Abstract
Background: Electric stimulation (ES) has been recognized as an effective method to improve motor function to paralysed patients with stroke. It is important for ES to synchronize with voluntary movement. To enhance this co-ordination, the finger-equipped electrode (FEE) was developed. The purpose of this study was to evaluate FEE in improving motor function of upper extremities (UEs) in patients with chronic stroke.
Methods and subjects: The study participants included four patients with chronic stroke who received FEE electronic stimulation (FEE-ES) plus passive and active training and three control patients who underwent training without FEE-ES. The patients were treated five times weekly for 4 weeks. UE motor function was evaluated before and after treatment using Fugl-Meyer Assessment (FMA) and Brunnstrom recovery staging.
Results: The mean age of patients in each group was 60-years and there was a mean of 49 months since the onset of symptoms. All patients had severe UE weakness. The patients receiving FEE-ES had greater improvement in UE function than control patients (total, proximal and distal FMA, p < 0.05; Brunnstrom staging of UE, p < 0.05).
Discussion: The results indicate that FEE-ES may be an effective treatment for patients with chronic stroke.
Introduction
Up to 85% of stroke patients show an initial deficit in an upper extremity (UE), with only 50% of stroke patients regaining some limited function of their paretic UE Citation[1], Citation[2]. Unsuccessful use of the paretic UE in stroke patients may cause ‘learned non-use phenomenon’, in which patients habitually rely on their non-paretic UE to perform daily activities Citation[3].
Several advanced rehabilitation techniques have emerged in recent years which have demonstrated that UE function may be improved in stroke survivors, even in those with chronic hemiplegia. Such techniques include constraint-induced movement therapy Citation[4], Citation[5], robot-assisted movement Citation[6], Citation[7], bilateral symmetric movement of the paretic and non-paretic UEs Citation[8], Citation[9], the mirror therapy Citation[10], Citation[11] and repetitive facilitation exercises Citation[12].
Electrical stimulation (ES)-mediated repetitive movement therapy has also been proposed as a means for facilitating post-stroke motor recovery Citation[13]–Citation[17]. For patients with no residual motor activity, ES may be delivered in a cyclical manner, without any input from the user. For those with some residual motor activity, volitionally-activated electromyography (EMG) can be used to trigger or modulate the ES, thus adding a cognitive component to ES therapy. The EMG-mediated ES technique has been shown to be more effective than cyclic ES Citation[13].
Although self-initiated stimulation can improve participants’ focus during training, it has proven to be impractical for patients with severe deficits, since it requires some residual movement capacity of the impaired shoulder, arm or hand; therefore, the system is not useful for severely disabled stroke survivors Citation[18], Citation[19].
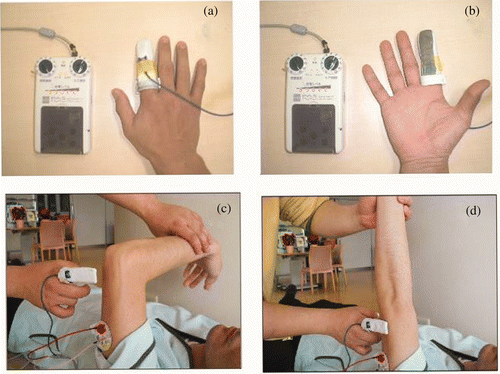
A new ES treatment system has been developed, using a finger-equipped electrode (FEE), for patients with severe paresis. It consists of an electrode fitted on a finger, much like a finger cap (see ). By touching the patient's paretic UE, the therapist applies the ES and thus can control its timing.
Figure 1. (a, b) Characteristics of the FEE, which is made of metal-coated fabric. (c, d) A treatment session (for elbow extension) using the FEE. One self-adhesive electrode was placed on the proximal side of the triceps brachii muscle. The therapist, outfitted with the FEE on a finger, said ‘Raise your hand as far as you can’ and started to assist the patient's motion (if any residual extension was possible). Then, the FEE was applied to the skin over the nerve which innervated the muscle to promote contraction. The muscle contraction was repeated 50-times.
Even in patients with no residual motor activity, motor activation can be induced after treatment with ES using the FEE. The purpose of this study was to evaluate FEE in improving UE motor function in chronic stroke patients with severe hemiplegia.
Methods
Patients
Seven patients with chronic stroke were enrolled in the study. As shown in , all patients had severe UE weakness. There were no significant differences in the clinical characteristics of patients undergoing FEE-ES and the control patients (see ).
Table I. Baseline clinical characteristics of two groups
Inclusion criteria for this study were the following:
no skin allergy to electric stimulation/electrodes;
the ability to follow simple commands;
first episode of stroke;
no consciousness disturbance; and
plateau in the maximum motor recovery after conventional rehabilitation.
All participants gave informed consent for study participation; study methods were approved by the institutional review board of Inobe Hospital.
Experimental design
The palmer surface of the FEE was made of metal-coated fabric (). This characteristic allowed the therapist to apply ES by touching the patient and, thus, to control the timing of the ES. The stimulation frequency was 30 Hz. The level of stimulation was increased until a comfortable gross muscle contraction was visible.
In the FEE-ES group, one treatment session lasted ∼1.5 hours. With patients lying on their backs, passive mobilization activities were performed for 10 minutes to facilitate active motion. After passive mobilization, ES with the FEE was initiated. For forward flexion of the shoulder, one self-adhesive electrode was placed on the proximal side of the patient's anterior deltoid muscle. The therapist, outfitted with the FEE on a finger, said ‘Raise your hand as far as you can’, and started to assist the patient's motion (if any residual motion remained).
The FEE was then applied to the skin over the nerve which innervated the muscle to promote full extension. Thus, the ES with the FEE could be synchronized with the voluntary motion; ES lasted until the FEE stimulation was stopped. The muscle contraction was repeated 50-times. After practice of shoulder motion, other motions were practiced, including flexion of the elbow by stimulation of the biceps brachii; extension of the elbow by stimulation of the triceps brachii; pronation and supination of the forearm by stimulation of the pronator teres and supinator, respectively; and extension of the wrist and finger by stimulation of the extensor carpi radialis and extensor digitorum communis, respectively. Each motion was practiced 50-times. Patients in the control group underwent the same training exercises with the therapist, but did not receive ES.
Both the patients undergoing FEE-ES and control patients underwent five treatment sessions per week for 4 weeks. UE motor function was assessed before and after the 4 weeks of treatment sessions using the Fugl-Meyer Assessment (FMA), according to six Brunnstrom stages, as follows: (1) flaccidity; (2) synergy development (minimal voluntary movements); (3) voluntary synergistic movement; (4) some movements deviating from synergy; (5) independence from basic synergies; and (6) isolated joint movements Citation[20]. Assessment also used the modified Ashworth scale (MAS), which measures spasticity (muscle tone) on a scale of 0, 1, 1+, 2, 3 and 4, with higher scores indicating more severe spasticity Citation[21].
Statistical analysis
The dependent t-test for paired samples was used to perform pre- vs. post-treatment comparisons of the variables of motor-function. The Wilcoxon-Mann-Whitney test was used to compare the patients undergoing FEE-ES and the control patients with regard to the changes in FMA scores of the upper limb and fingers before and after treatment. The Chi-square test was used to compare the Brunnstrom staging scores between the two groups of patients. P-values less than 0.05 were considered to be statistically significant.
Results
– depict the mean changes in total, proximal and distal UE function, respectively, as shown by FMA scores for assessments undertaken before and after 4 weeks of treatment.
Figure 2. Changes in Fugl-Meyer Assessment (FMA) total scores for the upper extremities of patients undergoing electronic stimulation by the finger-equipped electrode (FEE-ES) and control patients after 4 weeks of treatment. Data are mean ± SD of each individual patient of both groups (scores of post-treatment minus pre-treatment). The asterisk indicates that the Wilcoxon-Mann-Whitney test showed that the difference between the two groups was statistically significant (p = 0.032).
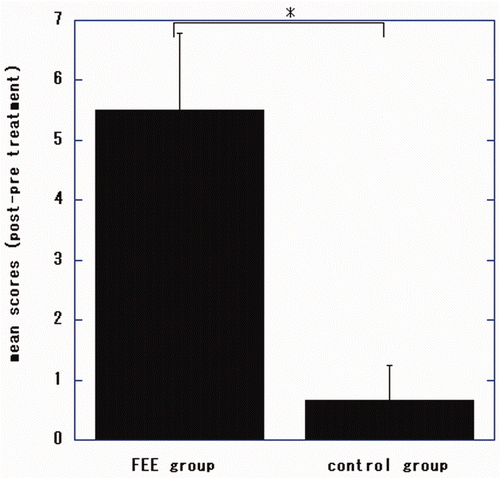
Figure 3. Changes in Fugl-Meyer Assessment (FMA) scores for the proximal upper extremities of patients undergoing electronic stimulation by the finger-equipped electrode (FEE-ES) and control patients after 4 weeks of treatment. Data are mean ± SD of each individual patient of both groups (scores of post-treatment minus pre-treatment). The asterisk indicates that the Wilcoxon-Mann-Whitney test showed that the difference between the two groups was statistically significant (p = 0.026).
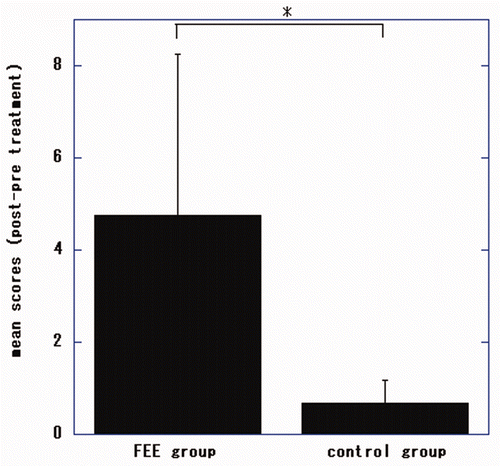
Figure 4. Changes in Fugl-Meyer Assessment (FMA) scores for the distal upper extremities of patients undergoing electronic stimulation by the finger-equipped electrode (FEE-ES) and control patients after 4 weeks of treatment. Data are mean ± SD of each individual patient of both groups (scores of post-treatment minus pre-treatment). The asterisk indicates that the Wilcoxon-Mann-Whitney test showed that the difference between the two groups was statistically significant (p = 0.026).
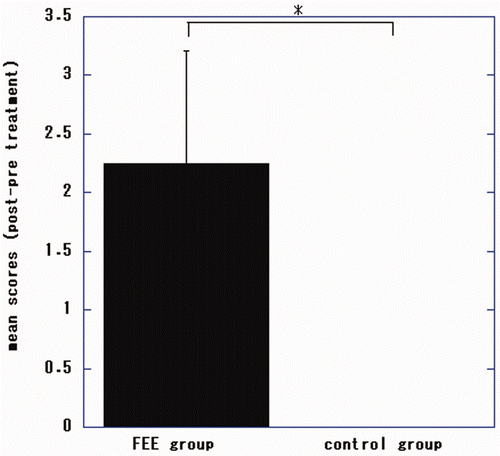
There were significant differences in total, proximal and distal FMA scores of the patients undergoing FEE-ES compared to the control patients (p = 0.032, U = 0.000; p = 0.026, U = 0.000; p = 0.026, U = 0.000, respectively).
In the FEE-ES group, the changes of FMA scores improved significantly from before treatment to the end of treatment (total FMA, p = 0.025, t = −4.1812; proximal FMA, p = 0.046, t = −3.2795; distal FMA, p = 0.015, t = −5.00 [dependent t-test]).
The Brunnstrom scores of the affected UEs of all patients before treatment were 3. Three of the four patients undergoing FEE-ES achieved Brunnstrom scores of 4 after treatment and the other patients (control patients and patient no. 4 of the FEE-ES group) were unchanged (p = 0.0472, Chi-square value = 3.938 [Chi-square test]).
The scores of MAS did not change from before treatment to afterward, except for patient no. 4 whose score of elbows decreased from 1+ to 1.
Discussion
The study data suggest that ES with FEE can induce significant motor recovery in even severely paretic UEs of patients with chronic stroke.
The severity of paresis and degree of treatment effectiveness observed in this study were similar to that reported by Chan et al. Citation[22], who employed functional ES for bilateral upper limb training. The FMA scores reported by Chan et al. Citation[22] for pre-training, post-training and the difference were 18.2, 25.9 and 7.7, respectively, as compared with the corresponding scores of 16.3, 23.3 and 7.0 in this study. Chan et al. Citation[22] used an ES unit that was self-triggered by the subject's unaffected hand via a motion-detection system based on an accelerometer. Although triggered ES might be more effective than non-triggered ES in facilitating UE motor recovery following stroke Citation[13], the severely paretic UE itself could not be used as a trigger. Thus, Chan et al. Citation[22] as well as Kuntson et al. Citation[18], Citation[19] used the unaffected UE as a self-trigger.
This system was neither self-triggered nor simple cyclic ES without triggering, but instead was motion-triggered by the therapist. As each patient voluntarily attempted to move the affected shoulder, hand, wrist and fingers (with the therapist's help), the therapist used ES to induce movement via touching the skin area covering the appropriate muscle with the FEE. Thus, the full movement achieved was a product of the patient's volitional motion and the ES triggered by the therapist. Because this treatment system did not require the patient to retain any residual ability to move the shoulder, hand, wrist and fingers, it might be more widely applicable than existing treatments. Unlike some other treatments, ES with the FEE can be used effectively by patients with severe deficits who have only limited or no residual capacity for movement.
This treatment system may owe its effectiveness, in part, to sensory-motor integration theory Citation[17], Citation[23], which asserts that sensory input from movement of an affected limb directly influences subsequent motor output. The sensory components of large afferent fibre activation, proprioceptive input and increased cognitive sensory attention all contribute to reducing spasticity and, thus, assist in the return of voluntary movement and increased function.
Recently, using multichannel near-infrared spectroscopy to non-invasively and dynamically measure haemoglobin levels in the brain during functional activity, Hara Citation[24] showed that cerebral blood flow in the sensory-motor cortex area on the injured side was increased during power-assisted functional ES session compared with cerebral blood flow during simple active movement or simple ES. Further studies are needed to investigate ways to improve increasing cerebral blood flow using the FEE system.
This was a preliminary study of a small number of patients. A study of a large treatment population and a randomized controlled study are required to confirm the beneficial effects of this new treatment. Studies are planned that will apply FEE-ES to other paretic muscle groups, such as the muscles of the lower extremities or those used for swallowing.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Skilbeck CE, Wade DT, Hewer RL, Wood VA. Recovery after stroke. Journal of Neurology, Neurosurgery and Psychiatry 1983; 46: 5–8
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: Measurement and recovery over the first three months. Journal of Neurology, Neurosurgery and Psychiatry 1987; 50: 714–719
- Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Experimental Neurology 1989; 104: 125–132
- Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke 2006; 37: 1045–1049
- Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D. The EXCITE trial: Retension on improvement upper extremity function among stroke survivors receiving CI movement theraphy. Lancet Neurology 2008; 7: 33–40
- Lum PS, Burgar CG, Shor PC, Majmunder M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Archives of Physical Medicine and Rehabilitation 2002; 83: 952–959
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Pharm D, Wagner TH, Krebs HI, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. New England Journal of Medinine 2010; 362: 1772–1783
- Whitall J, McCombe-Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 2000; 31: 2390–2395
- Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor exitability and enhaces upper limb motricity poststroke: Apilot study. Journal of Clinical Neuroscience 2004; 21: 124–131
- Yavuzer G, Selles R, Sezer N, Sutbeyaz S, Bussmann JB, Koseoglu F, Atay MB, Stam HJ. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2008; 89: 393–398
- Dohle C, Pullen J, Nakaten A, Kust J, Rietz C, Karb H. Mirror therapy promotes recovery from severe hemiparesisi: A randomized controlled trial. Neurorehabilitation and Neural Repair 2009; 23: 209–217
- Kawahira K, Shimodozono M, Etoh S, Kamada K, Noma T, Tanaka N. Effects of intensive repetition of a new facilitation technique on motor functional recovery of the hemiplegic upper limb and hand. Brain Injury 2010; 24: 1202–1213
- de Kroon JR, IJzerman MJ, Chae J, Lankhorst GJ, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. Journal of Rehabilitation Medicine 2005; 27: 65–74
- Hara Y, Ogawa S, Muraoka Y. Hybrid power-assisted functional electrical stimulation to improve hemiparetic upper-extremity fuction. American Journal of Physical Medicine and Rehabilitation 2006; 85: 977–985
- Hara Y, Ogawa S, Tsujiuchi K, Muraoka Y. A home-based rehabilitation program for the hemiplegic upper extremity by power-assisted functional electrical stimulation. Disability and Rehabilitatio 2008; 30: 296–304
- Fujiwara T, Kasashima Y, Honaga K, Muraoka Y, Tsuji T, Osu R, Hase K, Masakado Y, Liu M. Motor improvement and corticospinal modulation induced by hybrid assistive neuromuscular dynamic stimulation (HANDS) therapy in patients with chronic stroke. Neurorehabilitation and Neural Repair 2009; 23: 125–132
- Shin HK, Cho SH, Jeon H, Lee Y, Song JC, Jang SH, Lee C, Kwon YH. Cortical effect and functional recovery by the electromyography-triggered neuromuscular stimulation in chronic stroke patients. Neuroscience Letters 2008; 442: 174–179
- Knutson JS, Harley MY, Hisel TZ, Chae J. Improvement hand function in stroke survivors: A pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Archives of Physical Medicine and Rehabilitation 2007; 88: 513–520
- Kuntson JS, Hisel TZ, Harley MY, Chase J. A novel functional electrical stimulation treatment for recovery of hand function in hemiplegia: 12-week pilot study. Neurorehabilitation and Neural Repair 2009; 23: 17–25
- Brunnstrom S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Physical Therapy 1966; 46: 357–375
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy 1987; 67: 206–207
- Chan MK, Tong RK, Chung KY. Bilateral upper limb training with functional electric stimulation in patients with chronic stroke. Neurorehabilitation and Neural Repair 2009; 23: 357–365
- Kimberley T, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Experimental Brain Research 2004; 154: 450–460
- Hara Y. Neurorehabilitation with new functional electrical stimulation for hemiparetic upper extremity in stroke patients. Journal of Nippon Medical School 2008; 75: 4–14
