Abstract
Purpose: The aim of this study is to investigate endoluminal ultrasound applicator configurations for volumetric thermal ablation and hyperthermia of pancreatic tumours using 3D acoustic and biothermal finite element models. Materials and methods: Parametric studies compared endoluminal heating performance for varying applicator transducer configurations (planar, curvilinear-focused, or radial-diverging), frequencies (1–5 MHz), and anatomical conditions. Patient-specific pancreatic head and body tumour models were used to evaluate feasibility of generating hyperthermia and thermal ablation using an applicator positioned in the duodenal or stomach lumen. Temperature and thermal dose were calculated to define ablation (> 240 EM43 °C) and moderate hyperthermia (40–45 °C) boundaries, and to assess sparing of sensitive tissues. Proportional-integral control was incorporated to regulate maximum temperature to 70–80 °C for ablation and 45 °C for hyperthermia in target regions.
Results: Parametric studies indicated that 1–3 MHz planar transducers are the most suitable for volumetric ablation, producing 5–8 cm3 lesion volumes for a stationary 5-min sonication. Curvilinear-focused geometries produce more localised ablation to 20–45 mm depth from the GI tract and enhance thermal sparing (Tmax < 42 °C) of the luminal wall. Patient anatomy simulations show feasibility in ablating 60.1–92.9% of head/body tumour volumes (4.3–37.2 cm3) with dose < 15 EM43 °C in the luminal wall for 18–48 min treatment durations, using 1–3 applicator placements in GI lumen. For hyperthermia, planar and radial-diverging transducers could maintain up to 8 cm3 and 15 cm3 of tissue, respectively, between 40–45 °C for a single applicator placement. Conclusions: Modelling studies indicate the feasibility of endoluminal ultrasound for volumetric thermal ablation or hyperthermia treatment of pancreatic tumour tissue.
Introduction
Pancreatic cancer causes the fourth highest number of cancer-related mortalities in the USA, and the current 5-year survival rate is 4% [Citation1]. At clinical presentation, over 80% of patients are diagnosed with locally advanced or metastatic disease, which precludes curative treatment in the form of surgical resection [Citation1]. The current standard clinical treatment for these patients involves gemcitabine-based chemotherapy with or without adjuvant radiation therapy, but these therapies have been shown to result in only modest survival and palliative benefit [Citation2]. To relieve pain-related symptoms, which affects over 90% of advanced-staged patients, oral analgesics are typically administered; however, these require around-the-clock dosing for suitable palliation and have debilitating side effects, such as constipation [Citation3,Citation4]. Other current palliative techniques, such as alcohol ablation of the coeliac plexus, have limited efficacy in some patients and may cause severe complications [Citation4].
Thermal ablation has been applied for treatment of many malignancies, including cancers of the liver, lung, prostate, uterus, bone, and others [Citation5]. It has also been demonstrated to provide palliative relief in patients with advanced pancreatic cancer through tumour debulking and neurolysis/denervation mechanisms, even in cases where only partial tumour volume ablation is achieved [Citation6–9]. Ablation of locally advanced and metastatic pancreatic cancer can also potentially produce a survival advantage, as highlighted by recent efforts integrating high-intensity focused ultrasound (HIFU) ablation with chemotherapy [Citation10]. Many ablative modalities, including radiofrequency ablation (RFA), cryoablation, microwave ablation, HIFU, photodynamic therapy, and electroporation are currently being developed or clinically investigated for treatment of pancreatic cancer [Citation6]. With the exception of HIFU, all of these methods rely on invasive percutaneous or interstitial operation, which can be difficult to safely perform in the pancreas due to the close proximity of sensitive bowel tissues and major blood vessels, leading to many treatment-related complications [Citation11]. As a result, better RFA treatment safety has been achieved by applying it in a laparotomy setting, which is highly invasive [Citation11,Citation12]. While HIFU is much less invasive than these other techniques, its application through the abdomen can be challenging and is precluded in many patients due to respiratory motion and obstructions in the acoustic pathway to the pancreas, caused by intervening organs, bowel, and gas within the GI tract [Citation7,Citation8].
In addition to thermal ablation, there is also potential clinical benefit in developing hyperthermia treatments for advanced pancreatic cancer. Pancreatic tumours are often characterised by hypovascularity, hypoxia, and desmoplastic stroma, which have been hypothesised to minimise the effectiveness of standard chemotherapy and radiation treatments [Citation13]. By transiently increasing the perfusion and oxygenation of treated tissues, hyperthermia has been shown to be an effective adjuvant therapy for both chemotherapy and radiation treatments in many cancer models [Citation14–16]. Clinical studies evaluating the efficacy of combining regional hyperthermia and gemcitabine with or without cisplatin for patients with advanced pancreatic cancer have begun in both Europe and Japan [Citation17,Citation18]. Hyperthermia may also enhance the delivery of encapsulated drugs into desmoplastic pancreatic tumours through increasing local perfusion and, in the case of thermally sensitive drug-containing liposomes, directly triggering the release of the drug at the target site [Citation19].
Endoluminal and intracavitary catheter-based ultrasound applicators can provide a minimally invasive solution for delivery of thermal ablation or hyperthermia to tumours accessible from body lumens. Significant advantages of this ultrasound-based approach for thermal therapy include the high degree of control over the spatial distribution of energy as compared to other modalities, direct localisation of the energy source and therapy adjacent to the target zone, as well as compatibility with diagnostic ultrasound or MR guidance techniques for possible real-time treatment monitoring and feedback control [Citation20,Citation21]. Specific applicator configurations for placement in the upper gastrointestinal (GI) tract have been applied clinically for endoluminal ablation of oesophageal and biliary tumours [Citation22,Citation23]. An endoscope with a miniature spherically focused transducer at the tip has also been developed and experimentally validated to create ablation lesions in porcine pancreas and liver under endoscopic ultrasound guidance [Citation24,Citation25].
Simulation studies incorporating parametric analysis and patient-specific anatomical models have been applied for device design and performance evaluations of endoluminal and interstitial ultrasound technologies for thermal therapy (e.g. [Citation26–33]). The objectives of this study are to investigate the feasibility and to characterise the performance of delivering volumetric thermal therapy to target regions within the pancreas using an endoluminal ultrasound applicator positioned within the GI tract. Three-dimensional acoustic and biothermal finite element models were developed to calculate power deposition patterns and resulting temperature and thermal dose distributions for both ablation and hyperthermia treatment paradigms. Full 3D multi-component models were used to perform parametric studies across applicator design parameters and anatomical variations to determine favourable designs and establish ranges of expected performance. Comprehensive patient-specific models based on segmentation of CT images of pancreatic tumours and surrounding anatomy were developed to serve as generalised anatomy models that bracket a range of representative geometries and potential target volumes. Using specific device configurations, as informed by the parametric studies, both thermal ablation and hyperthermia treatments were independently simulated in these models to evaluate delivery strategies and capabilities for achieving volumetric coverage of pancreatic tumour tissue while minimising exposure to surrounding/intervening normal tissues.
Materials and methods
Endoluminal ultrasound applicator
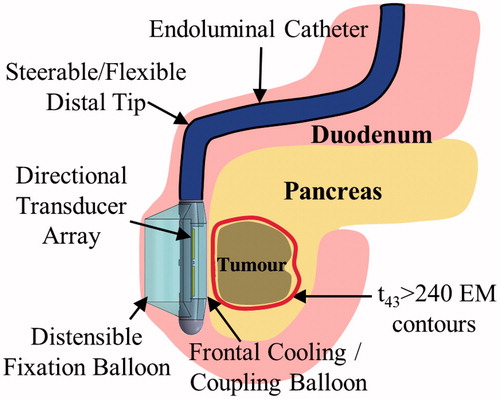
As shown in , the endoluminal applicator would be inserted orally through the oesophagus, and, akin to an endoscope, positioned in the stomach or duodenal lumen adjacent to the tumour-bearing region of the pancreas. Acoustic energy would be directed through the GI luminal wall into the tumour by an ultrasound transducer array on the distal tip of the applicator. A balloon covering the transducers, containing circulating cooled water, would provide coupling of the acoustic energy into the adjacent tissue and cool the wall tissue to protect it from thermal injury.
Figure 1. Schema and concepts of an endoluminal ultrasound applicator positioned in the GI tract for thermal therapy of pancreatic tumours. The applicator is illustrated as positioned in the duodenum for sonication of tumours in the head of the pancreas, following placement and insertion strategies common in endoscopy. Transducer arrays are configured for focused or diffuse patterns to provide a high degree of spatial control and volumetric heating.
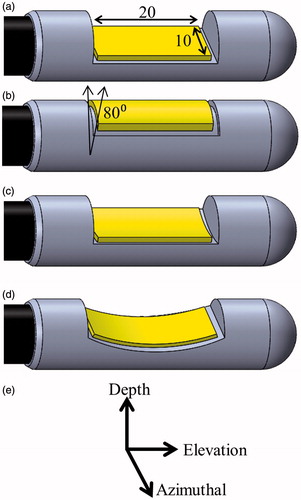
The dimensions of the applicator and transducer assembly were conservatively chosen to be within a 15-mm diameter limit, following general constraints of the gastrointestinal anatomy on dimensions of endoscopic devices [Citation34]. As illustrated in , four separate transducer configurations were considered for this study: planar, tubular section, curvilinear along the azimuthal axis of the applicator (lightly focused), and curvilinear along the elevation axis (strongly focused). The dimensions of the planar and curvilinear transducers were modelled as 10 mm width × 20 mm length, and the tubular transducer was modelled with a 20 mm length, 80° active acoustic sector angle, and 6 mm radius of curvature. These geometries were chosen both due to their practical implementation and to examine diffuse versus focal acoustic distributions. The distal tip of the applicator transducer assembly was modelled as a 12 mm diameter × 50 mm length cylinder with a single central transducer and the cooling balloon boundary situated 6.5 mm from the transducer surface, based off existing devices developed by our group.
Figure 2. Considered transducer (yellow) configurations: (a) planar, (b) tubular section, (c) lightly focused curvilinear along the azimuthal direction, and (d) strongly focused curvilinear along the elevation direction, with respect to the (e) transducer coordinate system. The planar and curvilinear transducers are 20 × 10 mm along the elevation and azimuthal directions, and the tubular section is an 80° sector with a 6-mm radius of curvature and a 20-mm length.
Theory
Three-dimensional acoustic and bioheat transfer models were implemented to calculate the acoustic intensity patterns and resulting transient temperature and thermal dose profiles produced by ultrasound applicators in an endoluminal anatomical setting. The preliminary modelling framework for endoluminal ablation of pancreatic tumours was developed by Prakash et al. [Citation35], and expanded upon herein. Temperature distributions were simulated using an implicit finite element method (FEM) solver (COMSOL Multiphysics 4.3, Burlington, MA) and the Pennes bioheat transfer equation (EquationEquation 1(1) ) [Citation36]:
(1)
where ρ (kg m−3) is tissue density, C (J kg−1 °C−1) is the specific heat of tissue, T ( °C) is tissue temperature, k (W m−1 °C−1) is thermal conductivity, ωb (kg m−3 s−1) is blood perfusion, Cb (J kg−1 °C−1) is the specific heat of blood, and Tb (°C) is capillary blood temperature (37 °C). Qac (W m−3) is the acoustic heat deposition in tissue, and is derived from the acoustic pressure field (EquationEquation 2
(2) ):
(2)
where α (Np m−1) is the acoustic absorption coefficient and p0 (Pa) is the acoustic pressure amplitude. The acoustic absorption coefficient was approximated as equal to the acoustic attenuation coefficient μ (Np m−1) for each tissue, as all scattered energy was assumed to be absorbed locally. The acoustic pressure field was calculated for all considered transducer configurations using the Rayleigh-Sommerfeld diffraction integral, through a MATLAB (MathWorks, Natick, MA) implementation of the rectangular radiator approximation (EquationEquation 3
(3) ) [Citation37]:
(3)
where c (m s−1) is the speed of sound, Δw and Δh (m) are the width and height of each rectangular sub-element, λ (m) is the wavelength, N is the total number of radiator sub-elements, R (m) is the distance from the radiator sub-element to the point in the tissue, k (m−1) is the wavenumber, and xn and yn (m) are the difference in azimuthal and elevation coordinates between the tissue point and the radiator center. un (m s−1) is the surface velocity for element n, and is calculated using the expression below (EquationEquation 4
(4) ):
(4)
where I is the transducer surface intensity (W m−2), which is proportional to the electrical power applied to the transducer by the acoustic efficiency.
Temperature distributions were calculated using a direct implicit stationary solver (PARDISO) in COMSOL. Dirichlet boundary conditions constrained the outer boundaries of the tissue to 37 °C, and a convective heat flux boundary condition was imposed at the balloon–tissue interface, with a heat transfer coefficient of h = 500 (W m−2 °C−1) [Citation38]. The range of temperatures for the cooling water flow was 7–25 °C, based on practical ranges typically used for ablation and hyperthermia devices. A cooling temperature of 7–10 °C was used for the ablation simulations to more efficiently reduce heating of the luminal wall. Water cooling at 20–25 °C was applied in the hyperthermia simulations, with inherently lower applied power levels and resulting temperature and thermal dose, to avoid over-cooling and to extend therapeutic temperatures to tissue regions close to the luminal wall. Heterogeneous tissue properties and perfusion values for stomach wall, duodenal wall, pancreas, tumour, and surrounding soft tissues were incorporated and are shown in [Citation39–44]. The material properties of pancreatic tumour tissue were assumed to be the same as pancreatic tissue, except for the attenuation coefficient where values equal to and slightly (1.25 times) higher than normal pancreatic tissue were included. This estimated higher value was used to account for the higher collagen content and fibrotic nature of the pancreatic tumour stroma, as collagen density has been shown to correlate with acoustic attenuation in other soft tissues [Citation2,Citation45,Citation46]. While a range of attenuation values for pancreatic tissue was extracted from literature, and is specifically examined along with the corresponding tumour attenuation value in the parametric studies, all other modelling incorporated the highest values of these ranges, as shown in , to give the most conservative estimates of achievable thermal lesion volumes and heating penetration. Proportional-set pointintegral (PI) feedback control of the applied power was integrated into the transient thermal solver, with the set point evaluated between 70–80 °C to control the maximum temperature in the tumour. This controller was implemented to provide a standard point of comparison between simulations and to simulate a feasible feedback control scheme for treatment monitoring under either invasive thermometry or MR temperature imaging guidance [Citation47]. Thermal dose distributions were calculated using the Sapareto-Dewey formulation (EquationEquation 5(5) ) [Citation48]:
(5)
where t43 is the equivalent minutes at 43 °C (EM43 °C), R = 0.5 above 43 °C and 0.25 otherwise, and T is the average temperature at each point over a time interval Δt. The thermally destroyed or ablated tissue region was defined as the tissue in which the dose exceeded 240 EM43 °C, which has been shown to correspond to an approximate threshold for coagulative necrosis in many soft tissues [Citation49–51]. In order to simulate the dynamic cessation of perfusion during ablation, a binary switch was implemented to reduce tissue perfusion from nominal to 0 kg m−3 s−1 during heating when tissue temperature exceeded 52 °C or thermal dose exceeded 300 EM43 °C [Citation52].
Table 1. Material properties used in acoustic and biothermal modelling.
Parametric studies
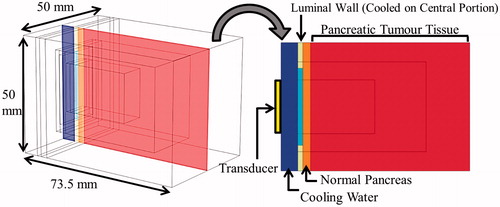
A simplified 3D model of a pancreatic head tumour, with surrounding normal pancreas and intervening tissue regions adjacent to the duodenum (), was created to provide the framework for parametric studies of general applicator design and performance. The applicator was positioned with the transducer offset 6.5 mm from the duodenal wall, with water-cooling of the luminal wall surface adjacent to the applicator cooled by 10 °C or 25 °C water for ablation and hyperthermia treatments, respectively. The proximal edge of the pancreatic tumour tissue was 5 mm deep from the inner duodenal wall, and the overall dimensions of the tissue block were 50 × 50 × 73.5 mm. The duodenal luminal wall was modelled as distended with a 2 mm thickness [Citation53]. Tissue thermal domains closer to the transducer were meshed at a finer resolution (equivalent to the acoustic wavelength) to appropriately capture the steep heating gradients near the applicator. The mesh size gradually increased to a maximum of 4 mm at the peripheral edges of the model. The acoustic calculations to obtain Qac were performed on a finer mesh (quarter-wavelength element length), which was then decimated to fit the thermal solver mesh. Convergence studies for both acoustic and bioheat transfer modelling were performed to ensure that the mesh element sizes were sufficiently small for accuracy and solution stability.
Figure 3. 3D generalised pancreatic tumour model employed for parametric studies of endoluminal ultrasound thermal therapy.
For parametric studies of ablation, the feedback controller was set to limit maximum tumour temperature to 80 °C. The following transducer design parameters were varied: the transducer configuration (planar, tubular section, lightly focused curvilinear, or strongly focused curvilinear), frequency (1–5 MHz), and the radius of curvature (ROC) for curvilinear geometries (15–100 mm). In order to capture the range of anatomical variation in adults, the duodenal wall thickness was varied between 1 and 5 mm. A range of values for the attenuation coefficient of pancreatic and tumour tissues, based on values reported in literature, was also incorporated. The range for pancreatic tissue was 4.37f1.2 Np/m – 11.9f 0.78 Np/m, and the tumour attenuation was varied between 1–1.25 times greater than the corresponding pancreatic value [Citation39,Citation54]. The effects of all of these parameters on the penetration depth of the thermal lesion, the total thermal lesion volume, and the maximum luminal wall temperature and thermal dose were evaluated. For each study, transient temperature solutions at a single applicator orientation were obtained with a variable time-stepping scheme for 5 min of total power application and heating time, followed by 3 min of cool-down with continued accrual of thermal dose and thermal spread of temperature contours. The PI controller proportional gain (Kp) and integral gain (Ki) parameters were adjusted for each case (Kp: 0.11–0.28 W °C−1, Ki: 0.0012–0.005 W °C−1 s−1) to achieve a 5-min rise time to the maximum set point temperature. This scheme was selected empirically to minimise power requirements and to set an appropriate time frame for image-guided control such as MRTI. It also resulted in minimal temperature overshoot and reduced thermal exposure of the luminal wall relative to shorter duration, high power schemes.
For parametric studies of hyperthermia, only the planar and tubular transducer configurations were considered, due to their larger volumetric coverage of heating. The frequency was varied between 1–4 MHz for both configurations, and the volumes and dimensions of the 40 °C and 43 °C contours were measured as end points. Simulations were designed to approach pseudo-steady state during 10-min long transient solutions. The controller parameters, Kp and Ki, were chosen to elevate the max temperature to 45 °C within 2–3 min and sustain it for 7–8 additional min. These final isothermal contours approximate the steady-state hyperthermia distributions that could be maintained for desired treatment lengths.
Patient anatomy models
Three-dimensional patient anatomy models were created based on axial CT imaging studies of patients with pancreatic cancer. Four geometries were incorporated, with details of each shown in . These examples, while limited in representing the full scope of disease manifestation, were chosen to capture a range of distinct geometries, in terms of tumour size, location, and proximity to sensitive structures. Example 1 included a ∼2 cm diameter pancreatic head tumour with boundaries immediately adjacent to the duodenum. Example 2 represents a ∼2 cm diameter pancreatic head tumour located deeper (∼5 mm from the inner duodenal wall to closest proximity) in the pancreas and adjacent to the superior mesenteric artery (SMA), pancreatic duct, and bile duct. A strategy of preserving the bile duct during ablation treatment by using a simulated cooling balloon catheter was evaluated in this model. Example 3 represents a very advanced case, consisting of a large (∼5 cm diameter) pancreatic head tumour in close proximity to the aorta, vena cava, portal vein, SMA, and superior mesenteric vein (SMV). While its size and proximity to vasculature may limit its candidacy for thermal therapy treatment, this model is incorporated in the analysis to investigate generalized heating performance and potential strategies that may be applicable in extreme cases. As such, multiple large position changes of the applicator along the duodenum were considered in this model to extend coverage of the tumour. Example 4 included a ∼4 cm diameter pancreatic body tumour peripheral to the stomach, portal vein, SMV, splenic artery, splenic vein, hepatic artery, and the right gastroepiploic vein. In this model sonication from the stomach lumen was simulated with a longer (10 × 30 mm) transducer geometry at two discrete applicator positions. For all cases, the applicator was rotated about the long (elevation) axis during therapy by discrete amounts in order to extend treatment coverage.
Table 2. General anatomical characteristics of patient anatomy models of pancreatic tumours.
Mimics (Materialise, Leuven, Belgium) was used to segment the CT images and generate 3D surface meshes of nearby and relevant anatomical structures, including the tumour, pancreas, duodenum/stomach, luminal wall, ducts, and nearby significant vasculature in each model. Using 3-matic (Mimics), models of the applicator within a 2 cm diameter cooling balloon were positioned in either the duodenum or stomach lumen adjacent to the target volume, and the 3D object contours were converted into finite element meshes. Applicators were empirically positioned into a section of lumen close in proximity to the targeted region, such that large volumetric coverage of tissue could be achieved by rotation or minute translation of the applicator, and such that there were not sensitive structures (such as ducts, vessels) along the beam path. For models with multiple applicator positions, these placements were selected with the additional criteria that they should afford minimal overlap of thermal coverage. Interior luminal surfaces adjacent to the applicator were modelled as smooth, under the assumption that the cooling balloon provides distension of luminal folds with no trapped gas present at the interface. Convergence studies of mesh size were performed to ensure solution stability and accuracy, with a maximum mesh size of 0.75 mm in the region adjacent to the transducer surface extending 3–4 cm over the tumour volume, gradually transitioning to a maximum mesh size of 5–8 mm at the outer peripheries of the models. Each model consisted of 1.5–3 million elements, and Example 3 had three separate meshes which corresponded to the three distinct positions of the applicator.
Specific transducer configurations, empirically selected according to the model geometry and informed by the general performance characteristics revealed from the parametric studies, were chosen for ablation and hyperthermia simulations for each patient anatomy model. Each finite element mesh was imported into MATLAB for calculation of the acoustic intensity field using the rectangular radiator method, and all biothermal simulations were performed in COMSOL 4.3. Heterogeneous tissue properties, as shown in , were incorporated. The attenuation coefficients of pancreatic tissue and pancreatic tumour tissue were selected as the highest value in the aforementioned range (11.9f 0.78 and 1.25 × 11.9f 0.78, respectively). When visibly distended, ducts were modelled with the properties of static water. Localised cooling effects of major blood vessels were modelled using heat flux boundary conditions at the inner surface of the blood vessels, with an ambient blood temperature of 37 °C, and the heat transfer coefficient h value was calculated using the method detailed by Haemmerich et al., with vasculature parameters obtained from literature [Citation55–60]. The duodenal and stomach walls were modelled as 2 mm and 3 mm thick, respectively [Citation53,Citation61,Citation62]. Feedback control over the maximum tumour temperature was applied for the ablation simulations and transient temperature solutions were obtained. For hyperthermia studies, a single applicator position was considered. A constant input power to the transducer was applied to obtain temperature distributions at steady-state. Thermal treatment parameters, including cooling water temperature, maximum set-point temperature for the feedback controller, heating time lengths, and applicator orientations underwent minor iteration for each case to limit thermal exposure to luminal and other sensitive tissues, and to extend volumetric coverage of tumour tissue, but were not rigorously optimised.
Results
Parametric studies
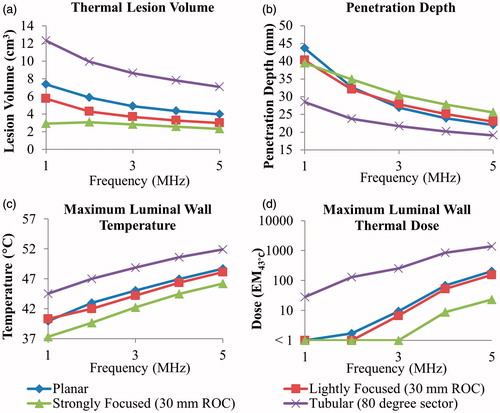
The characteristic temperature and thermal dose contours produced by each transducer configuration for ablation treatment simulations are shown in , for the frequency and curvatures specified in the caption. The effects of transducer configuration and frequency on ablation volume, penetration depth, and maximum luminal wall temperature/thermal dose are shown in . The ablation volume for these 5-min sonications ranged from 2.2–11.6 cm3, and the penetration depth from the inner luminal wall into the targeted tumour tissue ranged from 18.6–43.4 mm depending on which configuration and frequency was used. Both frequency and transducer configuration had a significant effect on the sparing of the luminal wall, with the maximum temperature ranging from 37.3–44.5 °C at 1 MHz and 46.2–51.9 °C at 5 MHz across configurations. Maximum thermal dose exposure of the luminal wall exceeded 15 EM43 °C for tubular transducers (at all frequencies), for planar and lightly curvilinear transducers operating at or above 4 MHz, and for strongly focused curvilinear transducers at 5 MHz.
Figure 4. Cross-sectional temperature distributions and 240 EM43 °C contours (black dashes) for ablation studies in the generalised tumour model for 10 × 20 mm planar, lightly focused, strongly focused, and 80° tubular transducer configurations operating at 2 MHz, after 5 min of active sonication.
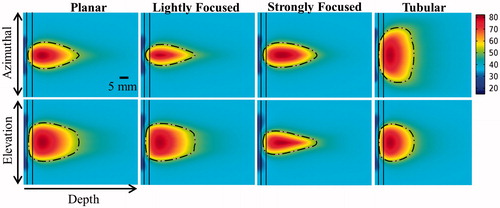
Figure 5. Effects of transducer configuration and operating frequency on (a) tumour ablation volume (thermal dose > 240 EM43 °C), (b) penetration depth (measured from inner luminal wall) of thermal lesion, (c) maximum temperature, and (d) maximum thermal dose of luminal wall tissue, after a single 5-min sonication with a temperature set point of 80 °C and 3 min of post-sonication cooling.
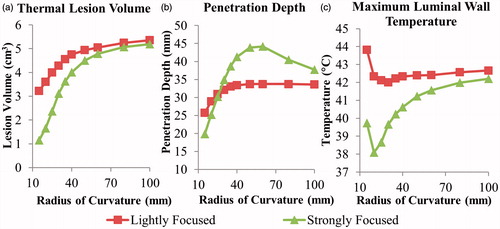
For curvilinear transducer geometries, the effect of the degree of focusing on thermal lesions was examined by varying the radius of curvature (ROC) at a set frequency of 2 MHz, as shown by . The influence of this parameter on the resulting lesion parameters was more pronounced with the strongly focused design. Increasing the radius curvature resulted in larger lesion volumes, ranging from 1.1–5.1 cm3 as the ROC varied from 15–100 mm. A maximum penetration depth of 44.2 and 33.6 mm were obtained for the strongly focused and lightly focused transducers, respectively, at a 60 mm ROC. Sparing of the luminal wall was improved with lower ROCs down to approximately 20 mm, until the geometric focus of the transducer was within 10 mm of the luminal tissue.
Figure 6. Effects of radius of curvature for 2 MHz curvilinear transducers on (a) tumour ablation volume (dose > 240 EM43 °C), (b) penetration depth (measured from inner luminal wall) of thermal lesion, and (c) maximum temperature of luminal wall tissue, after a single 5-min sonication with a temperature set point of 80 °C and 3-min post-sonication cooling.
As the luminal wall thickness increased from 1–5 mm, the maximal luminal wall temperature varied from 37.0–56.3 °C for planar, 37.0–54.8 °C for lightly focused curvilinear (30 mm ROC), 37.0–51.8 °C for strongly focused curvilinear (30 mm ROC), and 38.6–61.8 °C for tubular section transducers, all operating at 2 MHz. The relationship between luminal wall thickness and maximum wall temperature was approximately linear for all transducer configurations.
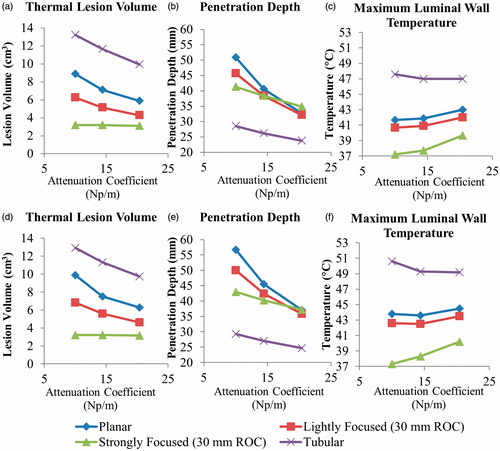
Sensitivity of the thermal lesion parameters to pancreatic and tumour attenuation coefficients varied with transducer configuration, as shown in . For 2 MHz planar transducers, the lesion volume decreased 8.4–5.0 cm3, the penetration depth decreased 50.5–34.5 mm, and the maximum luminal wall temperature increased 41.7–43 °C as pancreatic attenuation increased from 4.37f 1.2 Np/m – 11.9f 0.78 Np/m, with tumour attenuation 1.25 × greater. When tumour attenuation was equal to pancreatic attenuation, the penetration depth and thermal lesion volume increased by up to ∼5 mm and 1 cm3, respectively. The strongly focused transducer was insensitive to attenuation changes with regard to lesion volume, and the tubular section was relatively insensitive in terms of luminal heating. The penetration depths of both configurations were less sensitive to attenuation compared to the planar and lightly focused transducers.
Figure 7. Effects of pancreatic tissue attenuation coefficient on (a, d) tumour ablation volume (dose > 240 EM43 °C), (b, e) penetration depth (measured from inner luminal wall) of thermal lesion, and (c, f) maximum temperature of luminal wall tissue, after a single 5-min sonication at 2 MHz operating frequency, with a maximum temperature set point of 80 °C and 3-min post-sonication cooling period. The tumour attenuation coefficient is 1.25× the corresponding pancreatic tissue attenuation in (a–c), and equivalent to the pancreatic tissue attenuation in (d–f).
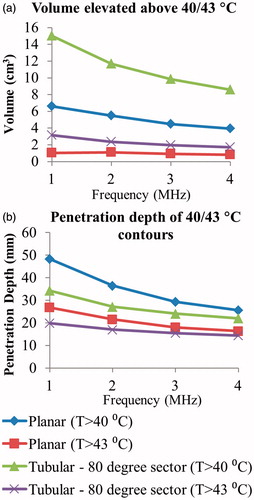
Parametric studies for hyperthermia treatments investigated the temperature distributions at steady-state, with maximum temperature set to 45 °C, and revealed similar trends as the ablation cases above. Tissue volumes elevated above 40 and 43 °C for the tubular section and planar transducers across 1–4 MHz frequencies are shown in , in addition to the penetration depth of each temperature contour. The azimuthal extent of tubular section 40 and 43 °C contours were an average of 34.3 and 22.1 cm3 across frequencies, while the same for the planar transducer were 14.2 and 7.4 cm3, respectively. The contours’ extents in the elevation direction were approximately equal (22.2 cm3 and 15.1 cm3 for tubular 40 and 43 °C contours versus 21.8 cm3 and 15.3 cm3 for planar), and frequency independent. Maximum luminal temperatures for all tested configurations were below 39.5 °C, with maximum temperatures occurring with the 4 MHz tubular section configuration.
Figure 8. Effects of transducer configuration and operating frequency on (a) tumour volume elevated above 40 and 43 °C, and penetration depth (measured from inner luminal wall) of 40 and 43 °C temperature contours, at steady-state with a maximum temperature set point of 45 °C for generation of moderate hyperthermia.
Patient anatomy models
Example 1: small head tumour
Ablation studies for this geometry () were simulated using an applicator placed in the duodenal lumen for two separate transducer configurations: a 2 MHz planar transducer and a 2 MHz lightly focused curvilinear transducer with an ROC of 25 mm. These were chosen due to the near proximity of the tumour boundary to the duodenal lumen and their capability to cover the majority of the tumour volume solely through rotation of the applicator. As shown in , which details ablation treatment delivery parameters and results for all four cases, use of either configuration demonstrated ablation of over 90% of the tumour volume under 25 min without exceeding a duodenal wall thermal dose of 5 EM43 °C. More (five versus three) discrete rotations of the applicator were necessary using the curvilinear transducer to extend tumour coverage. The curvilinear transducer resulted in a lower maximal thermal dose exposure of luminal tissue and the distal pancreatic duct.
Figure 9. (a) 3D model of the Example 1 anatomy, consisting of a small pancreatic head tumour directly adjacent to the duodenal lumen and positioned applicator. (b) 240 EM43 °C contours for ablation simulations using a 2 MHz planar (dashed contour) and 2 MHz lightly focused curvilinear transducer with a 25 mm ROC (dotted contour) across the central transverse slice through the applicator. (c) Temperature distributions and 40 and 43 °C contours (grey with dashes) are given for a hyperthermia simulation using a 1 MHz tubular section transducer. A: applicator, T: tumour, P: pancreas, DW: duodenal wall, PD: pancreatic duct.
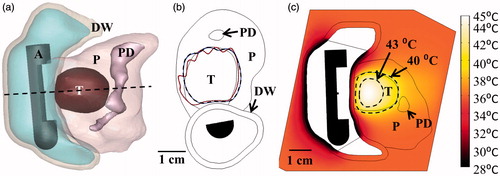
Table 3. Parameters and outcomes for patient anatomy ablation simulations.
Details of hyperthermia treatment simulations for all four geometries are shown in . A 1 MHz tubular transducer (80° active sector) was chosen for this geometry due to the relatively short depth of the tumour boundaries from the duodenum. With a constant applied surface intensity of 2.8 W/cm2, 95% of the tumour volume could be elevated above 40 °C, as shown in . No rotation or translation of the applicator was necessary for full tumour coverage.
Table 4. Parameters and outcomes for patient anatomy hyperthermia simulations.
Example 2: small head tumour
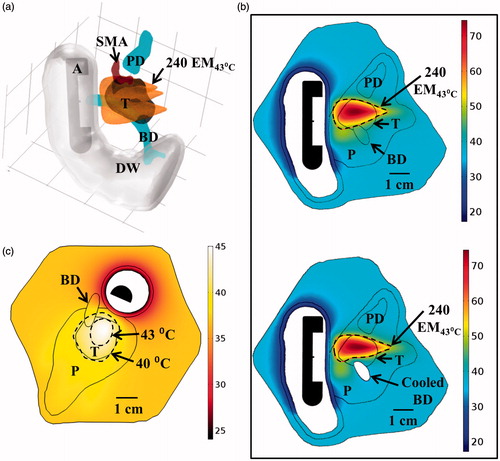
The ablation study was simulated using an applicator placed in the duodenal lumen with a 2 MHz strongly focused curvilinear transducer (ROC of 32.5 mm) to more effectively target the deeper tumour depth and limit exposure to the adjacent sensitive tissues. Both rotation and minor translation (< 1 cm) of the applicator were necessary to increase tumour coverage. Over 80% of the tumour volume could be ablated in less than 20 min, with minimal thermal exposure to the SMA and pancreatic duct, as shown in and . Intraductal cooling of the bile duct using a simulated balloon catheter, with circulating coolant at 33 °C, was necessary to achieve adequate sparing of the bile duct, as shown in , for the simulated applicator placements. Hyperthermia was performed with the applicator in a single position by rotating a 1.5 MHz planar transducer to cover the extent of the tumour, and could elevate 86.5% of the tumour volume above 40 °C at steady state, as shown in .
Figure 10. (a) Ablated lesion volume (dose > 240 EM43 °C) overlaid with the Example 2 model anatomy, consisting of a small head tumour surrounded by the SMA, PD, and BD, using a 2 MHz strongly focused curvilinear transducer. (b) Maximum temperatures and 240 EM43 °C contours across a sagittal plane for two ablation simulations: without (top) and with (bottom) simulated cooling of the bile duct, all other treatment parameters being identical. Sparing of the bile duct (BD) could be achieved by perfusing it with cooling water for the duration of the ablation treatment. (c) Temperature distributions and 40 and 43 °C contours (grey with dashes) for hyperthermia produced by rotating a 1.5 MHz planar applicator. A: applicator, T: tumour, P: pancreas, DW: duodenal wall, PD: pancreatic duct, BD: bile duct, SMA: superior mesenteric artery.
Example 3: large head tumour
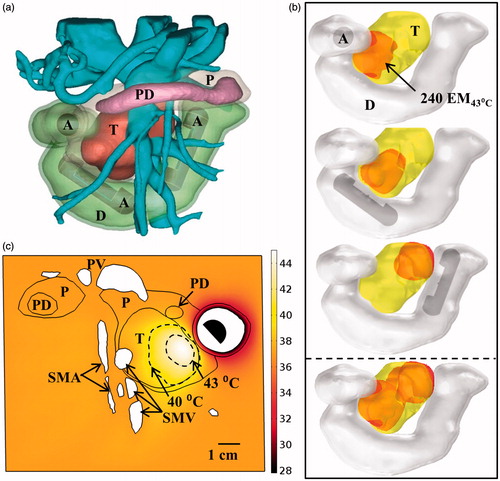
Three discrete positions of the applicator along the length of the duodenum were used for the ablation study, as shown in , with multiple rotations of the applicator at each position to increase tumour coverage. A planar 2 MHz transducer was chosen due to its large volumetric capabilities. Using this approach, about 60% of the 37 cm3 volume could be ablated within 50 min of cumulative heating and cooling time. The maximal thermal doses applied to the adjacent vasculature, pancreatic duct, and duodenal wall were all less than 15 EM43 °C. Within this same model, hyperthermia was simulated using a 1.5 MHz transducer at a single position shown in . With rotation of the applicator, 7.6 cm3 (20.4%) of the tumour volume could be elevated above 40 °C at steady state.
Figure 11. (a) 3D model of the Example 3 large pancreatic head tumour and surrounding anatomy, illustrating proximity of tumour to significant vasculature. (b) Ablated lesions were produced in the tumour by translating an applicator with a 2 MHz planar transducer to three individual positions along the length of the duodenum, with the cumulative thermal lesion shown at the bottom. (c) Temperature distribution and 40 and 43 °C contours (grey with dashes) across an axial plane for hyperthermia simulation using a 1.5 MHz planar transducer rotated at a single position in the duodenum. A: applicator, T: tumour, P: pancreas, D: duodenum, PD: pancreatic duct, PV: portal vein, SMV: superior mesenteric vein, SMA: superior mesenteric artery.
Example 4: body tumour
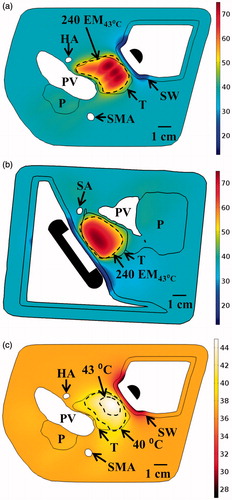
The applicator was positioned at two discrete locations in the stomach, and minutely translated and rotated at each to increase tumour coverage. For ablation, a 2 MHz planar transducer was chosen for volumetric capabilities, and generated ablation of 79.8% of the tumour volume in a 29-min total treatment time, as shown in . Thermal damage to the 3-mm thick stomach wall was minimised (<15 EM43 °C) by using a lower cooling water temperature of 7 °C, and there was no substantial heating of the surrounding major blood vessels in the region (all <1 EM43 °C) or the pancreatic duct (<5 EM43 °C). For hyperthermia, a 1.5 MHz planar transducer was used at a single applicator position, and could elevate 66.6% of the tumour volume above 40 °C at steady state by rotating the applicator to cover the tumour extent, as shown in .
Figure 12. Maximum temperature distributions and 240 EM43 °C contours (dashed) across (a) axial and (b) sagittal planes after ablation treatment for the Example 4 body tumour model, as treated with an applicator positioned in the stomach lumen with a 30 × 10 mm 2 MHz planar transducer. (c) Temperature distribution and 40 and 43 °C contours (grey) across an axial slice for hyperthermia by rotating a 1.5 MHz planar applicator. T: tumour, SW: stomach wall, P: pancreas, PV: portal vein, SMA: superior mesenteric artery, HA: hepatic artery, SA: splenic artery.
Discussion
This study has applied preliminary parametric and patient-specific acoustic and biothermal models to demonstrate the feasibility of generating ultrasound-based volumetric thermal ablation and hyperthermia within pancreatic tumours through the GI wall using an endoluminal delivery approach. Applicator transducer designs were investigated by evaluating four configurations (planar, lightly focused curvilinear, strongly focused curvilinear, and tubular section) across ranges of operating frequency and degree of focusing, with regard to heating capabilities and sparing of the luminal wall. Sweeps across transducer frequency for all configurations indicated that lower frequencies (1–3 MHz) were generally advantageous compared to higher frequencies (4–5 MHz), as they produced larger thermal lesion volumes and deeper penetration, while retaining the luminal wall at lower temperatures and thermal doses (). In consideration of increased applied power requirements due to decreased absorption, cavitation thresholds within the applicator cooling fluid and tissue, and possibly excessive penetration of energy toward distal tissues and interfaces at frequencies at or below 1 MHz, a nominal low frequency of 2 MHz was selected for ablation simulations of the generalized patient anatomy models (). With regard to heating characteristics of each transducer configuration, parametric studies revealed that the planar transducer has the most favourable general performance profile: deep (∼30–35 mm) penetration, high lesion volume capability (up to 6 cm3 for a single position and 5 min application), and suitable sparing (T < 45 °C, dose < 10 EM43 °C) of the luminal wall at frequencies below 3 MHz (). The strongly focused curvilinear transducer offers significantly superior sparing (3–4 °C lower maximum temperature, ∼tenfold less thermal dose) of luminal tissue and the capability of tailoring the penetration and thermal lesion volume through a priori selection of the ROC ( and ). These properties would make it appropriate as a more specialised design option, suitable for selective targeting of smaller target regions deeper from the GI tract or sonication through thicker (> 3 mm) luminal walls. Lightly curvilinear transducers represent an intermediate between the planar and strongly focused designs, allowing some control over heating depth and volumetric coverage ( and ), but without a distinct significant advantage. Ablation studies across varying normal pancreatic and tumour tissue attenuation coefficients revealed that heating characteristics of focused transducers were less influenced by tissue attenuation as compared to the more diffuse configurations (). Thus, in the absence of quantitative tissue property measurements, focused transducer designs could be advantageous due to their more predictable and consistent performance. Tubular section transducers, in contrast, were demonstrably the least suitable for ablation due to shallow heat penetration and comparably higher thermal exposure (4–7 °C higher maximum temperature) of the luminal wall relative to other configurations (). However, low-frequency tubular transducers could be appropriate for delivering hyperthermia to small target volumes within 3 cm of the luminal wall (). Planar transducer devices would require rotation to cover equivalent azimuthal extent, but could be advantageous for generating hyperthermia within larger/deeper targets due to greater penetration and potential volumetric coverage. As an alternative to having an assortment of these fixed transducer configurations available for a procedure, it may be possible to incorporate a more complex phased array transducer capable of dynamically producing variations of these focused and diffuse beam patterns, thereby permitting more flexibility from a single device in treatment delivery [Citation63–65].
One of the primary safety requisites for endoluminal ultrasound delivery of thermal ablation to pancreatic targets is to preclude thermal damage to the stomach or duodenal wall. This concern is relatively minimal for hyperthermia delivery, due to the much lower temperature elevation requirements, as shown by the parametric studies. While specific thermal dose thresholds of thermal damage for human duodenal or stomach wall tissues have not been distinctly established, approximate damage thresholds can be inferred from experimental investigations in porcine GI tract (oesophagus, small intestine, rectum) [Citation66–68]. These studies indicate that significant acute and chronic damage, including degenerative necrosis of the tissue or thromboses of blood vessels, occur at thermal dose levels greater than 80–120 EM43 °C and temperatures greater than 45–47 °C [Citation67,Citation68]. As such, the maximum luminal temperatures and doses obtained in the ablation simulations for the patient anatomy model studies (< 45 °C and < 15 EM43 °C) were considered to be within the safe limit to avoid significant thermal injury. As shown by the parametric studies, maximum luminal wall thermal exposures were strongly dependent on the luminal wall thickness. As such, sonicating through the stomach wall would likely have a higher risk of thermal injury as compared to through the duodenal wall, due to its greater thickness. However, there are many treatment parameters that could be adjusted to compensate for the increased risk of sonication through thicker GI luminal walls, including lowering the maximum set-point temperature or decreasing the cooling water flow temperature, as shown in the body tumour model (). Using more focused transducer configurations, tailoring the ROC, and/or using lower frequency transducers could also improve sparing of the luminal wall ( and ). In practice, it could be beneficial to distend the stomach or duodenum lumen to stretch and thin the luminal wall. This would be particularly important in the presence of folds or rugae, which could cause an increase in effective wall thickness or harbour air bubbles that could impact acoustic transmission and increase localised heating [Citation25]. Distension of the lumens may be achievable either through filling the stomach or small intestines with water, through endoscopic manipulation, or by incorporating large distensible balloons onto the applicator that would be inflated once the applicator was positioned [Citation69].
Simulations of these applicators incorporating patient anatomy models of pancreatic tumours and surrounding tissues illustrated feasibility of safely generating conformal ablation or hyperthermia across a range of tumour positions, sizes, and applicator positioning. Positioning the applicator within the duodenum provided the most access to tumours in the head of the pancreas (), whereas the body tumour was most accessible to an applicator placed in the stomach lumen (). All models necessitated sequential rotation and/or repositioning of the applicator to extend coverage of tumour volumes, leading to slightly scalloped or irregular outer boundaries of the lesions, as typical for heating with discretely rotating planar and curvilinear devices [Citation70]. Substantial portions (> 80% of total volume) of head tumours under 3 cm diameter could be targeted for ablative or hyperthermic temperature exposures in short (< 30 min) heating times without any major applicator repositions (). Furthermore, fewer manipulations were needed for applicators with a planar transducer as compared to lightly or strongly focused transducer configurations, due to its greater volumetric coverage (). However, tailoring the ROC of focused transducers to the target anatomy provided more preferential heating of the tumour and enhanced sparing of normal tissues proximal/distal to the tumour, as compared to the non-focused geometry (, ). Obtaining suitable volumetric ablation of large pancreatic tumours (> 4 cm diameter) places additional demands on applicator positioning and manipulation to access different portions of the tumour, as shown in . These large target volumes and repositional requirements could increase technical and time demands, and may be impractical for extended hyperthermia delivery, depending upon temperature range and time regimen required. In addition to size, the proximity of pancreatic tumours to sensitive anatomy, such as ducts, bowel, or significant vasculature, limits volumetric tumour coverage for ablation and imposes the need for highly accurate placement and manipulation of the applicator to ensure that energy is directed precisely towards target tissues. Integrating the applicator with an endoscope may provide sufficient control for positional accuracy [Citation25]. Image-guidance techniques, incorporating MRI or ultrasound guidance, could also be employed to verify positioning, monitor treatments, and permit feedback control [Citation25,Citation71,Citation72]. While the PI feedback control scheme employed herein permitted conformal lesion formation, more advanced schemes incorporating pilot/boundary-control points or model predictive control could further improve targeting and reduce undesirable thermal exposure [Citation52,Citation73]. Additional strategies to preserve sensitive tissues, such as internal cooling of major ducts using a cooling catheter, as shown in , may be considered. Further optimisation of designs and delivery strategies can be achieved with more extensive modelling inclusive of additional and broader ranges of patient anatomy cases.
In principle, this endoluminal ultrasound approach may offer significant advantages over existing techniques for delivering thermal therapy to the pancreas. It can provide an attractive alternative to percutaneous RF or microwave ablation by minimising complication risk through much more selective and targeted heating, and would not require major open surgery for its application [Citation6,Citation11,Citation12]. Compared to extracorporeal HIFU, endoluminal ultrasound could provide overall shorter ablation treatment times or enhanced hyperthermia coverage due to its local volumetric heating capability ( and ). It could also provide easier access to tumours near or invading the duodenum or bowels that are often acoustically inaccessible from extracorporeal sources [Citation7–9]. Further, although not investigated herein, application of these devices from within the body also suggests that in terms of energy delivery, compensating for respiratory or other sources of motion may be less challenging as compared to extracorporeal HIFU due to tissue and applicator motion being constrained together through endoscopic fixation. However, integrating the endoluminal approach with image-guidance treatment monitoring and feedback, e.g. by using MR thermometry, could still require respiratory gating or other motion-compensatory techniques [Citation71].
Conclusion
Three-dimensional acoustic and biothermal models illustrate the feasibility of generating ultrasound-based thermal therapy within pancreatic tumours using an endoluminal applicator placed in the duodenal or stomach lumen. Four specific applicator transducer configurations, ranging from curvilinear-focused to planar or radial-diverging, were demonstrated to provide appreciable volumetric coverage for thermal ablation of pancreatic tumours or the delivery of moderate hyperthermia. Planar and curvilinear configurations could produce ablative temperatures and lethal thermal doses for tissue volumes of up to 8 cm3 for a single applicator placement and 5 min sonication length, and for larger tumours additional rotations or translations of the applicator could be used to extend coverage. For moderate hyperthermia delivery, non-focused transducer geometries could sustain up to 16 cm3 of target tissue between a temperature range of 40–45 °C. Thermal simulations provide preliminary guidelines for appropriate frequency, transducer geometry, positioning, and treatment parameters to deliver conformal thermal therapy to pancreatic head or body target regions ranging from 20–45 mm deep from the GI wall while minimising thermal exposures (dose < 15 EM43 °C) to the intervening stomach or duodenal tissues. Additional modelling in the form of parametric studies and patient anatomy models can be applied for further optimisation of device designs and delivery approaches.
Declaration of interest
The work was supported by National Institutes of Health grant P01CA159992. This research was conducted with government support under and awarded by US Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. The authors alone are responsible for the content and writing of the paper.
References
- American Cancer Society. Cancer Facts & Figures, 2013. 2013. Available from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163–72
- Brescia FJ. Palliative care in pancreatic cancer. Cancer Control 2004;11:39–45
- Hameed M, Hameed H, Erdek M. Pain management in pancreatic cancer. Cancers (Basel) 2010;3:43–60
- Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia 2005;21:745–53
- Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 2014;20(9):2267–78
- Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract 2014;2014:1
- Anzidei M, Marincola BC, Bezzi M, Brachetti G, Nudo F, Cortesi E, et al. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: preliminary experience for pain palliation and local tumor control. Invest Radiol 2014;49:759–65
- Wu F, Wang Z, Zhu H, Chen W, Zou J, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology 2005;236:1034–40
- Vidal-Jove J, Perich E, del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem 2015;27:703–6
- Pezzilli R, Ricci C, Serra C, Casadei R, Monari F, D’Ambra M, et al. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers 2010;2:1419–31
- Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010;97:220–5
- Rasheed ZA, Matsui W, Maitra A. Pathology of pancreatic stroma in PDAC. In: Grippo PJ, Munshi HG, editors Pancreatic Cancer and Tumor Microenvironment. Trivandrum, India: Transworld Research Network; 2012
- Diederich CJ, Hynynen K. Ultrasound technology for hyperthermia. Ultrasound Med Biol 1999;25:871–87
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008;44:2546–54
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem B, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 2010;11:561–70
- Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, et al. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia 2013;29:8–16
- Ishikawa T, Kokura S, Sakamoto N, Ando T, Imamoto E, Hattori T, et al. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperthermia 2012;28:597–604
- Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev 2001;53:285–305
- Salgaonkar VA, Diederich CJ. Catheter-based ultrasound technology for image-guided thermal therapy: Current technology and applications. Int J Hyperthermia 2015;31:203–15
- Lafon C, Melodelima D, Salomir R, Chapelon JY. Interstitial devices for minimally invasive thermal ablation by high-intensity ultrasound. Int J Hyperthermia 2007;23:153–63
- Melodelima D, Prat F, Fritsch J, Theillere Y, Cathignol D. Treatment of esophageal tumors using high intensity intraluminal ultrasound: first clinical results. J Transl Med 2008;6:28
- Lafon C, Theillere Y, Prat F, Arefiev A, Chapelon J, Cathignol D. Development of an interstitial ultrasound applicator for endoscopic procedures: Animal experimentation. Ultrasound Med Biol 2000;26:669–75
- Hwang JH, Farr N, Morrison K, Wang Y, Khokhlova T, Ko B, et al. Development of an EUS-guided high-intensity focused ultrasound endoscope. Gastrointest Endosc 2011;73(4):AB155
- Li T, Khokhlova T, Maloney E, Wang Y, D’Andrea S, Starr F, et al. Endoscopic high-intensity focused US: Technical aspects and studies in an in vivo porcine model (with video). Gastrointest Endosc 2015;81:1243–50
- Prakash P, Salgaonkar VA, Diederich CJ. Modelling of endoluminal and interstitial ultrasound hyperthermia and thermal ablation: Applications for device design, feedback control and treatment planning. Int J Hyperthermia 2013;29:296–307
- Chen X, Diederich CJ, Wootton JH, Pouliot J, Hsu I. Optimisation-based thermal treatment planning for catheter-based ultrasound hyperthermia. Int J Hyperthermia 2010;26:39–55
- Scott SJ, Salgaonkar V, Prakash P, Burdette EC, Diederich CJ. Interstitial ultrasound ablation of vertebral and paraspinal tumours: Parametric and patient-specific simulations. Int J Hyperthermia 2014;30:228–44
- Chopra R, Burtnyk M, Haider MA, Bronskill MJ. Method for MRI-guided conformal thermal therapy of prostate with planar transurethral ultrasound heating applicators. Phys Med Biol 2005;50(21):4957–75
- Wootton JH, Prakash P, Hsu IJ, Diederich CJ. Implant strategies for endocervical and interstitial ultrasound hyperthermia adjunct to HDR brachytherapy for the treatment of cervical cancer. Phys Med Biol 2011;56(13):3967–84
- Chopra R, Burtnyk M, N’djin WA, Bronskill M. MRI-controlled transurethral ultrasound therapy for localised prostate cancer. Int J Hyperthermia 2010;26:804–21
- Burtnyk M, Chopra R, Bronskill MJ. Quantitative analysis of 3D conformal MRI-guided transurethral ultrasound therapy of the prostate: Theoretical simulations. Int J Hyperthermia 2009;25:116–31
- Burtnyk M, Chopra R, Bronskill M. Simulation study on the heating of the surrounding anatomy during transurethral ultrasound prostate therapy: A 3D theoretical analysis of patient safety. Med Phys 2010;37:2862–75
- Varadarajulu S, Banerjee S, Barth BA, Desilets DJ, Kaul V, Kethu SR, et al. GI endoscopes. Gastrointest Endosc 2011;74:1–6.e6
- Prakash P, Salgaonkar VA, Scott SJ, Jones P, Hensley D, Holbrook A, et al. MR guided thermal therapy of pancreatic tumors with endoluminal, intraluminal and interstitial catheter-based ultrasound devices: Preliminary theoretical and experimental investigations. Proc SPIE 2013;8584:85840V
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948;1:93–122
- Ocheltree KB, Frizzel L. Sound field calculation for rectangular sources. IEEE Trans Ultrason Ferroelectr Freq Control 1989;36:242–8
- Wootton JH, Hsu IJ, Diederich CJ. Endocervical ultrasound applicator for integrated hyperthermia and HDR brachytherapy in the treatment of locally advanced cervical carcinoma. Med Phys 2011;38:598–611
- Francis A. Duck. Physical Properties of Tissues: A Comprehensive Reference Book. London: Academic Press, 2013
- Hasgall P, Neufeld E, Gosselin M. IT’IS database for thermal and electromagnetic parameters of biological tissues. Version 2.4, July 30th, 2013
- Kersting S, Konopke R, Kersting F, Volk A, Distler M, Bergert H, et al. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology 2009;137:1903–11
- Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res 1987;43:14–20
- Delrue L, Blanckaert P, Mertens D, Van Meerbeeck S, Ceelen W, Duyck P. Tissue perfusion in pathologies of the pancreas: assessment using 128-slice computed tomography. Abdom Imaging 2012;37:595–601
- Kandel S, Kloeters C, Meyer H, Hein P, Hilbig A, Rogalla P. Whole-organ perfusion of the pancreas using dynamic volume CT in patients with primary pancreas carcinoma: acquisition technique, post-processing and initial results. Eur Radiol 2009;19:2641–6
- O’Donnell M, Mimbs J, Miller J. The relationship between collagen and ultrasonic attenuation in myocardial tissue. J Acoust Soc Am 1979;65:512–17
- Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang Z, et al. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas 1995;11:357–64
- Smith N, Merrilees N, Hynynen K, Dahleh M. Control system for an MRI compatible intracavitary ultrasound array for thermal treatment of prostate disease. Int J Hyperthermia 2001;17:271–82
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787–800
- McDannold N, Hynynen K, Wolf D, Wolf G, Jolesz F. MRI evaluation of thermal ablation of tumors with focused ultrasound. J Magn Reson Imaging 1998;8:91–100
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009;25:3–20
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 2011;27:320–43
- Prakash P, Diederich CJ. Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: implications for treatment planning, monitoring and control. Int J Hyperthermia 2012;28:69–86
- Masselli G, Picarelli A, Di Tola M, Libanori V, Donato G, Polettini E, et al. Celiac disease: Evaluation with dynamic contrast-enhanced MR imaging. Radiology 2010;256:783–90
- Jones JP, Behrens M. In vivo measurement of frequency dependent attenuation in normal liver, pancreas, and spleen. Ultrason Imaging 1981;3:205–6
- Haemmerich D, Wright A, Mahvi D, Lee Jr F, Webster J. Hepatic bipolar radiofrequency ablation creates coagulation zones close to blood vessels: A finite element study. Med Biol Eng Comput 2003;41:317–23
- Arienti V, Califano C, Brusco G, Boriani L, Biagi F, Giulia Sama M, et al. Doppler ultrasonographic evaluation of splanchnic blood flow in coeliac disease. Gut 1996;39:369–73
- Perišić MD, Ćulafić D, Kerkez M. Specificity of splenic blood flow in liver cirrhosis. Rom J Intern Med 2005;43:141–51
- Hübner GH, Steudel N, Kleber G, Behrmann C, Lotterer E, Fleig WE. Hepatic arterial blood flow velocities: assessment by transcutaneous and intravascular Doppler sonography. J Hepatol 2000;32:893–9
- Chato J. Heat transfer to blood vessels. J Biomech Eng 1980;102:110–18
- Gabe IT, Gault JH, Ross J Jr, Mason DT, Mills CJ, Schillingford JP, et al. Measurement of instantaneous blood flow velocity and pressure in conscious man with a catheter-tip velocity probe. Circulation 1969;40:603–14
- Nylund K, Hausken T, Ødegaard S, Eide G, Gilja O. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med 2012;33:E225
- Zuber-Jerger I, Muller A, Kullmann F, Gelbmann CM, Endlicher E, Muller-Ladner U, et al. Gastrointestinal manifestation of systemic sclerosis – thickening of the upper gastrointestinal wall detected by endoscopic ultrasound is a valid sign. Rheumatology 2010;49:368–72
- Melodelima D, Salomir R, Mougenot C, Moonen C, Cathignol D. 64-element intraluminal ultrasound cylindrical phased array for transesophageal thermal ablation under fast MR temperature mapping: an ex vivo study. Med Phys 2006;33:2926–34
- Pichardo S, Hynynen K. New design for an endoesophageal sector-based array for the treatment of atrial fibrillation: a parametric simulation study. IEEE Trans Ultrason Ferroelectr Freq Control 2009;56:600–12
- Werner J, Park E, Lee H, Francischelli D, Smith NB. Feasibility of in vivo transesophageal cardiac ablation using a phased ultrasound array. Ultrasound Med Biol 2010;36:752–60
- Date RS, Biggins J, Paterson I, Denton J, McMahon RF, Siriwardena AK. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas 2005;30:266–71
- Dewhirst M, Viglianti B, Lora-Michiels M, Hanson M, Hoopes P. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- Li D, Zhou S, Qiu S, Qiao S. Thermodamage, thermosensitivity and thermotolerance of normal swine oesophagus. Int J Hyperthermia 1987;3:143–51
- Bhutani MS, Deutsch JC. EUS Pathology with Digital Anatomy Correlation: A Text and Atlas. Shelton, CT: PMPH-USA, 2009
- Ross AB, Diederich CJ, Nau WH, Rieke V, Butts RK, Sommer G, et al. Curvilinear transurethral ultrasound applicator for selective prostate thermal therapy. Med Phys 2005;32:1555–65
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90
- Ebbini ES, Ter Haar G. Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia 2015;31:77–89
- Arora D, Cooley D, Perry T, Skliar M, Roemer RB. Direct thermal dose control of constrained focused ultrasound treatments: phantom and in vivo evaluation. Phys Med Biol 2005;50:1919–35

