomized phase III study. Late breaking abstract. European Cancer Congress, Vienna, Austria, 25–29 September 2015
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol (CCT) 1991;14:133–41
- Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors: a prospectively controlled randomized study by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1996;34:1097–104
- Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, Oliynychenko P, et al. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2005;61:145–53
- Bicher HI, Wolfstein RS, Lewinsky BS, Frey HS, Fingerhut AG. Microwave hyperthermia as an adjunct to radiation therapy: summary experience of 256 multifraction treatment cases. Int J Radiat Oncol Biol Phys 1986;12:1667–71
- Van der Zee J, Peer-Valstar JN, Rietveld PJM, de Graaf-Strukowska L, van Rhoon GC. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys 1998;40:1205–12
- Sneed PK, Dewhirst MW, Samulski T, Blivin J, Prosnitz LR. Should interstitial thermometry be used for deep hyperthermia? Int J Radiat Oncol Biol Phys 1998;40:1015–17
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005;23:3079–85
- Franckena M, Fatehi D, de Bruijne M, Canters RAM, van Norden Y, Mens JW, et al. Hyperthermia dose–effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 2009;45:1969–78
- Fotopoulou C, Cho CH, Kraetschell R, Gellermann J, Wust P, Lichtenegger W, et al. Regional abdominal hyperthermia combined with systemic chemotherapy for the treatment of patients with ovarium cancer relapse: results of a pilot study. Int J Hyperthermia 2010;26:118–26
- Kapp DS, Cox RS. Thermal treatment parameters are most predictive of outcome in patients with single tumor nodules per treatment field in recurrent adenocarcinoma of the breast [Comments]. Int J Radiat Oncol Biol Phys 1995;33:887–99
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993;25:289–97
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: data from a phase III trial. Int J Radiat Oncol Biol Phys 1997;39:371–80
- Issels RD, Prenninger SW, Nagele A, Boehm E, Sauer H, Jauch KW, et al. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: a phase II study. J Clin Oncol 1990;8:1818–29
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, et al. Preoperative radio-chemotherapy in locally advanced recurrent rectal cancer: regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000;48:381–91
- De Bruijne M, van der Holt B, van Rhoon GC, van der Zee J. Evaluation of CEM43T90 thermal dose in superficial hyperthermia. A retrospective analysis. Strahlenther Onkol 2010;186:436–43
- Field SB, Raaphorst GP. Thermal dose. In: Field SB, Hand JW, editors. An Introduction to the Practical Aspects of Clinical Hyperthermia. London: Taylor & Francis; 1990. pp 69–76
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994;10:457–83
- Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage process. Int J Hyperthermia 2013;29:262–80
- Field SB, Bleehen NM. Hyperthermia in the treatment of cancer. Cancer Treat Rev 1979;6:63–94
- Field SB, Morris CC. The relationship between heating time and temperature: its relevance to clinical hyperthermia. Radiother Oncol 1983;1:179–86
- Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977;123:463–74
- Sapareto SA, Hopwood LE, Dewey WC. Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res 1978;73:221–33
- Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia 2003;19:252–66
- Roti Roti JL. Cellular responses to hyperthermia (40–46 °C): cell killing and molecular events. Int J Hyperthermia 2008;24:3–15
- Field SB. Hyperthermia in the treatment of cancer. Phys Med Biol 1987;32:789–811
- Westra A. De invloed van straling op het vermogen tot proliferatie van in vitro gekweekte zoogdiercellen (The inpact of radiation on the proliferation potential of in vitro grown mammalian cells). PhD thesis, University of Amsterdam, 1971
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol 2001;77:399–408
- Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol 2015;10:165–78
- Konings AWT. Interaction of heat and radiation in vitro and in vivo. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Principles and Practice of Thermoradiotherapy and Thermochemotherapy. Berlin: Springer Verlag; 1995. pp 89–102
- Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 2012;28:509–17
- Vujaskovic Z, Song CW. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia 2004;20:163–74
- Reinhold HS, van den Berg AP. Effects of hyperthermia on blood flow and metabolism. In: Field SB, Hand JW, editors. An Introduction to the Practical Aspects of Clinical Hyperthermia. London: Taylor & Francis; 1990. pp 77–106
- Lokshina AM, Song CW, Rhee JG, Levitt SH. Effect of fractionated heating on the blood flow in normal tissues. Int J Hyperthermia 1985;1:117–29
- Song CW, Patten MS, Chelstrom LM, Rhee JG, Levitt SH. Effect of multiple heatings on the blood flow in RIF-1 tumours, skin and muscle of C3H mice. Int J Hyperthermia 1987;3:535–45
- Song CW, Choi IB, Nah BS, Sahu SK, Osborn JL. Microvasculature and perfusion in normal tissue and tumors. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Principles and Practice of Thermoradiotherapy and Thermochemotherapy. Berlin: Springer Verlag; 1995. pp 139–56
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21:761–67
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006;22:197–203
- Reinhold HS, Endrich B. Tumour microcirculation as a target for hyperthermia. Int J Hyperthermia 1986;2:111–37
- Van den Berg-Blok AE, Reinhold HS. Time–temperature relationship for hyperthermia induced stoppage of the microcirculation in tumors. Int J Radiat Oncol Biol Phys 1984;10:737–40
- Vaupel P, Kallinowski F. Physiological effects of hyperthermia. Recent results in cancer research 1987;104:71–109
- Iwata K, Shakil A, Hur WJ, Makepeace CM, Griffin RJ, Song CW. Tumor pO2 can be increased markedly by mild hyperthermia. Br J Cancer 1996;74:S217–221
- Horsman MR, Overgaard J. Can mild hyperthermia improve tumor oxygenation? Int J Hyperthermia 1997;13:141–7
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996;56:5347–50
- Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, et al. Thermochemotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 2004;10:4287–93
- Vaupel P, Kelleher DK. Pathophysiological and vascular characteristic of tumours and their importance for hyperthermia: heterogeneity is the key issue. Int J Hyperthermia 2010;26:211–23
- Song CW, Park H, Griffin RJ. Review: improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001;155:515–28
- Shakil A, Osborn JL, Song CW. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Radiat Oncol Biol Phys 1999;43:859–65
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000;46:179–85
- Griffin RJ, Dings RPM, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: role of tumor vascular thermotolerance and relevant physiological factors. Int J Hyperthermia 2010;26:265–3
- Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. PNAS 2010;107:20477–82
- Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncologica 1995;34:313–16
- Myerson RJ, Singh AK, Bigott HM, Cha B, Engelbach JA, Kim J, et al. Monitoring the effect of mild hyperthermia on tumour hypoxia by Cu-ATSM PET scanning. Int J Hyperthermia 2006;22:93–115
- Dewhirst MW, Sim DA, Gross J, Kundrat MA. Effect of heating rate and normal tissue microcirculatory function. In: Overgaard J, ed. Hyperthermic Oncology, Vol1. London: Taylor & Francis; 1984. pp 177–80
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 2011;26:1–26
- Van Rhoon GC, Samaras T, Yarmolenko PS, Dewhirst MW, Neufeld E, Kuster N. CEM43 °C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol 2013;23:2215–27
- Vaupel P. Pathophysiological mechanisms of hyperthermia in cancer therapy. In: Gautherie M, editor. Biological Basis of Oncologic Thermotherapy. Berlin: Springer; 1991. pp 73–134
- Nah BS, Choi IB, Oh WY, Osborn JL, Song CW. Vascular thermal adaptation in tumors and normal tissue is rats. Int J Radiat Oncol Biol Phys 1996;35:95–101
- Song CW. Effect of local hyperthermia in blood flow and microenvironment: a review. Cancer Res 1984;44:S4721–30
- Rappaport DS, Song CW. Blood flow and intravascular volume of mammary adenocarcinoma 13762A and normal tissues of rat during and following hyperthermia. Int J Radiat Oncol Biol Phys 1983;9:539–47
- Song CW, Rhee JG, Levitt SH. Blood flow in normal tissues and tumors during hyperthermia. J Natl Cancer Inst 1980;64:119–24
- van der Zee J, Vujaskovic Z, Kondo M, Sugahara T. The Kadota Fund International Forum 2004 – clinical group consensus. Int J Hyperthermia 2008;24:111–22
- Huilgol NG, Gupta S, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia 2010;26:21–5
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: a phase III clinical study. Int J Hyperthermia 2011;27:180–6
- Zhao C, Chen J, Yu B, Chen X. Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non-invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol 2014;90:853–8
- Datta NR, Gómez Ordóñez S, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53
- Cihoric N, Tsikkinis A, van Rhoon GC, Crezee J, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia early online 2015;31:609–14
- Perez CA, Gillespie B, Pajak T, Hornback NB, Emami B, Rubin P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: a report by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1989;16:551–8
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the RTOG. Am J Clin Oncol 1991;14:133–41
- Van der Zee J, Van der Holt B, Rietveld PJM, Helle PA, Wijnmaalen AJ, Van Putten WLJ, et al. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer 1999;79:483–90
- Jones EL, Prosnitz LR, Dewhirst MW, Vujaskovic Z, Samulski TV, Oleson JR, et al. In regard to Vasanthan et al. Int J Radiat Oncol Biol Phys 2005;63:644
- Van der Zee J, Van Rhoon GC, Wust P. In regard to Dr Vasanthan et al. Int J Radiat Oncol Biol Phys 2005;62:940–1
- Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, et al. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: prognostic factors in 196 tumors. Int J Radiat Oncol Biol Phys 1998;40:365–75
- Leopold KA, Dewhirst MW, Samulski TV, Dodge RK, George SL, Blivin JL, et al. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1993;25:841–7
- Dinges S, Harder C, Wurm R, Buchali A, Blohmer J, Gellermann J, et al. Combined treatment of inoperable carcinomas of the uterine cervix with radiotherapy and regional hyperthermia. Strahlenther Onkol 1998;174:517–21
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma: a multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996;12:3–20
- Wust P, Stahl H, Dieckmann K, Scheller S, Loeffel J, Riess HJahnke V, et al. Local hyperthermia of N2/N3 cervical lymph node metastases: Correlation of technical and thermal parameters with response. Int J Radiat Oncol Biol Phys 1996;34:635–46
- Perez CA, Patterson JH, Emami B. Evaluation of 45 °C hyperthermia and irradiation. Studies in a murine rhabdomyosarcoma model. Am J Clin Oncol 1993;16:469–76
- Tilly W, Wust P, Rau B, Harder C, Gellermann J, Schlag P, et al. Temperature data and specific absorption rates in pelvic tumours: predictive factors and correlations. Int J Hyperthermia 2001;17:172–88
- Maguire PD, Samulski TV, Prosnitz LR, Jones EL, Rosner GL, Powers B, et al. A phase II trial testing the thermal dose parameter CEM43°T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int J Hyperthermia 2001;17:283–90
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, et al. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiation therapy. Clin Cancer Res 2005;11:5206–14
- Lagendijk JJW, van Rhoon GC, Hornsleth SN, Wust P, de Leeuw ACC, Schneider CJ J, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998;14:125–33
- Dewhirst MW, Phillips TL, Samulski TV, Stauffer P, Shrivastava P, Paliwal B, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys 1990;18:1249–59
- Bruggmoser G, Bauchowitz S, Canters R, Crezee J, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011;187:605–9
- Bruggmoser G, Bauchowitz S, Canters R, Crezee J, Ehmann M, Gellermann J, et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: quality management in regional deep hyperthermia. Strahlenther Onkol 2012;188(Suppl2):198–211
- Wust P, Gellermann J, Harder C, Tilly W, Rau B, Dinges S, et al. Rationale for using invasive thermometry for regional hyperthermia of pelvic tumors. Int J Radiat Oncol Biol Phys 1998;41:1129–37
- Van Der Gaag ML, De Bruijne M, Samaras T, Van Der Zee J, Van Rhoon GC. Development of a guideline for the water bolus temperature in superficial hyperthermia. Int J Hyperthermia 2006;22:637–56
- Arunachalam K, Maccarini PF, Stauffer PR. A thermal monitoring sheet with low influence from adjacent waterbolus for tissue surface thermometry during clinical hyperthermia. IEEE Tran Biomed Eng 2008;55:2397–406
- Birkelund Y, Jacobsen S, Arunachalam K, Maccarini P, Stauffer PR. Flow patterns and heat convection in a rectangular water bolus for use in superficial hyperthermia. Phys Med Biol 2009;54:3937–53
- Arunachalam K, Maccarini PF, Craciunescu OI, Schlorff JL, Stauffer PR. Thermal characteristics of thermobrachytherapy surface applicators for treating chest wall recurrence. Phys Med Biol 2010;55:1949–69
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 2007;183:479–86
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, et al. First results of triple modality treatment by combining radiotherapy, chemotherapy and hyperthermia for treatment of stage IIB-III-IVA cervical cancer. Cancer 2005;104:763–70
- Fatehi D, van der Zee J, van der Wal E, van Wieringen WN, van Rhoon GC. Temperature data analysis for 22 patients with advanced cervical carcinoma treated in Rotterdam using radiotherapy, hyperthermia and chemotherapy: a reference point is needed. Int J Hyperthermia 2006;22:353–63
- Breedveld S. Towards automated treatment planning in radiotherapy. Doctoral thesis, Erasmus University, Rotterdam, the Netherlands, 2013
- Laginha KM, Verwoert S, Charrois GJR, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murice breast cancer tumors. Clin Cancer Res 2005;11:6944–9
- Kok HP, Crezee J, Franken NAP, Stalpers LJA, Barendsen GW, Bel A. Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distribution. Int J Rad Oncol Biol Phys 2014;88:739–45
- Crezee J, van Leeuwen CM, Oei AL, Stalpers LJA, Bel A, Franken NA, et al. Thermoradiotherapy planning: integration in routine clinical practice. Int J Hyperthermia 2015 Dec 15, 1–9 early online
- Verhaart RF, Verduijn GM, Fortunati V, Rijnen Z, van Walsum T, Veenland JF, et al. Accurate 3D temperature dosimetry during hyperthermia therapy by combining invasive measurements and patient specific simulations. Int J Hyperthermia 2015;31:686–92
- Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia correlation with response and direct thermometry. Cancer 2006;107:1373–82
- Craciunescu OI, Stauffer PR, Soher BJ, Wyatt CR, Arabe O, Maccarini P, et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med Phys 2009;36;4848–58

