Abstract
Objective: To longitudinally examine objective and self-reported outcomes for recovery of cognition, communication, mood and participation in adults with traumatic brain injury (TBI) and co-morbid post-traumatic sleep/wake disorders.
Design: Prospective, longitudinal, single blind outcome study.
Setting: Community-based.
Participants: Ten adults with moderate–severe TBI and two adults with mild TBI and persistent symptoms aged 18–58 years. Six males and six females, who were 1–22 years post-injury and presented with self-reported sleep/wake disturbances with onset post-injury.
Interventions: Individualized treatments for sleep/wake disorders that included sleep hygiene recommendations, pharmacological interventions and/or treatments for sleep apnea with follow-up.
Main outcome measures: Insomnia Severity Index, Beck Depression and Anxiety Inventories, Latrobe Communication Questionnaire, Speed and Capacity of Language Processing, Test of Everyday Attention, Repeatable Battery for the Assessment of Neuropsychological Status, Daily Cognitive-Communication and Sleep Profile.
Results: Group analysis revealed positive trends in change for each measure and across sub-tests of all measures. Statistically significant changes were noted in insomnia severity, p = 0.0003; depression severity, p = 0.03; language, p = 0.01; speed of language processing, p = 0.007.
Conclusions: These results add to a small but growing body of evidence that sleep/wake disorders associated with TBI exacerbate trauma-related cognitive, communication and mood impairments. Treatment for sleep/wake disorders may optimize recovery and outcomes.
Introduction
Traumatic brain injury (TBI) can result in both short- and long-term physical, cognitive and neurobehavioural impairments for survivors. These impairments can result in devastating personal and societal consequences, impacting return to the community and pre-injury lifestyle [Citation1]. Complaints of disturbed sleep, excessive daytime sleepiness and disorders of arousal have been well-established as being among the most pervasive, enduring and common sequelae of TBI [Citation1, Citation2]. Sleep/wake disturbances are prevalent across all levels of severity and across the continuum of recovery, often evolving over time. This is critical from the perspective of recovery from TBI, as adequate sleep is essential for general healthy functioning even in a normative population [Citation3]. Recent studies of healthy adults reveal that, following days of chronically restricted sleep of duration below 7 hours per night, significant daytime cognitive dysfunction accumulates including lapses of attention, slowed working memory, reduced cognitive processing, depressed mood and perseveration of thought [Citation3]. Additionally, laboratory studies of healthy adults subjected to sleep restriction have found adverse effects on endocrine functions, as well as metabolic and inflammatory responses, suggesting that sleep restriction produces unhealthy physiological consequences [Citation3]. Furthermore, sleep plays a crucial role in neuro-synaptic plasticity and neurogenesis [Citation4, Citation5]. However, up to 70% of TBI survivors report symptoms of insomnia including difficulty falling asleep, staying asleep, sleep fragmentation and difficulties with early awakening, across the continuum of recovery [Citation2, Citation6–9]. Thus, the investigation of sleep following TBI and its relationship to recovery and outcomes merits investigation.
While it is difficult to fully determine a causal relationship between the brain injury and sleep/wake disturbances, the majority of studies and clinical reports indicate that, at the very least, their date of onset, regardless of the type of disorder, is post-injury. Their presentation and aetiology is likely multi-factorial and complex and may in part be due to neurological factors and confounding non-neurological, injury-related factors such as depression, pain, substance use, poor sleep hygiene, weight gain and reduced activity. From a neurological perspective, the occurrence of these disorders may be a result of complex interactions between brain lesions, including damage to the basal forebrain and the reticular activating system, hypothalamic injury with associated decreases in levels of wake-promoting neurotransmitters such as orexin (hypocretin), endocrine changes associated with injury, trauma-related alterations in the circadian cycle due to injury of the suprachisamatic nuclei of the anterior hypothalamus and co-morbid pain, anxiety and depression and genetic predisposition [Citation10–14].
Studies involving polysomnography (PSG), an objective measure of sleep, report that 50% of those with moderate–severe TBI present with other treatable sleep disorders and also report changes in sleep architecture amongst those with chronic TBI (>1 year) [Citation6, Citation13, Citation15]. Despite this, however, the relationship between sleep/wake disorders and their impact on recovery and outcomes, particularly outcomes for cognition and communication, is only beginning to receive scientific attention [Citation1, Citation16].
Imaging studies have confirmed the importance of sleep in hippocampal function, learning and the formation of memory in humans [Citation17–19]. Furthermore, reduced hippocampal volume [Citation20] and decreased cognitive performance has been found in individuals with chronic insomnia [Citation19]. In the animal literature, prolonged sleep restriction and or fragmentation has been found to reduce hippocampal cell proliferation and neurogenesis [Citation21–24]. Thus, it has been hypothesized that chronically restricted or fragmented sleep may impact the generation and maturation of new neurons in the adult human brain and that the functional consequences and cognitive disturbances associated with chronic sleep disturbance may be related to reductions in neuro-synaptic plasticity and neurogenesis [Citation25]. Therefore, it is not surprising that sleep/wake disturbances have been shown to further impact already-impaired cognitive and communication abilities post-TBI [Citation1, Citation26]. Individuals with cognitive, communication and sleep impairments often shy away from situations involving multiple conversation partners and/or background noise as they lack the attentional, executive and information processing resources to cope with the complex demands of communication in these environments [Citation1, Citation27]. Disruptions in cognitive processes such as attention, concentration, memory, reasoning and language processing can affect communication processes, such as the ability to attend to and understand conversation [Citation27].
There is recent data suggesting that sleep wake disorders exacerbate impairments in attention post-TBI [Citation28]. Bloomfield et al. [Citation28] concluded that individuals post-TBI with poor sleep quality (by self-report) demonstrated significantly poorer sustained attention than those with TBI who reported good quality of sleep. Sleep/wake disturbances are also associated with memory impairments that continue to be evident at least 3 months post-injury [Citation10, Citation29]. In 2004, Mahmood et al. [Citation16] conducted an investigation of the relationship between sleep disturbance and neurocognitive ability in 87 adults with TBI across all levels of severity, who had been admitted to a comprehensive outpatient rehabilitation programme. They reported that ‘as would be expected, Pittsburgh Sleep Quality Index (PSQI) scores and Beck Depression scores were significantly correlated because of the partial overlap of depression symptoms and insomnia’ ([Citation16], p. 382). They also found that measures sensitive to higher-order executive functioning and speed of information processing showed a positive relationship with PSQI scores. Their findings supported a hypothesis of a predictive relationship between performance on neuropsychological tests and reports of sleep disturbance in adults with TBI.
In 2007, Wilde et al. [Citation30] examined the impact of co-morbid Obstructive Sleep Apnea (OSA) diagnosed after the TBI on the cognitive functioning of patients with TBI, in comparison to patients with TBI but without OSA. Those with TBI with OSA performed significantly worse on verbal and visual delayed recall measures and had more attention lapses than the TBI without OSA patients. The authors concluded that TBI with OSA is associated with greater impairments in sustained attention and memory than those with TBI without OSA.
Despite the clearly-defined association between post-traumatic sleep/wake disorders and post-traumatic impairments in cognition and communication, studies examining interventions for sleep and wakefulness have not included measures of communication and few have included measures of cognition as outcomes. A recent pilot study examined the efficacy of acupuncture to treat insomnia in patients with TBI and used two neuropsychological outcome measures [Citation31]. A recent systematic review of the literature on sleep/wake disorders after TBI [Citation2], that addressed a number of sub-topics including intervention, found that, of the six intervention study papers reviewed, only four included outcome measures assessing non-sleep parameters such as mental health and sustained motor performance [Citation32–35]. However, a recently-published case study demonstrated that successful diagnosis and intervention to optimize sleep and wakefulness post-severe TBI can optimize recovery of cognition and communication. In this case, long-term treatment of sleep and arousal beginning at 1-year post-injury resulted in significant self-reported improvements in attention, memory, language processing and mood [Citation1]. Gains were maintained over time and objective improvements were identified at 31 months post-injury in a follow-up neuropsychological examination.
Although there is mounting awareness that sleep/wake disorders impede the recovery process and exacerbate other trauma-related impairments, there has been limited research examining the impact of successful diagnosis and management of these disorders on outcomes for cognition and communication. Thus, this is the first study with a meaningful sample size that treats the sleep (or wake) disorder and then assesses the effects on cognition. The objective then was to longitudinally examine objective and self-reported outcomes of aspects of cognition, communication and mood in adults with chronic TBI after treatment of post-traumatic sleep/wake disorders.
It was hypothesized that proper assessment and treatment to optimize sleep and wakefulness would facilitate clinically and functionally meaningful recovery and outcomes for aspects of cognition (speed of information processing, working memory, attention), communication (increased ability to participate in conversation, increased social communication) and mood. Further, it was hypothesized that optimization of sleep and/or wakefulness would not result in changes to other specific aspects of cognition and communication such as visual spatial abilities and vocabulary, as this relationship has not been identified in the literature. Finally it was hypothesized that optimization of sleep and wakefulness and optimization of cognition, communication and mood, would increase self-reported participation outcomes (i.e. involvement in life situations, according to the World Health Organization, International Classification of Functioning, Disability and Health) [Citation36].
Methods
The study was approved by the research ethics review board of the University of Toronto, Sunnybrook Health Sciences Centre, Toronto, and three other hospitals where recruitment also took place. Participants were recruited from across Southern Ontario through clinicians working in major rehabilitation centres in Toronto, community-based rehabilitation practitioners and an announcement on the Brain Injury Association of Canada website. Clinicians completed a referral form that was sent to the Principal Investigator. All participants were required to have a clinician referral for consideration of participation.
In order to be included in the study, participants had to meet the following criteria: (1) be between the ages of 18–60 years; (2) have a diagnosis of moderate–severe TBI as determined by one or more of the following: Glasgow Coma Scale (GCS) Rating ≤12 [Citation37], post-traumatic amnesia ≥24 hours, retrograde amnesia; evidence of intracranial bleed, required neurosurgery or complicated mild traumatic brain injury defined as GCS Rating 12–15 with ongoing unresolved symptoms; (3) be a minimum of 1-year post-injury in order to minimize the possible confound of spontaneous recovery; (4) have reported cognitive-communication impairments including challenges with attention, memory, information processing and social communication. Social communication included things such as being able to participate in multi-speaker conversations, follow conversation in noisy environments and on the telephone and be able to respond quickly and appropriately in conversation; (5) have self-reported post-traumatic sleep and or wake disturbance (excessive daytime sleepiness), defined as dissatisfaction with sleep such that it was a cause of significant distress that had not been diagnosed or treated; (6) have obtained a score of 15 or greater on the Insomnia Severity Index (ISI) [Citation38], during the initial interview. This represents ‘Clinical Insomnia’ (see Measures) for those with night sleep problems. For those with hypersomnia; a score of 8–14 was acceptable for inclusion, as this represents ‘Sub-threshold Insomnia’; (7) be functioning at a Level 8 or greater (Purposeful, Appropriate: Stand-by Assistance) on the Rancho Los Amigos Cognitive Scale Revised [Citation36], such that they were able to understand the purpose of the study and comply with the protocol; (8) be able to speak and read English; and (9) have provided written informed consent to participate.
Individuals were excluded from the study if they (1) had a history of psychosis; (2) were actively using substances such as alcohol or non-prescription drugs; (3) had a pre-injury diagnosis of attention deficit disorder; or (4) were actively re-training the cognitive and communication domains being measured (i.e. exercises designed to retrain attention and memory and communication); or (5) had alcohol or caffeine the day and night before their PSG.
Study protocol
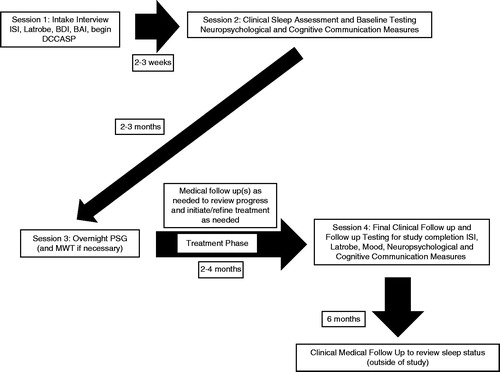
A visual representation of the study protocol and timeline is provided in . During the first session, participants were administered the ISI [Citation37], the Latrobe Communication Questionnaire (Latrobe-QC) [Citation39], the Beck Depression and Anxiety Inventories (BDI/BAI) [Citation40, Citation41] and were requested to begin regular completion of the Daily Cognitive-Communication and Sleep Profile (DCCASP) [Citation1, Citation42].
Session two, which followed 2–3 weeks after session one, involved a comprehensive clinical evaluation of sleep and wakefulness by a neurologist who was also a diplomat of the American Board of Sleep Medicine. This was followed on the same day by a baseline evaluation of neuropsychological status and cognitive-communication status. Scoring of the measures was conducted by a qualified psychometrist/research assistant who was blind to the brain injury diagnosis and research status of the participants.
Session three, which occurred within 2–3 months after session two, involved overnight PSG and a Multiple Wake Test (MWT) [Citation43] the following day where indicated. The scheduling of this session varied somewhat according to availability of participants and appointments at the sleep lab. Participants returned for a follow-up appointment with the sleep specialist to discuss findings, recommendations and treatment. Treatment was individualized and was not part of the experimental protocol. Treatment specifics were noted as part of the detail recorded for each participant but were not used in any analysis.
Once treatment was initiated, participants continued to complete the DCCASP and report to the investigator on a weekly-basis on progress, barriers and any issues impacting success of the treatment. Participants were able to electronically complete this measure and submit the completed forms to a database bi-weekly. This methodology was chosen to increase ease and compliance with completion. If it was observed that a participant had not submitted any data during a specific time period, the Principal Investigator followed up by telephone. It was anticipated that there would be a different number of weeks of completed DCCASP data available for each participant as the length of total time in the study (in particular, between sessions 3 and 4) would vary across participants depending on the nature of the sleep diagnosis, intervention and clinical progress.
Session four involved a follow-up appointment, between 2–4 weeks after session three, to discuss progress with the sleep specialist. Once sleep and/or wakefulness were optimized (as determined collaboratively with the neurologist and the participant), participants returned for a final follow-up assessment of neuropsychological status, mood and cognitive-communication status. Some participants required a number of follow-up visits with the sleep specialist to optimize their treatment. Information regarding treatment is also provided (see ) in order to further understand the heterogeneity and complexity of the disorders across clients. The sleep hygiene programme involved participant-specific strategies to facilitate good sleep-promoting habits, such as maintaining a regular sleep–wake schedule.
Table I. Participant demographics and baseline characteristics.
Measures
Neuropsychological and communication battery
Measures were chosen that were repeatable, assessed cognitive functions and mood states, were sensitive to sleep and sleep-deprivation, identified performance in the cognitive domains necessary for communication including auditory attention, information processing and working memory and evaluated communication function.
The test battery consisted of objective and self-report measures:
Repeatable Battery for the Assessment of Neuropsychological Performance (RBANS) [Citation44]. The RBANS is one of the most commonly-used measures of neuro/cognitive functioning in brain injury research, with well-established and documented psychometric properties. It provides standardized scores and has two parallel versions, which allows for re-testing at any time [Citation45].
Speed and Capacity of Language Processing (SCOLP) [Citation46]. This test is sensitive to the slowing of language and cognitive functioning that often occur following brain injury. The SCOLP is composed of two brief tests, Speed of Comprehension Test and Spot-the-Word Vocabulary Test. Four parallel versions of the Speed of Comprehension Test are available for re-test purposes. The psychometric properties of this test have been well established [Citation47].
Test of Everyday Attention (TEA) [Citation48]. The TEA was designed to assess several independent attention systems serving different functions in everyday behaviour. The TEA provides norm-referenced scores on tests and has three parallel versions for test–re-test purposes. Further, the primary mode of stimulus presentation is auditory, which underlies language processing and communication. Its psychometric properties have been well documented [Citation47].
Latrobe QC [Citation39]. This self-report instrument measures perceived communication ability in a variety of domains including comprehension, expression, speed of processing, social comfort and communication competency. Content and test–re-test reliability and discriminant validity have been demonstrated with adults following TBI [Citation49].
BDI–II [Citation40]. This 21-question multiple-choice self-report inventory is one of the most widely used instruments for measuring the severity of depression over the past 2 weeks. Its psychometric properties have been well established [Citation40].
BAI [Citation41]. This 21-item questionnaire is used to assess the severity of an individual’s anxiety by evaluating the somatic and cognitive symptoms of anxiety over the last 7 days. The BAI has also been widely used in clinical research with well-documented psychometric properties [Citation41].
DCCASP [Citation1, Citation42]. This profile is a series of seven self-report 5-point Likert rating scales developed for use in clinical practice and as a research tool, as a means of monitoring daily fluctuations in cognitive-communication function in relation to quality of sleep. It was initially developed specifically for an ABI population as well as to fill a measurement gap in evaluating sleep function by self-report and the impact of sleep (or lack thereof) on daytime function. Its preliminary reliability and validity have been established in a normative population [Citation42]. Work is underway to determine the reliability and validity in a large sample of persons with brain injury.
ISI [Citation37]. This index is designed to be both a brief screening measure of insomnia and an outcomes measure for use in treatment research. Its reliability and validity have been established and it has also been found to be sensitive to change following pharmacologic and/or behavioural intervention for primary insomnia [Citation51].
Sleep measures
Overnight polysomnography (PSG) was performed followed by a Maintenance of Wakefulness Test (MWT) the following day where clinically indicated [Citation43]. The PSG and MWT test were scored by a registered PSG technologist and the diagnoses of sleep disorders were determined by the physician based on available data.
Polysomnography. All PSGs were recorded on digital equipment using standard recording and scoring methods [Citation52]. During each study, monitoring of the following took place: Electroencephalogram (EEG; electrodes C3, C4, O1, O2), A1, A2 (reference leads at the mastoids), electro-oculogram (EOG; LOC [below and lateral to left eye], ROC [above and lateral to right eye]), surface electromyography (EMG; mentalis/submentalis, anterior tibialis), respiratory measures (abdominal and thoracic effort [measured with respiratory inductive plethysmography belts], nasal/oral pressure [measured with a nasal/oral pressure transducer], nasal/oral flow [measured with a thermistor]), oxygen saturation and a 2-lead ECG. Expanded EEG or EMG montages were used if nocturnal seizures or parasomnias were suspected. All studies were videotaped and audiovisual recordings were time-synchronized to the remainder of the data. Sleep was manually scored according to the American Academy of Sleep Medicine [AASM] criteria [Citation53]. Sleep disorder diagnoses were made after clinical assessment according to the ICSD-2 [Citation54].
Maintenance of Wakefulness Test (MWT) [Citation43]. This test involves giving the participant four opportunities to maintain wakefulness during the day at 9 am, 11 am, 1 pm, 3 pm for 40 min each time. They are placed in a slightly reclined position, in a dimly lit room, with the instructions ‘Please sit quietly and try to remain awake’. The latency to sleep onset was recorded. The MWT has also been confirmed as an important test to quantify Excessive Daytime Sleepiness, as well as to provide an indicator of future risk of accidents [Citation43, Citation54]. Patients with TBI are typically administered the Multiple Sleep Latency Test [Citation55], however the MWT is more appropriate as excessive daytime sleepiness is the more common sleep concern for those with severe TBI [Citation56]. Furthermore, excessive daytime sleepiness impairs function and puts patients at risk of sustaining further injury [Citation54].
Epworth Sleepiness Scale (ESS) [Citation57]. This eight question scale was designed to evaluate daytime sleepiness and asks the respondent the likelihood that they would fall asleep under various scenarios. This questionnaire was administered as part of the routine clinical evaluation of sleep. Most rested individuals have a score under 10. The reliability and validity of the ESS has been well established [Citation58].
Participation measures
Formal measures of participation were not administered. However, change on the following items was collected: employment status, participation in school (academic status), relationship status, participation in rehabilitation, level of independence and socialization. A series of six questions was asked of the participants that related to each of the above areas. Each question was in the following format: ‘Have there been any changes in “your employment status” since we first met?’ If changes were reported, probes were asked to determine the nature of the change. Answers were recorded in note format that were reviewed and categorized once all participants had completed follow-up measures.
Statistical analyses
Data were analysed in two formats: as aggregate data for the purpose of examining trends and as a single case series, including descriptive information. The assessment of results obtained from objective measures and self-report questionnaires was made through statistical analysis using paired non-parametric Wilcoxon Signed Rank tests in SAS 9.2 and examination of group change scores at baseline and follow-up assessment [Citation59]. The Wilcoxon Signed Rank test was chosen due to the small sample size and heterogeneity, both at baseline and at follow-up among the sample (i.e. no normal distribution of the sample). In order to take a conservative approach to the analysis, a Bonferroni correction was then applied to correct for the large number of measures in the protocol and thus, control for the possibility of inflating type 1 errors. Grouped DCCASP results were analysed by ARIMA modelling interrupted time series and multivariate time series to determine correlations between the domains. The week averages, of the last 4 weeks of observation prior to intervention, were selected as the baseline period and the week averages for the last 4 weeks of observation as the post treatment period for all seven sleep quality domains. This time period was chosen as the time to establish treatment efficacy varied across participants. The last 4 weeks of treatment therefore, represents the time point by which all participants had responded to their individualized treatments, thus this was viewed as an equilibrium point from reaching treatment efficacy to the last period of follow-up in the study. A repeated measure segmented regression model was used to assess the impact of the treatment on the sleep quality measures. The Generalized Estimation Equation (GEE) method was used to model the covariance structure of the repeated measures over time.
Although there were numerous measures included in this study, there was limited chance of having missing data. The only opportunity was in completion of the DCCASP, filled out by participants on their own throughout the duration of the study. The minimum completion was 4 weeks of baseline prior to treatment implementation and then the last 4 weeks of treatment at the completion of the study. In order for the same amount of data to be analysed per participant, data collected in between the minimum baseline period and the minimum treatment period were not included in the analysis. The amount of data discarded varied across participants as treatments and course of treatment were individualized and, thus, varied accordingly. Data on this measure were analysed weekly so if there were more than 3 days in a given week where the form was not completed the week of DCCASP would be dropped from analysis. It was anticipated that participants would be more likely to miss out an entire day rather than one or more of the seven questions that were required to be answered each day. If more than three individual questions were not completed then that particular day would be treated as missing data.
Results
Twenty-one individuals with TBI were referred for participation over a 2-year enrolment period. Minimum enrolment was set at 12 so that participants could be staggered into the study at 2-monthly intervals to minimize congestion of appointments at the sleep lab. Seven individuals did not meet the inclusion criteria, so 14 individuals were enrolled in the study. Of those who did not meet criteria for inclusion, three were from out of province and their travel costs for participation could not be covered, two had cognitive impairments due to brain tumour rather than TBI, one was actively using substances and one was aged 17 (minimum age was 18 years). Two were lost to follow-up after some baseline testing data were obtained. One completed baseline cognitive testing but withdrew before having overnight polysomnography. This participant was unable to make the commitment to attend the medical appointments. The other individual who was lost to follow-up began actively using substances and developed psychosis. They were removed from the study, but provided with follow-up medical care. Recruitment proved challenging due to the time commitment required of participants for a thorough investigation and treatment. Twelve participants completed the study; 10 individuals with moderate–severe TBI and two with mild TBI and persistent symptoms. indicates the socio-demographic information and clinical characteristics. The mean age was 38.0 (median = 34.5; SD = 14.2; range = 18–58) years and the mean years of education were 15.6 (median = 16 years, SD = 3.0, range = 10–21) years. There were six females and six males; five were married, one was widowed, one was divorced, one was in a relationship and four were single. The mean time post-injury was 4.8 years (median = 2.0 years, SD = 7.2, range = 1–22 years).
Sleep
When the aggregated sleep findings were considered from the perspective of TBI in comparison to normal healthy adults sleepers, a number of trends were observed, including reduced total sleep time, reduced sleep efficiency (amount of time asleep as compared to time in bed), increased sleep onset latency (time to fall asleep), increased stage N1 sleep, decreased N3 sleep, alterations in REM percentage and latency (). Specific details of the sleep findings will be reported elsewhere.
Table II. Statistical analysis of pre–post measures group data.
All 12 participants had a (relatively) treatable sleep/wake disorder with reported onset after the TBI (). While subjects were recruited on this basis, this finding, in-and-of-itself, was important given that two of the participants were more than 15 years post-injury and both had been struggling with unaddressed trauma-related sleep disorders. All participants except one reported that the sleep difficulties began after their brain injury. One person was assessed to have mild OSA that did not warrant treatment but, after the brain injury, developed central sleep apnea, severe insomnia and exacerbated OSA that warranted treatment. Further, one participant who was 2 years post-injury had undergone PSG at another centre and was told no intervention was appropriate. Two participants, who were actively driving at the onset of the study, had inadequate alertness for safe driving as indicated by a sleep intrusion on the MWT. The standards of failure for the MWT and driving for the medical centre sleep lab are such that any sleep intrusion regardless of the duration is considered a fail and driving is medically suspended by the sleep physician sending a letter to the Ministry of Transportation. The suspension was in place until they could demonstrate sufficient alertness with treatment and a repeat MWT with a pass (i.e. no sleep intrusions). The time to respond to treatment varied among participants according to diagnosis, comorbidities and level of support and some required a number of follow-up appointments to optimize sleep and wakefulness. All were actively followed for at least 1 month after their sleep and daytime wakefulness was optimized in order to complete the study and were seen again at 6-months after completion of the study for medical follow-up (or earlier if problems arose). Participants were in the study an average of 8 months, range 4–13 months.
Objective measures
With respect to aggregate data, positive changes were noted on each sub-test of every measure, after response to treatment of sleep and/or wakefulness (). The greatest change, however, was observed in the areas of speed of information processing (SCOLP Speed of Comprehension sub-test, corrected p = 0.007), language (RBANS language, corrected p = 0.014), as well as the RBANS total test score, which includes attention and memory sub-tests (corrected p = 0.014) and mood (BDI, corrected p = 0.031) (). These findings are consistent with the hypothesis that those cognitive domains underlying successful communication; that is, sustained auditory attention and speed of information processing would be most sensitive to changes in sleep and or wakefulness. Furthermore, mood is highly sensitive to sleep restriction and so it was anticipated that mood would improve with improvements in quantity and quality of sleep and or improvements in quality of wakefulness and arousal [Citation60]. It was expected that improvements would be seen in social communication as measured by the Latrobe QC. Although improvements were seen in scores (and reported functional improvements), the magnitude of change was not statistically significant. These findings also support the hypothesis of little or no change in vocabulary, in visual spatial function and visual spatial attention.
Table III. Mood and sleep raw scores.
Post-treatment, all 12 participants rated their sleep as being improved to some degree (ISI) and 10 of 12 reported improvements in their overall level of insomnia severity (). Eleven participants reported improvements in mood raw scores for either depression and/or anxiety; however, one individual’s (participant 8) depression worsened and was assessed in the severe category post-intervention (BDI; ) Anxiety improved for 10 of 12 participants (BAI), but two participants reported an increase, which they attributed to pressures of their employment status. In regards to communication, eight of 12 reported improved perception of their overall communication competence (Latrobe QC), two of 12 perceived their overall communication competence as being the same and two of 12 perceived their communication competence as being somewhat worse. All continued to report that they found group conversations difficult to follow. However, while they tended to avoid this type of social interaction whenever possible at baseline, 11 of 12 reported an increase in comfort in this situation at follow-up.
When individual participant scores are examined, it is seen that 11 of 12 participants displayed a statistically significant improvement in speed and capacity of language processing (SCOLP-speed). On sub-tests of the RBANS, seven of 12 participants showed improvements in their immediate memory and 10 of 12 showed improvements in their delayed memory scores. Ten of 12 participants showed improvements in language, driven primarily by improvements in semantic fluency, (which is a reflection of processing speed). Eight of 12 showed improvements in attention. As previously stated, the authors were not expecting to see much change in visual spatial ability, nor is it directly related to communication (with the exception of facial recognition and perception of emotion) so it was not an area of particular interest. However, five of 12 participants showed improvements in scores. On overall RBANS raw scores and percentile scores, 10 of 12 participants showed improvements. The TEA was where the most variability of performance was seen upon follow-up. As this study was interested in the impact of attention as it pertains to communication, the greatest improvements were anticipated in auditory verbal working memory (elevator counting with distraction and elevator counting with reversal sub-tests) and sustained auditory attention/vigilance (lottery sub-test) in response to improvements in sleep. For these sub-tests, seven of 12 participants improved their auditory verbal working memory scores. Nine of 12 showed large improvements in sustained auditory attention.
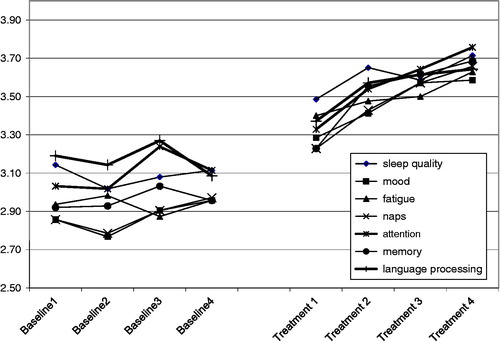
Daily cognitive-communication and sleep profile
All 10 participants who completed the DCCASP reported self-perceived changes in cognitive function (attention, memory) and communication (language processing) in response to optimization of sleep (). For those who required different treatments (i.e. those who required changes to or ‘fine-tuning’ of their treatment), there was a clearly observed improvement in function when treatment was optimized. The trend before treatment was not significant, whereas the trend significantly increased after treatment by 0.12, p = 0.022 and the level also increased by 0.35, p = 0.014 (see ). The covariance structure was modelled by AR(1) with parameter alpha equal to 0.75. Two participants were unable to complete the DCCASP, one due to significant memory impairments and the amount of support required and another due to fatigue and the response burden. The completion rate for the remaining participants was 96%, resulting in very few instances of missing data. The data that were missing did not require that any weeks of DCCASP be removed from the analysis.
The DCCASP was particularly helpful in determining when an individual was not responding to the intervention prescribed and needed to be re-evaluated. Further, those 10 participants who completed the DCCASP reported that it facilitated their awareness of their sleep and its direct relationship with their daytime cognitive and communication function, their mood and level of fatigue.
Participation
At the onset of the study, three participants were in school, one with full-time 1–1 support and two with partial support. Five were unable to work, two were working in paid employment, one was working in a volunteer capacity and one was attempting a graduated return to work. Six were still in active rehabilitation. All reported reduced socialization in comparison to pre-injury levels due to fatigue and cognitive-communication difficulties. Eleven participants reported being uncomfortable and/or lacking confidence in situations requiring social communication. Seven reported reduced levels of independence at baseline. Upon completion of the study, one of the participants previously not working returned to work part-time, one began to pursue return to work and one of the students pursuing a part-time online college degree was able to increase his course load. One of the younger participants, who had been living at home with his family, was able to move into a partially supervized independent living situation (a group home). One participant whose spouse had been actively involved in rehabilitation, reported that the spouse was able to ‘pull back’ and return to more of a partner role. Thus, although we did not formally measure this construct, it was positive to note that, for five of the 12 participants, including one participant who was 22 years post-injury, there were positive changes in participation. The others did not report any changes in participation.
Discussion
This study builds upon the previous case study results published by Wiseman-Hakes et al. [Citation1] and collectively these are the first studies to longitudinally examine the impact of treatment of sleep/wake disorders associated with TBI, on recovery of cognition, communication and mood. Prior studies have been either cross-sectional and retrospective in nature without objective examination of sleep [Citation16] or have been limited to examining one aspect of cognition [Citation24] or only one sleep diagnosis [Citation30]. Other studies have examined sleep disorders but have not looked at their impact on outcomes. These results suggest that the proper identification, diagnosis and treatment of sleep/wake disorders associated with TBI can facilitate and in some cases optimize, outcomes in the areas of sustained attention (vigilance), divided attention, working memory and speed and capacity of language (and information) processing, even for those individuals many years post-injury.
The authors have chosen to present the results with both aggregate data and individually with the view that it strengthens the analysis and interpretation. Through analysis and examination of aggregate data, this study is able to present changes and trends in an area that has not been studied longitudinally to date. Furthermore, presentation of individual findings captures the considerable heterogeneity of this population. Together, these two approaches to the data analysis may help to inform future research questions in this young field of study. In addition, it is at the individual level that those with TBI experience these debilitating symptoms and this is an important consideration when investigating outcomes. Despite their consistent diagnosis of chronic TBI and sleep disorders, participants were very different in their presentations at baseline and throughout the study. All had, and were aware of, cognitive and communication impairments, yet some were very adept at compensating and were quite high functioning at baseline. Two of the participants had extremely high objectively-measured neuropsychological scores at baseline and, for these two individuals, a ceiling effect was noted for some measures, even at baseline. However, their perception of sleep and function as measured by the DCCASP () does capture the impact of these impairments and their response to intervention. It is possible that for the six involved in active rehabilitation at the time of the study their participation in rehabilitation may have played a role in their increased participation. However, all reported that they perceived their increase as being a result of improved sleep and wakefulness. The authors acknowledge the possibility that some of the change may be due in part to participation in rehabilitation. However, this study attempted to be as rigorous as possible to control for this and those participants who were in TBI rehabilitation were not involved in any direct training of the cognitive or communication parameters under investigation. Their rehabilitation programmes were functional, individualized and contextually-based; involving helping with school work, activities of daily living such as scheduling, facilitating social integration and physiotherapy. The Principal Investigator was in regular contact with the rehabilitation professionals working with the participants to make sure that no exclusions from the study were warranted. Furthermore, the authors looked to see if there was any difference among participants who were in active rehabilitation and those who were not. Thus, the mean change score was analysed for each sub-group and it was discovered that there were no meaningful statistically significant changes between the group involved in rehabilitation and those who were not.
All participants, regardless of their stage of recovery, showed clinically and functionally relevant improvements across most measures in response to intervention, that in some cases reached statistical significance. Some of the sub-tests on the TEA appeared to be quite difficult for a number of participants and it was on this test that the greatest impact of self-reported cognitive fatigue was seen. It is not possible, however, to determine if this was a true indication of fatigue or possibly reduced attentional capacity. It is also important to note that the objective of this study was not to determine treatment efficacy or optimal methods of treatment, but rather to examine the impact of treatment on cognitive and communication outcomes. Thus, the results also suggest that communication competence and mood may be enhanced in response to optimization of sleep and wakefulness. These findings are particularly important and provide evidence that functional improvements can be made in response to optimization of sleep and or wakefulness, even for those who are many years post-injury. Although this study design did not allow for the establishment of clear causal relationships, the findings, as well as the fact that treatments were individualized according to the specific disturbance, do highlight the critical relationship between sleep, mood, cognitive function and communication-competence. All of these areas are often compromised following TBI and so, by failing to screen for and treat sleep/wake disturbances, patients may not be able to reach their full potential for recovery.
Study limitations
Given the small sample size and lack of control group, these findings may not be generalizable to a larger population of individuals with TBI. The authors did not have access to cognitive-behavioural therapies for insomnia, which may have heightened the success of some of the interventions. Non-specific factors unrelated to the intervention such as changes in employment status, levels of stress and illness were not formally accounted for in the results; however, this information was collected daily via the DCCASP and associated with short-term variations in perceived function in response to these factors. It is also acknowledged that, despite attempts to minimize the confound of spontaneous recovery, it is possible some of the changes experienced by the participants may be due in part to natural recovery. However, these participants ranged from 1–22 years post-injury and, despite this time frame, the cognitive problem continued to persist and remained fairly stable across baseline measurements. It was only in response to treatment of sleep that we saw this ‘sudden’ improvement. This supports an effect of the treatment and makes it less likely a spontaneous change.
The study measures were carefully chosen to evaluate aspects of cognition, communication and mood that are sensitive to restrictions in and changes in sleep. As with any assessment there is no ‘ideal battery’ and each measure had its strengths and weaknesses. Like any battery, the objective measures may not have been sensitive enough to pick up subtle cognitive deficits in those very high functioning participants and so a ceiling effect was noted for some measures at baseline. The DCCASP also has a heavy response burden as it should be completed daily (although it only takes 5 min) and some participants required regular support to complete it. As noted earlier there were two participants who were unable to complete the measure. However, all those who did complete the DCCASP found it educational and informative. It is also possible that the DCCASP hyper-sensitized participants to the relationship between their sleep and day-time function and, thus, may have impacted their self-reported scores. Finally, the study was quite ‘measure-intensive’, although the addition of a sensitive quality-of-life measure and a measure of occupation may have been helpful as positive changes were observed in these areas.
Conclusions
To the best of the authors’ knowledge, this is the first study to examine change in objective performance and self-reported function in adults with chronic TBI and sleep/wake disorders, in response to treatment for sleep. Furthermore, the PSG and MWT results of this study showed sleep disorders to be highly prevalent in this sample at baseline. Although this study recruited on the basis of clinician and or self-report of sleep/wake problems, it was striking that participants ranged from 1–22 years post-injury and only one had had a prior assessment of sleep with no accompanying diagnosis or treatment plan. Every participant was diagnosed with one or more treatable sleep disorders. These findings reinforce the necessity for routine screening for sleep disorders in persons with TBI. Moreover, when pharmacological management is prescribed for sequelae of TBI, as well as other co-morbid health issues, it is essential to consider the possible effects of prescribed medications on sleep and wakefulness, both desirable and undesirable.
These results add to a small but growing body of evidence that sleep/wake disorders associated with TBI remain prevalent many years post-injury if left untreated. Furthermore, based on understanding of the animal and human literature regarding the role of sleep restriction or fragmentation, these disorders may, in fact, compromise recovery and outcomes, by exacerbating trauma-related cognitive, communication and mood impairments. As such, these findings underscore a clear need for the systematic evaluation and treatment of sleep and wakefulness post-TBI. There is a need for further study and understanding of the mechanisms by which sleep and wake disorders impact cognition, communication and mood outcomes in individuals with TBI.
Declaration of interest
The authors report no conflicts of interest. The study was funded through a fellowship in Clinical Research from the Canadian Institutes for Health Research and a University of Toronto Open Fellowship. Other support came from the Toronto Rehabilitation Institute who receives funding through the Ontario Ministry of Long Term Care. Support was also provided through the Ontario Work Study Program.
Acknowledgements
We are grateful to Dr Peter Meerlo, PhD, for his review of this work and insightful comments.
References
- Wiseman-Hakes C, Victor JC, Brandys C, Murray BJ. Impact of post-traumatic hypersomnia on functional recovery of cognition and communication. Brain Injury 2011;25:1256–1265
- Wiseman-Hakes C, Colantonio A, Gargaro J. Sleep and wake disorders following traumatic brain injury: A systematic review. Critical Reviews in Physical and Rehabilitation Medicine 2009;21:317–374
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine 2007;3:519–528
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience 2005;133:911–917
- Meerlo P, Mistlberger RE, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proceedings of the National Academy of Science 2006;103:19170–19175
- Ouellet MC, Morin CM. Subjective and objective measures of insomnia in the context of traumatic brain injury: A preliminary study. Sleep Medicine 2006;7:486–497
- Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2006;87:278–285
- Ponsford JL, Ziino C, Parcell D, Shekleton JA, Roper M, Redman JR, Rajaratnam SMW. Fatigue and sleep disturbance following traumatic brain injury: Their nature, causes and potential treatments. Journal of Head Trauma Rehabilitation 2012;27:224–233
- Rao V, Spiro J, Vaishnavi S, Rastogi P, Mielke M, Noll K, Cornwell E, Schretlen D, Makely M. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Injury 2008;22:381–386
- Baumann CR. Traumatic brain injury and disturbed sleep and wakefulness. Neuromolecular Medicine 2012;14:205–212
- Gosselin N, Tellier M. Patients with traumatic brain injury are at high risk of developing chronic sleep-wake disturbances. Journal of Neurology, Neurosurgery & Psychiatry 2010;81:1297
- Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury: Areview. CNS Drugs 2011;25:175–185
- Parcell DL, Ponsford JL, Redman JR, Rajaratnam SM. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: A preliminary study. Archives of Physical Medicine & Rehabilitation 2008;89:843–850
- Baumann CR, Werth E, Stocke R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: A prospective study. Brain 2007;130:1873–1883
- Shekleton JA, Parcell DL, Redman JR, Ponsford JL, Rajaratnam SMW. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 2010;74:1732–1738
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. Journal of Head Trauma Rehabilitation 2004;19:378–390
- Peigneux P, Laurey S, Fuch S, Colltte F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 2004;44:535–545
- Orban P, Rauchs G, Balteau E, Degueldre C, Luxen A, Maquet P, Peigneux P. Sleep after spatial learning promotes covert reorganization of brain activity. Proceedings of the National Academy of Sciences USA 2006;103:7124–7129
- Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biological Psychiatry 2006;60:1324–1330
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Medicine 2003;4:451–454
- Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, Hennig J, Perlis ML, Tebartz van Elst L, Feige B. Chronic insomnia and MRI-measured hippocampal volumes: A pilot study. Sleep 2007;30:955–958
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. European Journal of Neuroscience 2005;22:2111–2116
- Novati A, Hulshof HJ, Koolhaas JM, Lucassen PJ, Meerlo P. Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience 2011;190:145–155
- Guzman-Marin R, Suntsova N, Bahir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the Hippocampal denate gyrus of the adult rat. Sleep 2008;31:167–175
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Medicine Reviews 2009;13:187–194
- Struchen MA, Clark AN, Sander AM, Mills MR, Evans G, Kurtz D. Relation of executive functioning and social communication measures to functional outcomes following traumatic brain injury. NeuroRehabilitation 2008;23:185–198
- MacDonald S, Wiseman-Hakes C. Knowledge translation in ABI rehabilitation: A model for consolidating and applying the evidence for cognitive-communication interventions. Brain Injury 2010;24:486–508
- Bloomfield ILM, Espie CA, Evans JJ. Do sleep difficulties exacerbate deficits in sustained attention following traumatic brain injury? Journal of the International Neuropsychological Society 2010;16:17–25
- Lundin A, de Boussard C, Edman G, Borg J. Symptoms and disability until 3 months after mild TBI. Brain Injury 2006;20:799–806
- Wilde MC, Castriotta RJ, Lai JM, Atanasov S, Masel BE, Kuna ST. Cognitive impairment in patients with traumatic brain injury and obstructive sleep apnea. Archives of Physical Medicine and Rehabilitation 2007;88:1284–1288
- Zollman FS, Larson EB, Wasek-Throm LK, Cyborski CM, Bode RK. Acupuncture for treatment of Insomnia in patients with traumatic brain injury: A pilot intervention study. Journal of Head Trauma Rehabilitation 2012;27:135–142
- Ouellet MC, Morin CM. Cognitive behavioral therapy for insomnia associated with traumatic brain injury: A single-case study. Archives of Physical Medicine and Rehabilitation 2004;85:1298–1302
- Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: A single-case experimental design. Archives of Physical Medicine and Rehabilitation 2007;88:1581–1592
- Castriotta RJ, Atanasov S, Wilde MC, Masel BE, Lai JM, Kuna ST. Treatment of sleep disorders after traumatic brain injury. Journal of Clinical Sleep Medicine 2009;5:137–144
- Shan LP, Ashworth NL. Comparison of lorazepam and zopiclone for insomnia in patients with stroke and brain injury: A randomized, crossover, double-blinded trial. American Journal of Physical Medicine and Rehabilitation 2004;83:421–427
- WHO. International classification of functioning, disability and health: ICF. Geneva: World Health Organization; 2001. p 299
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. The Lancet 1974;304:81–84
- Morin CM. Insomnia: Psychological assessment and management. New York, NY: Guilford Press; 1993
- Hagen C, Malkmus D, Stenderup-Bowman K. The Rancho levels of cognitive functioning. 2nd ed. Downey, CA: Rancho Los Amigos National Rehabilitation Centre; 1973. Available online at: http://www.rancho.org/Research_RanchoLevels.aspx, accessed 29 April 2013
- Douglas J, O'Flaherty C, Snow P. Measuring perception of communicative ability: The development and evaluation of the La Trobe Communication Questionnaire. Aphasiology 2000;14:251–268
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review 1988;8:77–100
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology 1988;56:893–897
- Fung C, Nguyen M, Wiseman-Hakes C, Colantonio A. Preliminary validation of a new instrument for monitoring sleep, wakefulness and daytime function: Daily cognitive-communication and sleep profile. Sleep Medicine 2011;12(Suppl 1):S127
- Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, Belisle C, Erman MK, Hayduk R, Hosn R, et al. A normative study of the maintenance of wakefulness test (MWT). Electroencephalography and Clinical Neurophysiology 1997;103:554–562
- Randolph C. Repeatable battery for the assessment of neuropsychological status (RBANS): Manual. San Antonio, TX: The Psychological Corporation; 1998
- McKay C, Casey JE, Wertheimer J, Fichtenberg NL. Reliability and validity of the RBANS in a traumatic brain injured sample. Archives of Clinical Neuropsychology 2007;22:91–98
- Baddeley AD, Emslie H, Nimmo-Smith I. The speed and capacity of language processing (SCOLP) test: Manual. Bury St. Edmunds, England; London, UK: Harcourt Assessment; 1992
- Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests: Administration, norms and commentary. 3rd ed. Oxford, England: Oxford University Press; 2006
- Robertson H, Ward T, Ridgeway V, Nimmo-Smith I. Test of everyday attention. Flempton, England: Thames Valley Test; 1994
- Douglas J, Bracy C, Snow P. Measuring perceived communicative ability after traumatic brain injury: Reliability and validity of the La Trobe Communication Questionnaire. Journal of Head Trauma Rehabilitation 2007;22:31–38
- Bastien CH, Vallie'res A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2000;2:297–307
- Iber C, Ancoli-Israel S, Chesson A Jr, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007
- American Academy of Sleep Medicine (AASM). Manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine Publications; 2007
- American Academy of Sleep Medicine (AASM). International Classification of Sleep Disorders. 2nd ed. Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005
- Coelho FM, Narayansingh M, Murray BJ. Testing sleepiness and vigilance in the sleep laboratory. Current Opinion in Pulmonary Medicine 2011;17:406–411
- Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the Multiple Sleep Latency Test (MSLT): A standard measure of sleepiness. Sleep 1986;9:519–524
- Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, Bassetti CL. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 2005;65:147–149
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991;14:540–545
- Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep 1992;15:376–381
- SAS Institute Inc. SAS system for windows v9.2. Cary, NC: SAS Institute Inc.; 2008