Abstract
It has long been established that hyperthermia increases the therapeutic benefit of radiation and chemotherapy in cancer treatment. During the last few years there have been substantial technical improvements in the sources used to apply and measure heat, which greatly increases enthusiasm for the clinical use of hyperthermia. These advances are converging with a better understanding of the physiological and molecular effects of hyperthermia. Therefore, we are now at a juncture where the parameters that will influence the efficacy of hyperthermia in cancer treatment can be optimised in a more systematic and rational manner. In addition, the novel insights in hyperthermia’s many biological effects on tumour cells will ultimately result in new treatment regimes. For example, the molecular effects of hyperthermia on the essential cellular process of DNA repair suggest novel combination therapies, with DNA damage response targeting drugs that should now be clinically explored. Here, we provide an overview of recent studies on the various macroscopic and microscopic biological effects of hyperthermia. We indicate the significance of these effects on current treatments and suggest how they will help design novel future treatments.
Introduction
Hyperthermia is an anti-cancer treatment in which tumours are heated using an exogenous energy source. Heat can directly kill cancer cells, but also greatly synergises with radiotherapy and/or chemotherapy to increase the therapeutic window [Citation1,Citation2]. Although the effect of heat on the body has been studied for many decades, if not centuries, ‘modern’ hyperthermia has only been applied in the clinic since the 1980s. Early biological and physical studies revealed that the various physiological and cellular changes induced by hyperthermia are dose dependent [Citation3]. Therefore, heat treatment can be defined by the temperature that is applied: hyperthermia (39–45 °C), with temperatures <42 °C further defined as mild temperature hyperthermia, and thermal ablation (>45 °C). A second distinction in hyperthermia treatment is based on which part of the body is heated. Whole-body hyperthermia, as the name suggests, subjects the complete body to increased temperature. In regional hyperthermia an isolated part of the body, such as a body cavity, limb or organ, is heated, and during local hyperthermia only the tumour is heated [Citation4].
To understand how hyperthermia is applied nowadays, it is essential to realise that this treatment has an extensive history. Although the modern varieties of hyperthermia application can be traced back to the 1960s, elevated temperatures as a single modality have been employed to treat cancer for a much longer time, and can even be dated as far back as 5000 bc [Citation5]. In the 18 th and 19 th centuries, hyperthermia treatment started to be more evidence based. The use of heat then was based on the observation that tumours from patients started to shrink when the patients suffered from febrile diseases such as malaria or erysipelas. As a result, the surgeon Fehleisen started to infect cancer patients with bacteria, thus causing erysipelas, with the aim of eliminating tumours. Around 1900, William B. Coley developed special toxins, Coley’s toxins, to achieve the same effect, and he performed many studies on its effectiveness [Citation6]. The following years were extremely important for the modern use of hyperthermia. It was the period in which exogenous sources such as heated water baths started to be used to increase the temperature of gynaecological tumours [Citation7]. Moreover, radiotherapy was introduced around the same time, and when clinicians and researchers started combining it with heat, they found that hyperthermia increased sensitivity to radiation in tumours [Citation8]. This breakthrough stimulated an increase in the number of both clinical and fundamental studies from the 1950s until the present day. To this day, mild hyperthermia is used as a sensitiser for radiotherapy, but also for chemotherapy.
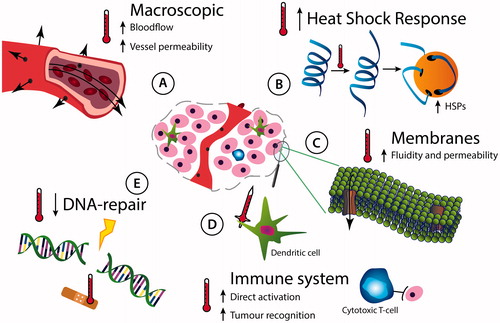
Initially, research on hyperthermia revolved around finding an optimal treatment temperature and time, or in short, an optimal thermal dose. These translational studies focused on creating a therapeutic window with maximum benefits of hyperthermia with minimal side effects. However, those early studies assumed that hyperthermia was only effective if it would directly kill cells, which only occurred when temperatures exceeded at least 42 °C. This made hyperthermia less feasible and thus less attractive in a clinical setting, causing a dampened enthusiasm for the treatment [Citation9]. As a result, there was a strong demand to improve hyperthermia technology, and a second line of hyperthermia research, focused on the physics of heating, quickly emerged. As of now, this field of research continues to improve not only the hyperthermia application techniques, but also the method of locally depositing heat and measuring it in the patient [Citation10]. The measuring tools aid clinicians in treating their patients optimally and it helps them document treatment outcomes for clinical research. Another line of hyperthermia research that has raised enthusiasm for cancer treatment employing heat has a biological and fundamental nature. Biological research strives to understand the biological mechanisms of hyperthermia at every temperature. Although this type of research may not always be directly applicable to clinical treatment per se, it will ultimately result in revolutions in the way we use hyperthermia in the clinic, by exploitation of the many cellular and physiological effects that hyperthermia has in tumours () [Citation5].
Figure 1. Biological effects of local hyperthermia in the tumour. A tumour, represented by the pictogram in the centre, and the diverse physiological and molecular effects of heat are indicated. (A) Hyperthermia alters physiology of the centrally located tumour by affecting its vasculature: heat increases the blood flow and vessel permeability. (B) Heat causes proteins to unfold and increases the intracellular amount of protein-chaperoning heat-shock proteins. (C) Temperatures exceeding 37 °C result in increased cell membrane fluidity, thereby influencing their permeability. Specific biological effects of heat on membranes may be further affected by altering properties of membrane-bound-proteins. (D) Local heat helps to activate the immune system and causes it to attack the tumour directly, but might also cause a systemic effect by which immune cells attack tumour cells distant from the heated tumour. (E) Hyperthermia affects DNA damage repair pathways by deactivating specific repair proteins.
This review aims to illustrate the importance of biological research to mild hyperthermia, since hyperthermia in this temperature range is the most prominent in the clinic. To demonstrate the broad potential of heating tumours, we present an overview of the biological effects of mild hyperthermia and describe their relevance in current treatment regimes. Moreover, we will describe how the biological research continues to influence the way we think about hyperthermia by presenting examples of treatment innovations that are not, or not yet, clinically applied, but exploit one or more biological effects of heat.
Macroscopic effects of hyperthermia
When a tumour is heated, a number of physiological changes occur. One of the earliest recognised physiological changes induced by hyperthermia is its effect on the vascular system. Hyperthermia causes an increase in blood flow in the heated area, and by expanding the vessels the heat improves their permeability () [Citation11–13]. Most of the physiological changes upon hyperthermia treatment are secondary to effects on tumour blood flow [Citation14]. In fact, blood flow is one of the predominant factors governing tumour response to heating [Citation15,Citation16]. It is a potent cooling mechanism which thus influences the delivery of heat to a tissue. Blood flow in tumours is also a principal factor responsible for the micro-environmental conditions within tumours, and since cells under oxygen-deprived and highly acidic conditions are more responsive to the effects of heat [Citation17,Citation18], blood flow will play a major role in influencing the cellular heat response, and subsequently that of the tumour.
How quickly the macroscopic changes of hyperthermia are seen and their degree of change generally depends on the time and temperature of heating. As soon as heat is applied to a tumour the blood flow increases, but these effects are cancelled upon treatment with temperatures that surpass 44.5 °C [Citation11,Citation19–25]. However, the degree of increase and how long it is maintained is very heterogeneous depending on both the temperature applied and the tumour model used [Citation11,Citation26]. The primitive construction and poor organisation of tumour vessels results in them being more permeable than normal tissue vessels [Citation27]. As flow increases in response to heat, fluid will begin to leak out of the vessels into the extracellular space [Citation25]. This oedema will eventually increase interstitial fluid pressure which subsequently causes vascular compression and a decrease in perfusion. This could explain the observations that vascular perfusion starts to decrease after about 30 min of heating the tumour to 42.5 °C and above [Citation19,Citation23–25]. However, it should be noted that at least one study found that this did not occur until a temperature of 44.5 °C was achieved [Citation22]. In addition, temperatures beyond 44.5 °C will directly kill endothelial cells, and this will cause vascular damage, resulting in haemorrhage that accentuates the already decreased perfusion [Citation11,Citation28]. In some examples the inherent vascular damage in the tumour can be so severe that a total vascular shut down occurs, even at temperatures as low as 42.5 °C [Citation25]. After heating, flow typically returns to normal at lower temperatures, but continues to decrease in those tumours where vascular damage was already initiated [Citation19,Citation22,Citation24,Citation25].
As mentioned above, the tumour vasculature is structurally and functionally abnormal and thus fails to meet the demands of the growing tumour mass, which is why tumours are characterised by regions of oxygen and nutrient deprivation, high lactate levels and extracellular acidosis [Citation27,Citation29]. Heat-induced vascular changes will further modify the micro-environmental parameters, although the mechanism is also dependent on the applied temperature. High temperatures cause vascular collapse which will further reduce oxygen and nutrient delivery to tumour cells. This is reflected by an escalation in energy deprivation [Citation30], lactic acid accumulation [Citation31], acidity [Citation30], and hypoxia [Citation32,Citation33]. For lower temperatures the increase in perfusion is associated with an increased oxygen delivery [Citation32,Citation34–37], but this greater availability of oxygen leads to an increased consumption of oxygen [Citation14,Citation38,Citation39]. Even though an enhanced oxygen consumption could be expected to decrease the diffusion distance of oxygen and thus elevate the level of tumour hypoxia, this has not been shown.
Although the macroscopic effects of hyperthermia can be classified as well-studied, there is controversy about the improvement in oxygenation to how long those effects last. Some studies have shown that this improvement can last for up to 24 h after heating at mild temperatures [Citation34,Citation36], and this has been suggested as one of the reasons why hyperthermia enhances radiation administered clinically in a fractionated schedule [Citation40]. However, this prolonged improvement in tumour oxygenation is difficult to explain, because the physiological changes that are likely to account for improved oxygenation with mild heat treatments are unlikely to be maintained after the blood flow increase has ceased. More consistent with those physiological effects, other studies have shown that tumour oxygenation actually rapidly returns to normal when the heating at mild temperatures ceases [Citation35]. Nonetheless, the several macroscopic effects of hyperthermia have established a central position in its modern use. Moreover, the transient increase in blood flow and resulting vascular leakage forms the basis for generating methods to increase drug delivery to a place of interest, for example, via the application of thermosensitive liposomes [Citation41–44].
The heat shock response
Heat activates a cellular mechanism that defends against protein stress. This heat shock response consists of a rapid production of heat shock proteins (HSPs), a specific group of proteins that chaperone denatured proteins and thereby prevent formation of toxic protein aggregates () [Citation45–47]. This defence mechanism is not limited to the response to heat, but is also activated by several other forms of stress, such as hypoxia and infection, and is therefore of vital importance for life [Citation47]. However, when it comes to treating cancers with mild hyperthermia, the heat shock response has an undesirable side effect: it causes tumours to become thermotolerant. Thermotolerance is a phenomenon that can be described as insensitivity to heat treatments within 48–72 h after the initial treatment. It has a pivotal role in the hyperthermia field because it demonstrates the importance of properly scheduling hyperthermia sessions for patients.
HSPs have been recognised to play a role in the development of thermotolerance for a long time, because they are thought to protect tumour cells from protein denaturation induced by hyperthermia [Citation48–51]. Moreover, tumours sometimes have a constitutively high level of HSPs that protect them from innately higher levels of protein stress, causing the tumours to be fully dependent on these high levels. Therefore, specific HSP inhibitors have been developed and have found their way into the clinic [Citation52]. Because of their availability and clinical use, employing these types of drugs together with hyperthermia should be considered in order to increase the effective therapeutic window and prevent thermotolerance. Although the detailed effects on thermotolerance are still rather unclear, the combination of heat and HSP inhibitors does increase cell sensitivity towards hyperthermia [Citation53]. It has also been proposed that activation of the heat shock response is inhibited by acute acidification, and indeed, sensitivity of cells towards heat is increased when it is combined with drugs that lower the pH in the cell, such as lonidamine, especially when cells were slightly acidic already [Citation54].
However, as will be described below, HSPs also occupy an important role in cancer immunology and in that context their role is actually thought to be beneficial for the patient. Therefore it will be essential to thoroughly investigate the implications of combining different variants of HSP inhibitors with hyperthermia on treatment efficacy, thermotolerance, and immunological effects, before applying them in a clinical setting.
Cellular membrane and drug uptake
One of the ways hyperthermia contributes to sensitising cells towards chemotherapeutics, is by increasing fluidity of the cytoplasmic membrane (). The cytoplasmic membrane consists of a phospholipid bilayer and proteins, and it forms the outer layer of the cell. Interactions between proteins and lipids in the membrane cause it to respond to temperature changes in a very dynamic fashion [Citation55]. Regulation of the membrane fluidity is essential for homeostasis and therefore conserved in many species. It is clear that the membrane plays a considerable role in the stress response, including the response to heat-shock [Citation56–58]. Membrane stress triggers various signalling cascades which transduce their signal to the heat shock transcription factor HSF1, which ultimately increases expression of the HSPs [Citation58,Citation59].
When the membrane becomes more fluid due to heat, its physical permeability for some compounds will increase [Citation60]. This is probably one of the reasons why some chemotherapeutics will be able to pass the cell barrier more effectively when the cells are treated with hyperthermia. For example, several reports show that the concentration of the chemotherapeutic cisplatin increases in the cell when it is treated with hyperthermia [Citation61–63]. Moreover, heat also contributes to structural changes in the membrane by altering the behaviour of membrane-embedded proteins, and this will also increase cellular cisplatin concentrations; this is illustrated by heat facilitating multimerisation of a copper transporter (CTR1) that is responsible for cisplatin uptake [Citation64]. Knowledge about the altered behaviour of the cell membrane in conditions of hyperthermia has mainly been used in regional hyperthermia treatment, where a heated chemotherapeutic is flushed on a specific part of the body such as the peritoneum (HIPEC) or the bladder (HIVEC) [Citation43,Citation65]. However, the temperature-mediated effects on the cell membrane will also play a role with the implementation of thermosensitive liposomes [Citation42].
The effects of hyperthermia on the membrane might be counteracted by an evolutionary conserved adaptation response. The exact mechanism by which cells compensate their membrane fluidity to heat is not known, but recent studies have revealed that the acyl-CoA dehydrogenase down-regulates a lipid desaturase upon heat in the model system Caenorhabditis elegans. This effectively creates a more rigid membrane structure that compensates for the increased fluidity [Citation66,Citation67]. Such an adaptive response might be relevant for the development of thermotolerance, thus it will be of importance to elucidate whether this mechanism also occurs in human cells after hyperthermia. In this context, it is important to realise that the composition of the membrane as well as its reaction to environmental changes can be deregulated in tumours, since this might have consequences for the treatment regime [Citation68]. Ultimately, research done on the subject will deliver new strategies to either mimic or enhance the effects of hyperthermia on the membrane, or to prevent unwanted side effects.
The immune system and hyperthermia
Fever and hyperthermia are both characterised by increased temperatures, and both contribute to the activation of the immune system () [Citation69,Citation70]. Treatments of cancer patients by using hyperthermia and stimulating their immune system have always been closely intertwined, as can be exemplified by the fact that William B. Coley is regarded as one of the fathers in the hyperthermia field, but is also seen as one of the pioneers in immunotherapy [Citation6,Citation69]. The many intriguing ways in which hyperthermia stimulates the immune system has great impact on the rationale of hyperthermia treatment in oncology, as is clear from the many reviews on the subject [Citation5,Citation69–73]. Currently, it is thought that there are two closely related ways in which local hyperthermia can modulate the immune system [Citation5]. The first effect of hyperthermia on immunity is localised to the heated tumour, but, secondly, local hyperthermia can also stimulate a systemic antitumour reaction that can strike tumour cells that are distant from the heated tumour [Citation69,Citation70].
Increased temperatures affect both adaptive and innate immunity [Citation70,Citation73]. The way temperature regulates the immune system is not only dependent on the magnitude of the temperature increase that is applied or achieved, but also on the duration [Citation70,Citation72,Citation74]. However, some effects occur quickly and are therefore relevant in hyperthermia treatment. The increased blood flow resulting from mild heating of the tumour can promote attraction of immune cells via improving trafficking between the tumour and the draining lymph nodes [Citation69]. It has also been shown that heat causes changes in adhesion molecules of the tumour vasculature. Specifically, the expression of the glycoprotein intracellular adhesion molecule 1 (ICAM-1) is increased via heat-induced increased interleukin 6 (IL-6) signalling. ICAM-1 then attracts effector/memory T-cells [Citation69,Citation75,Citation76]. Hyperthermia not only alters expression of vascular adhesion molecules, but also induces an increased expression of surface molecules on tumour cells. At 39 °C, heat increases the MHC class I polypeptide-related sequence A (MICA), a molecule which increases cell sensitivity to natural killer cells [Citation69,Citation77]. At a temperature of 43 °C, heat will increase the level of MHC class I molecules, which attract cytotoxic T-cells [Citation69,Citation78]. Febrile temperatures also enhance functions of dendritic cells, and their enhanced antigen-presenting function increases stimulation of T-cells [Citation70,Citation79–81]. It is thought that the heat-mediated increase in membrane fluidity has effects on organising the response of the adaptive immune system, promoting activation of T-cells in areas where the temperature is increased [Citation82–84].
The most interesting connection between immunity and the heat-shock response is the function of the increased HSPs, especially that of Hsp70. Like other HSPs, Hsp70 is produced upon heat treatment, but it can be released from cells. In the extracellular environment, Hsp70 will bind various immune cell surface receptors, which will in turn release various pro-inflammatory molecules. In this environment, Hsp70 stimulates dendritic cells and macrophages by acting as a damage-associated molecular pattern (DAMP) [Citation70]. Moreover, Hsp70s can stimulate the adaptive immune system by transferring chaperoned tumour proteins to antigen-presenting cells, which evokes a tumour-specific T-cell response [Citation69,Citation85–87]. This T-cell response presumably has the ability to target all tumour cells, including metastases [Citation88].
Although the role of hyperthermia in immunity has revolutionised the rationale behind using hyperthermia in oncology treatment, the connection has only recently gained more interest with the acknowledgement of the role for immunotherapy in oncology [Citation89]. To exploit the effects of hyperthermia on the immune system in the future, it will be especially important to fully understand the temperature dynamics and corresponding effects of the HSPs, since their role provokes the most tumour-specific immune response [Citation90,Citation91]. This is illustrated by the finding that Hsp70 can be used as an anti-cancer vaccine to mimic and maximise the response of hyperthermia [Citation92,Citation93].
Hyperthermia and DNA repair
It has long been known that hyperthermia increases cancer cell sensitivity for agents that cause DNA damage or interfere with DNA metabolism. One of the earliest discoveries and well-studied examples of an agent that causes DNA damage, and synergises with heat, is ionising radiation [Citation94]. However, heat also increases the degree of cell killing caused by certain types of chemotherapy, such as cisplatin and alkylating agents [Citation95]. The mechanism by which hyperthermia sensitises to DNA damaging agents has been extensively studied, but the results are often difficult to interpret because of the use of different temperatures and an overlap in the several DNA damage repair pathways. The many hypotheses that consider what effects hyperthermia has on DNA have recently been reviewed [Citation96].
Many researchers have tried to elucidate the mode of action of hyperthermia on DNA. Some early reports indicated that hyperthermia directly caused DNA damage and claimed that there were more chromosomal aberrations and DNA breaks after hyperthermia treatment [Citation97,Citation98]. Moreover, heating cells to 41.5 °C increased the amount of phosphorylation of histone H2AX (γH2AX), which is considered a marker for DNA double strand breaks [Citation99]. However, there is also a large body of literature that describes hyperthermia as having no direct damaging effect on DNA, but rather interferes with the activity of proteins important for repairing DNA caused by an exogenous agent, such as radiotherapy () [Citation96].
Identifying specific DNA repair pathways that are targeted by hyperthermia is not straightforward. This task has been attempted by employing a genetic approach, in which the sensitivity towards the combination of exogenously applied DNA damage and heat in wild-type cells is compared to cells that are deficient for a specific repair pathway. If hyperthermia results in inactivation of a repair pathway required for the repair of the induced DNA lesions, then heated wild-type cells will be more sensitive towards the DNA damaging agent than cells that are not heated. In the scenario that the repair deficient cell line is deficient for a pathway that is targeted by hyperthermia, then the cells will not be further sensitised by the applied heat. However, the spectrum of different lesions induced by a single DNA damaging agent, as well as the possibility that hyperthermia targets multiple DNA repair pathways, prevents conclusive interpretation of data obtained using this genetic approach. Indeed, multiple DNA repair pathways, such as base excision repair [Citation66] for single strand DNA lesions and non-homologous end joining for double strand breaks [Citation100], are believed to be inhibited by hyperthermia [Citation96]. However, the results obtained in these studies are often based on experiments done with temperatures above 43 °C, so it remains unclear whether inhibition of these DNA repair pathways add to the effects of mild hyperthermia. There is therefore still significant experimental ground to be covered before the mystery of how mild hyperthermia influences DNA metabolism is solved.
Nonetheless, when we can identify the effects of hyperthermia on DNA in cancer cells, the information obtained can help the hyperthermia field with questions such as how to minimise side effects and toxicity to healthy tissues, and how to maximise DNA damage load in the tumour. For example, it was discovered that hyperthermia functionally inhibits the DNA repair pathway of homologous recombination [Citation101,Citation102]. This repair pathway acts in the S-phase and G2-phase of the cell cycle to faithfully restore double strand breaks by using an intact copy of broken DNA as a template for repair. When such a double strand break occurs, it triggers a cascade that results in nucleolytic processing of the double strand DNA ends into single strand DNA, which is subsequently coated by the single-strand binding protein RPA. This is then replaced by RAD51 with the help of BRCA2. This RAD51 recombinase protein is believed to be of vital importance for the subsequent search for homologous DNA and the invading of this DNA, and is therefore regarded as the most essential component in homologous recombination. After the missing DNA is restored based on the sequence information of its identical sister chromatid in the cell, the intertwined DNA structure is resolved leaving two whole DNA molecules [Citation103,Citation104]. In the aforementioned study, it was shown that hyperthermia (>40 °C) inhibits the accumulation of the RAD51 at sites of DNA damage via targeting the BRCA2 protein for proteasomal degradation [Citation101].
Homologous recombination is involved in repair of breaks caused by radiation treatment, and therefore the finding that hyperthermia inhibits this DNA repair pathway provides at least part of the explanation for hyperthermia’s sensitising potential towards ionising radiation. However, homologous recombination is not only responsible for repairing double strand breaks that result from exogenous sources, but also acts to repair breaks that result from collapsing of replication forks. The finding that hyperthermia inhibits homologous recombination is therefore of particular interest, because it opens up new possibilities of treatments that can be combined with hyperthermia, such as PARP-1 inhibitors [Citation105]. PARP-1 inhibitors are a relatively new class of chemotherapy which cause collapse of replication forks [Citation106]. These PARP-1 inhibitors gained clinical interest because they specifically kill cells that are deficient in homologous recombination, while showing little toxicity to normal cells [Citation106,Citation107]. Thus, PARP-1 inhibitors are a prime example of a precision treatment for tumours of BRCA1/2 mutation carriers, as the tumour cells of these patients, but not their normal cells, are homologous recombination deficient. The demonstration that mild heat phenocopies BRCA deficiency and induces homologous recombination deficiency provides a rational for extending PARP-1 inhibitor treatment outside of the limited group of BRCA mutation carriers. Clinical trials should now be considered in which PARP-1 inhibitors are combined with hyperthermia to locally induce homologous recombination deficiency in tumours. This combination may have minimal side effects, because both treatments, which have little toxicity on their own, will only need to be applied temporarily. Therefore, we predict that it will only be a matter of time before this combination therapy will be applied in a clinical setting.
The future of hyperthermia
Hyperthermia has been used in the clinic for decades, and clinical studies have clearly demonstrated that heating tumours has benefit when added to radiation or to chemotherapy [Citation5,Citation108–110]. Moreover, hyperthermia is now readily available to treat a broad range of tumours, similar to radiotherapy and broad-spectrum chemotherapy, but unlike the latter two, hyperthermia has no severe side effects [Citation10,Citation111]. These beneficial features of hyperthermia together with the physiological and molecular effects that we have summarised in this review (), illustrate the potential of hyperthermia treatment in oncology.
The impact of hyperthermia in cancer treatment will only increase in the future, as we learn how to more effectively exploit the multiple biological effects of the heat on the tumour. However, the time has already arrived to translate the biological findings about hyperthermia into benefits for cancer patients. The importance of thermal dose has always been recognised in the context of hyperthermia treatment, and finding the optimal thermal dose for each biological effect will result in insights that will enhance the efficacy of the heat, and eventually in one or more doses that fit the treatment regimen envisioned by the practitioner. The possibilities to optimise hyperthermia treatment are greatly aided by the current advantages in the application techniques for hyperthermia: the heating systems have not only become more specialised and effective in heating, but it is now also possible to measure real-time heat deposition by MRI [Citation5]. Although hyperthermia itself is advancing beyond previous possibilities, the collection of possible synergising agents are also emerging. With the expanding interest in the use of proton therapy in oncology, it has been suggested that cells harbouring defects in homologous recombination are more sensitive towards proton irradiation than to photon irradiation [Citation112,Citation113]. Since, as we explained above, heat causes a defect in homologous recombination, there is a rational basis for the combination of hyperthermia with proton therapy. Ultimately, the collective efforts of clinicians, physicists and biologists will result in an effective, versatile and evidence-based use of hyperthermia in oncology that is bound to gain more popularity in the future.
Acknowledgements
This study has been supported by an Erasmus MC Mrace grant, by Cancer Genomics Netherlands, by the Dutch Cancer Society (DDHK 2013-6072), by the Danish Cancer Society, and the Danish Council for Independent Research: Medical Sciences.
Disclosure statement
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001;17:1–18.
- Van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84.
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994;10:457–83.
- Van der Zee J, Vujaskovic Z, Kondo M, Sugahara T. The Kadota Fund International Forum 2004 – clinical group consensus. Int J Hyperthermia 2008;24(2):111–22.
- Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53.
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154–8.
- Westermark KF. Uber die Behandlung des Ulcerirenden Cervix Carcinoma Mittels Konstanter Warme [On the treatment of ulcerating cervix carcinoma with constant heat.]. Zentralbl Gynaekol 1898;22:1335–9.
- Muller C. Therapeutische Erfahrungen an 100 mit kombination von Rontgenstrahlen un Hochfrequenz, resp. Diathermie behandeleten bosartigen Neubildungen [Therapeutic experiences with 100 combination therapies of X-rays and high frequency, respectively, diathermy treated malignant neoplasms.]. Munchener Medizinische Wochenschrift 1912;28:1546–9.
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005;21:779–90.
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97.
- Reinhold HS, Endrich B. Tumour microcirculation as a target for hyperthermia. Int J Hyperthermia 1986;2:111–37.
- Meyer RE, Braun RD, Rosner GL, Dewhirst MW. Local 42 degrees C hyperthermia improves vascular conductance of the R3230Ac rat mammary adenocarcinoma during sodium nitroprusside infusion. Radiat Res 2000;154:196–201.
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21:761–7.
- Vaupel P, Kallinowski F. Physiological effects of hyperthermia. Recent results Cancer Res 1987;104:71–109.
- Jain RK, Grantham FH, Gullino PM. Blood flow and heat transfer in Walker 256 mammary carcinoma. J Natl Cancer Inst 1979;62: 927–33.
- Patterson J, Strang R. The role of blood flow in hyperthermia. Int J Radiat Oncol Biol Phys 1979;5:235–41.
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology 1977;123:511–14.
- Gerweck LE, Nygaard TG, Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res 1979;39:966–72.
- Müller-Klieser W, Vaupel P. Effect of hyperthermia on tumor blood flow. Biorheology 1984;21:529–38.
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984;44(10 Suppl):S4721–30.
- Willett CG, Urano M, Suit HD, Strauss HW, Kahn J, Okunieff PG. Effect of temperature on blood flow and hypoxic fraction in a murine fibrosarcoma. Int J Radiat Oncol Biol Phys 1987;13:1309–12.
- Song CW, Patten MS, Chelstrom LM, Rhee JG, Levitt SH. Effect of multiple heatings on the blood flow in RIF-1 tumours, skin and muscle of C3H mice. Int J Hyperthermia 1987;3:535–45.
- Maeta M, Karino T, Inoue Y, Hamazoe R, Shimizu N, Koga S. The effect of angiotensin II on blood flow in tumours during localized hyperthermia. Int J Hyperthermia 1989;5:191–7.
- Vaupel P. Effects of physiological parameters on tissue response to hyperthermia: New experimental facts and their relevance to clinical problems. In: Gerner EW, TC C, editors. Hyperthermic Oncology 1992, plenary and symposium lectures. Volume 2. Tucson: Arizona Board of Regents; 1993. pp 17–23.
- Gyldenhof B, Horsman MR, Overgaard J. Hyperthermia-induced changes in the vascularity and histopathology of a murine tumour and its surrounding normal tissue. In: Franconi C., Arcangeli G. CR, editor. Hyperthermic Oncology 1996. VolumeII. Rome, Italy: Tor Vergata; 1996. pp 780–2.
- Shchors K, Evan G. Tumor angiogenesis: Cause or consequence of cancer? Cancer Res 2007;67:7059–61.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res 1989;49:6449–65.
- Fajardo LF, Schreiber AB, Kelly NI, Hahn GM. Thermal sensitivity of endothelial cells. Radiat Res 1985;103:276–85.
- Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008;8:425–37.
- Vaupel P, Okunieff P, Neuringer LJ. In vivo 31P-NMR spectroscopy of murine tumours before and after localized hyperthermia. Int J Hyperthermia 1990;6:15–31.
- Lee SY, Ryu KH, Kang MS, Song CW. Effect of hyperthermia on the lactic acid and beta-hydroxybutyric acid content in tumour. Int J Hyperthermia 1986;2:213–22.
- Otte J, Manz R, Thews G, Vaupel P. Impact of localized microwave hyperthermia on the oxygenation status of malignant tumors. Adv Exp Med Biol 1982;157:49–55.
- Urano M, Kahn J. The change in hypoxic and chronically hypoxic cell fraction in murine tumors treated with hyperthermia. Radiat Res 1983;96:549–59.
- Iwata K, Shakil A, Hur WJ, Makepeace CM, Griffin RJ, Song CW. Tumour pO2 can be increased markedly by mild hyperthermia. Br J Cancer Suppl 1996;27:S217–21.
- Horsman MR, Overgaard J. Can mild hyperthermia improve tumour oxygenation? Int J Hyperthermia 1997;13:141–7.
- Vujaskovic Z, Song CW. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia 2004;20:163–74.
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 2009;25:91–5.
- Durand RE. Potentiation of radiation lethality by hyperthermia in a tumor model: Effects of sequence, degree, and duration of heating. Int J Radiat Oncol Biol Phys 1978;4:401–5.
- Lepock JR, Cheng KH, Al-Qysi H, Sim I, Koch CJ, Kruuv J. Hyperthermia-induced inhibition of respiration and mitochondrial protein denaturation in CHL cells. Int J Hyperthermia 1987;3:123–32.
- Oleson JR. Eugene Robertson Special Lecture. Hyperthermia from the clinic to the laboratory: A hypothesis. Int J Hyperthermia 1995;11:315–22.
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia 1999;15:345–70.
- Li L, ten Hagen TLM, Hossann M, Suss R, van Rhoon GC, Eggermont AMM, et al. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J Control Release 2013;168:142–50.
- Owusu RA, Abern MR, Inman BA. Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Biomed Res Int 2013;2013:262313.
- Mallory M, Gogineni E, Jones GC, Greer L, Simone CB. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit Rev Oncol Hematol 2015;97:56–64.
- Lepock JR. Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperthermia 2004;20:115–30.
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006;22:191–6.
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 2010;11:545–55.
- Subjeck JR, Sciandra JJ, Chao CF, Johnson RJ. Heat shock proteins and biological response to hyperthermia. Br J Cancer Suppl 1982;5:127–31.
- Landry J, Chretien P, Bernier D, Nicole LM, Marceau N, Tanguay RM. Thermotolerance and heat shock proteins induced by hyperthermia in rat liver cells. Int J Radiat Oncol Biol Phys 1982;8:59–62.
- Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA 1991;88:1681–5.
- Nollen EAA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol 1999;19:2069–79.
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010;10:537–49.
- Miyagawa T, Saito H, Minamiya Y, Mitobe K, Takashima S, Takahashi N, et al. Inhibition of Hsp90 and 70 sensitizes melanoma cells to hyperthermia using ferromagnetic particles with a low Curie temperature. Int J Clin Oncol 2014;19:722–30.
- Coss RA, Storck CW, Wells TC, Kulp KA, Wahl M, Leeper DB. Thermal sensitisation by lonidamine of human melanoma cells grown at low extracellular pH. Int J Hyperthermia 2014;30:75–8.
- Saita EA, de Mendoza D. Thermosensing via transmembrane protein-lipid interactions. Biochim Biophys Acta 2015;1848:1757–64.
- Vigh L, Nakamoto H, Landry J, Gomez-Munoz A, Harwood JL, Horvath I. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann NY Acad Sci 2007;1113:40–51.
- Csoboz B, Balogh GE, Kusz E, Gombos I, Peter M, Crul T, et al. Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperthermia 2013;29:491–9.
- Török Z, Crul T, Maresca B, Schütz GJ, Viana F, Dindia L, et al. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochim Biophys Acta 2014;1838:1594–618.
- Balogh G, Horváth I, Nagy E, Hoyk Z, Benkõ S, Bensaude O, et al. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J 2005;272:6077–86.
- Lande MB, Donovan JM, Zeidel ML. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J Gen Physiol 1995;106:67–84.
- Wallner KE, DeGregorio MW, Li GC. Hyperthermic potentiation of cis-diamminedichloroplatinum(II) cytotoxicity in Chinese hamster ovary cells resistant to the drug. Cancer Res 1986;46:6242–5.
- Ohtsubo T, Saito H, Tanaka N, Matsumoto H, Sugimoto C, Saito T, et al. Enhancement of cisplatin sensitivity and platinum uptake by 40 °C hyperthermia in resistant cells. Cancer Lett 1997;119:47–52.
- Gabano E, Colangelo D, Ghezzi AR, Osella D. The influence of temperature on antiproliferative effects, cellular uptake and DNA platination of the clinically employed Pt(II)-drugs. J Inorg Biochem 2008;102:629–35.
- Landon CD, Benjamin SE, Ashcraft KA, Dewhirst MW. A role for the copper transporter Ctr1 in the synergistic interaction between hyperthermia and cisplatin treatment. Int J Hyperthermia 2013;29:528–38.
- Stamou K, Tsamis D, Pallas N, Samanta E, Courcoutsakis N, Prassopoulos P, et al. Treating peritoneal mesothelioma with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. A case series and review of the literature. Int J Hyperthermia 2015;31:850–6.
- Fantini D, Moritz E, Auvré F, Amouroux R, Campalans A, Epe B, et al. Rapid inactivation and proteasome-mediated degradation of OGG1 contribute to the synergistic effect of hyperthermia on genotoxic treatments. DNA Repair (Amst). 2013;12:227–37.
- Ma DK, Li Z, Lu AY, Sun F, Chen S, Rothe M, et al. Acyl-CoA dehydrogenase drives heat adaptation by sequestering fatty acids. Cell 2015;161:1152–63.
- Baritaki S, Apostolakis S, Kanellou P, Dimanche-Boitrel M-T, Spandidos DA, Bonavida B. Reversal of tumor resistance to apoptotic stimuli by alteration of membrane fluidity: therapeutic implications. Adv Cancer Res 2007;98:149–90.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015;15:335–49.
- Frey B, Weiss E-M, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42.
- Repasky E a, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res 2013;1:210–16.
- Lauber K, Brix N, Ernst A, Hennel R, Krombach J, Anders H, et al. Targeting the heat shock response in combination with radiotherapy: sensitizing cancer cells to irradiation-induced cell death and heating up their immunogenicity. Cancer Lett 2015;368:209–29.
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett 2008;271:191–204.
- Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 2001;97:2727–33.
- Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest 2011;121:3846–59.
- Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol 2007;82:1322–31.
- Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, et al. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003;94:308–13.
- Knippertz I, Stein MF, Dörrie J, Schaft N, Müller I, Deinzer A, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 2011;27:591–603.
- Bachleitner-Hofmann T, Strohschneider M, Krieger P, Sachet M, Dubsky P, Hayden H, et al. Heat shock treatment of tumor lysate-pulsed dendritic cells enhances their capacity to elicit antitumor T cell responses against medullary thyroid carcinoma. J Clin Endocrinol Metab 2006;91:4571–7.
- Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, Formstecher P, et al. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol 2007;81:1179–87.
- Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-gamma production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012;28:9–18.
- Kobayashi Y, Ito Y, Ostapenko V V, Sakai M, Matsushita N, Imai K, et al. Fever-range whole-body heat treatment stimulates antigen-specific T-cell responses in humans. Immunol Lett 2014;162:256–61.
- Zynda ER, Grimm MJ, Yuan M, Zhong L, Mace TA, Capitano M, et al. A role for the thermal environment in defining co-stimulation requirements for CD4(+) T cell activation. Cell Cycle 2015;14:2340–54.
- Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA 1997;94:13146–51.
- Todryk S, Melcher A, Hardwick N, Linardakis E, Bateman A, Colombo M, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol 1999;163:1398–408.
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJS, Kuppner MC, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol 2002;169:5424–32.
- Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, et al. Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. Nanomed Nanotech Biol Med 2014;10:1273–85.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10.
- Repasky EA. Progress in development of biomedical applications of heat shock proteins and thermal stress. Int J Hyperthermia 2013:29 359–61.
- Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia 2013;29:399–408.
- Calderwood SK, Gong J, Stevenson MA, Murshid A. Cellular and molecular chaperone fusion vaccines: targeting resistant cancer cell populations. Int J Hyperthermia 2013;29:376–9.
- Epple LM, Bemis LT, Cavanaugh RP, Skope A, Mayer-Sonnenfeld T, Frank C, et al. Prolonged remission of advanced bronchoalveolar adenocarcinoma in a dog treated with autologous, tumour-derived chaperone-rich cell lysate (CRCL) vaccine. Int J Hyperthermia 2013;29:390–8.
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26.
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008;44:2546–54.
- Oei AL, Vriend LEM, Crezee J, Franken N a. P, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol 2015;10:165.
- Anai H, Maehara Y, Sugimachi K. In situ nick translation method reveals DNA strand scission in HeLa cells following heat treatment. Cancer Lett 1988;40:33–8.
- Warters RL, Henle KJ. DNA Degradation in Chinese hamster ovary cells after exposure to hyperthermia. Cancer Res 1982;42:4427–32.
- Takahashi A, Mori E, Somakos GI, Ohnishi K, Ohnishi T. Heat induces gammaH2AX foci formation in mammalian cells. Mutat Res 2008;656:88–92.
- Ihara M, Takeshita S, Okaichi K, Okumura Y, Ohnishi T. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia 2014;30:102–9.
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA 2011;108:9851–6.
- Genet SC, Fujii Y, Maeda J, Kaneko M, Genet MD, Miyagawa K, et al. Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time- and temperature-dependent manner. J Cell Physiol 2013;228:1473–81.
- Zelensky A, Kanaar R, Wyman C. Mediators of homologous DNA pairing. Cold Spring Harb Perspect Biol 2014;6:a016451.
- Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol 2013;5:a012740.
- Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 2012;28:509–17.
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434(7035):913–17.
- Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson D a, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434(7035):917–21.
- Rampersaud EN, Vujaskovic Z, Inman BA. Hyperthermia as a treatment for bladder cancer. Oncology (Williston Park) 2010;24:1149–55.
- Soria F, Allasia M, Oderda M, Gontero P. Hyperthermia for non-muscle invasive bladder cancer. Expert Rev Anticancer Ther 2015;16:313–21.
- Bahouth Z, Halachmi S, Moskovitz B, Nativ O. The role of hyperthermia as a treatment for non-muscle invasive bladder cancer. Expert Rev Anticancer Ther 2016;16:189–98.
- Geijsen ED, de Reijke TM, Koning CC, Zum Vorde Sive Vording PJ, de la Rosette JJ, Rasch CR, et al. Combining mitomycin C and regional 70 MHz hyperthermia in patients with nonmuscle invasive bladder cancer: A pilot study. J Urol 2015;194:1202–8.
- Grosse N, Fontana AO, Hug EB, Lomax A, Coray A, Augsburger M, et al. Deficiency in homologous recombination renders mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys 2014;88:175–81.
- Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA, et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol 2015;116:374–80.

