Abstract
Background: The role of fixed airflow obstruction (FAO) in asthma is unclear. Objective: To assess the relationship between FAO and clinical features of asthma and the effect of FAO on treatment response. Methods: Post hoc descriptive analysis of data stratified by FAO category (screening post-albuterol FEV1/FVC <lower limit of normal [LLN] [FAO+] or ≥LLN [FAO−]) from two 12-week, randomized, placebo-controlled studies of budesonide/formoterol or the monocomponents in mild−moderate (study I; aged ≥6 years; NCT00651651; placebo run-in) or moderate−severe (study II; ≥12 years; NCT00652002; budesonide run-in) asthma patients. Results: At baseline, FAO+ versus FAO− patients were more likely male and had longer asthma duration and worse pulmonary function. During the treatment period, lung function and asthma control measures with placebo were generally worse in FAO+ versus FAO− patients. Budesonide was effective on most end points in both FAO+ and FAO− patients. In contrast to FAO− patients, FAO+ patients were unresponsive to formoterol monotherapy in both study populations. Consistently greater improvements in most end points (including worsening of asthma as predefined by specific lung function parameters or clinical symptoms) were observed moving from formoterol to budesonide to budesonide/formoterol in both FAO+ and FAO− patients, with generally greater than additive effects on lung function with budesonide/formoterol in FAO+ patients. Conclusions: FAO+ patients tended to be more impaired and at greater risk for an asthma event versus FAO− patients. While FAO+ patients were non-responsive to formoterol monotherapy, they retained responsiveness to budesonide and had the greatest lung function and control responses to budesonide/formoterol that were similar to or greater than responses of FAO− patients.
Introduction
Asthma is characterized by variable and recurring symptoms, airway inflammation, bronchial hyperresponsiveness and variable airflow obstruction [Citation1]. Patients meeting a conventional asthma definition but with persistent airflow limitation that is not fully reversible (hereafter: fixed airflow obstruction [FAO]) are well recognized by practicing clinicians and asthma researchers. The prevalence of FAO in severe or difficult-to-treat asthma ranges from 55% to 60% based on the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) using a post-bronchodilator threshold of ≤70% [Citation2,Citation3]. FAO in asthma has been attributed to structural changes due to airway remodeling [Citation4–6].
There is no accepted definition of FAO in asthma. The following two definitions have been recommended for chronic obstructive pulmonary disease (COPD), where the persistence of post-bronchodilator airway obstruction is a defining feature: FEV1/FVC < 0.70 [Citation7] or FEV1/FVC < lower limit of normal (LLN) [Citation8]. Neither definition has been extensively clinically validated. However, the LLN definition may reduce misclassification of FAO because the LLN value is derived from reference equations specific to the population under study, taking into account age, sex and ethnicity [Citation8,Citation9]. The reliability of the LLN definition depends on appropriate selection of reference equations and accurate interpretation of pulmonary function results [Citation10]. Use of the LLN definition may be particularly important in younger patients to improve the likelihood of early identification of FAO that might be missed using a fixed threshold of <0.70 [Citation11].
Here, we present a description of baseline demography, current asthma control characteristics and treatment outcomes from two 12-week pivotal US Food and Drug Administration asthma trials spanning a broad range of asthma severity. These trials were selected because they are the only ones that included placebo, inhaled corticosteroid (ICS), long-acting β2-adrenergic agonist (LABA) and ICS/LABA fixed-dose combination arms within the same trial. Patients were stratified post hoc according to FAO status based on FEV1/FVC <LLN (with data for FEV1/FVC <0.70 presented in the Supplementary material). The two trials were analyzed separately because of differences in patient severity and the run-in period (budesonide versus placebo). The findings from this descriptive analysis show that patients with FAO tend to have a longer duration of disease and to have more severe asthma based on asthma control characteristics at study entry compared with asthmatic patients without FAO. Patients with FAO did not respond to LABA monotherapy and were most responsive to ICS/LABA combination therapy, where a more than additive effect on lung function was observed.
Methods
Patients, study design and treatments
This post hoc analysis included data from two previously reported double-blinded, double-dummy randomized, 12-week studies (study I: NCT00651651 [Citation12]; study II: NCT00652002 [Citation13]) conducted in patients with mild-to-moderate (study I; aged ≥6 years) or moderate-to-severe (study II; aged ≥12 years) asthma with a smoking history of ≤10 pack-years. At screening, patients must have demonstrated reversibility from baseline FEV1 of ≥12% and ≥0.20 L in both studies. Asthma was defined according to standard American Thoracic Society criteria [Citation14]; asthma severity was based on ICS dose before entry and an FEV1 percent predicted value of ≥60% to ≤90% in study I and ≥45% to ≤85% in study II. Both studies included 2-week run-in periods (study I: placebo; study II: budesonide pressurized metered-dose inhaler [pMDI] 160 µg twice daily; both studies: albuterol as-needed) and 12-week randomized treatment periods, including the following treatments: budesonide/formoterol pMDI (study I: 160/9 µg twice daily; study II: 320/9 µg twice daily), budesonide pMDI 320 µg + formoterol dry powder inhaler (DPI) 9 µg twice daily (study II only; not presented in this report), budesonide pMDI (study I: 160 µg twice daily; study II: 320 µg twice daily), formoterol DPI (both studies: 9 µg twice daily) and placebo (both studies). In both studies, patients were eligible for randomization if they had documented daytime or nighttime asthma symptom scores >0 (where 0 indicates no symptoms and 3 indicates severe symptoms) on ≥3 of 7 consecutive days during the run-in period. To maintain blinding, patients received a pMDI and a DPI containing active treatment or placebo. Study protocols were approved by an institutional review board for each of the clinical sites and conducted in conformance with guidelines for the ethical treatment of human subjects, good clinical practice and applicable local regulations.
FAO definitions and LLN equations
Patients were classified as FAO+ or FAO− based on a single determination of post-albuterol lung function performed at screening according to two definitions: (1) FEV1/FVC < LLN (FAO+) or ≥LLN (FAO−) [Citation8] and (2) FEV1/FVC <0.70 (FAO+) or ≥0.70 (FAO−) [Citation7]. Screening spirometry assessments were performed ≥6 h after the last dose of inhaled short-acting β2 agonist (SABA) and ≥24 h after taking a LABA. FEV1/FVC LLN was based on gender- and race/ethnicity-specific regression equations developed from the third National Health and Nutrition Examination Survey (NHANES III) (Supplementary Table E1), which included males and females in three major racial/ethnic groups (White, African–Americans and Mexican–Americans) with an age range of 8–80 years [Citation9]. Patients who were not in these three major racial/ethnic groups were not included in this analysis. The equations were extrapolated for patients outside of the 8–80-year-old age range (nine patients in study I aged <8 years and one patient in study II aged >80 years).
Assessments
Reversibility was defined as the percentage improvement in FEV1 from the prebronchodilator assessment ∼15−30 min after albuterol pMDI (two to four actuations [90 µg/actuation]) or up to 2.5 mg nebulized albuterol at screening. Baseline asthma control measures were assessed on the day of randomization for pulmonary function variables and during the run-in period for diary-derived variables (rescue medication use [inhalations/day], asthma symptom score [0 = none to 3 = severe] and awakening-free nights [%]).
Treatment outcomes were assessed based on the least squares mean changes from baseline to the mean during the randomized treatment period in pulmonary function variables (predose FEV1 [L] and FVC [L]) and daily diary-derived variables (rescue medication use [inhalations/day], % awakening-free nights and % asthma control days [symptom-free day with no daytime or nighttime rescue medication use]). Study withdrawal because of a predefined asthma event also was assessed ().
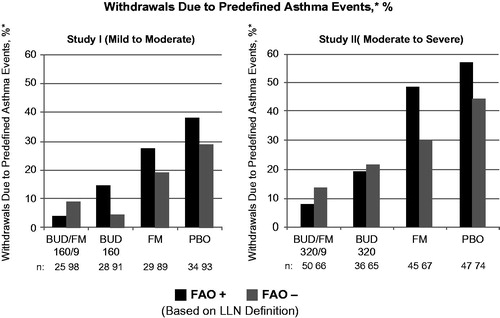
Figure 1. Adjusted* mean changes from baseline in % of withdrawals due to predefined asthma events± by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. ±Predefined criteria for an asthma event included: (1) decrease in am predose FEV1 ≥20% from randomization or a decrease to <45% (study I) or <40% (study II) of predicted normal, (2) ≥12 actuations of albuterol/day on ≥3 days within a 7-day period, (3) decrease in am PEF ≥20% from baseline on ≥3 days within a 7-day period, (4) ≥2 nights with an awakening due to asthma requiring rescue medication within any 7-day period and (5) clinical exacerbation requiring emergency treatment, hospitalization or use of an asthma medication not allowed by the protocol. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; LLN, lower limit of normal; PBO, placebo.
Statistical analysis
No formal statistics were performed in this analysis. Agreement between the LLN and 0.70 definitions, with regard to classifying FAO+ and FAO− status, was examined overall and categorically by age intervals that had a similar frequency of observations (data presented in the Supplementary material). Data were stratified by FAO category based on the LLN definition using descriptive statistics. Data from treatment groups were combined for demographics and baseline assessments and were analyzed separately for assessment of treatment outcomes in each study.
Results
Demographic and screening characteristics (LLN definition)
Of 511 patients included in study I and 596 included in study II, a total of 487 patients (study I; 6−11 years of age, n = 30; ≥12 years of age, n = 457) and 559 patients (study II; all ≥12 years of age) were included in FAO classification. In study I, 24 patients were excluded from the analysis because of race not accommodated by the LLN prediction equation (race other than Caucasian, African–American or Mexican–American; n = 21) or missing FEV1/FVC value (n = 3); in study II, 37 patients were excluded for race. Using the LLN definition, 116 (24%) and 226 (40%) patients in studies I and II, respectively, were classified as FAO+. Information on the overlap between FAO definitions is presented in the Supplementary material (Table E2 and Figure E1).
Demographic and clinical characteristics at screening are shown in by study and FAO status. FAO+ and FAO− patients had similar age and body–mass index. Patients who were FAO+ were more likely to be male, have asthma of longer duration and have lower prebronchodilator FEV1 % predicted values than FAO− patients. Percent reversibility and FVC % predicted were generally similar in FAO+ versus FAO− patients in both studies at screening.
Table 1. Demographic and screening disease characteristics by FAO category (LLN definition).
Baseline lung function and asthma control measures
Lung function and asthma control measures at baseline (day of randomization following a 2-week run-in period) are shown in . In both studies, FAO+ patients were more impaired than FAO− patients based on prebronchodilator FEV1 % predicted values, forced expiratory flow between 25% and 75% (FEF25–75%) predicted values, FEV1/FVC and rescue medication use. No consistent effects of FAO were observed for asthma symptoms or nighttime awakenings at baseline. The decrease in absolute FEV1 observed between screening and baseline values in study I (mild-to-moderate) patients is consistent with the per protocol step-down to a SABA-only during run-in; study II (moderate-to-severe) patients were stepped down to low-dose budesonide, so that this change is less manifest.
Table 2. Baseline disease characteristics and control measures by FAO category (LLN definition).
Treatment outcomes (LLN definition)
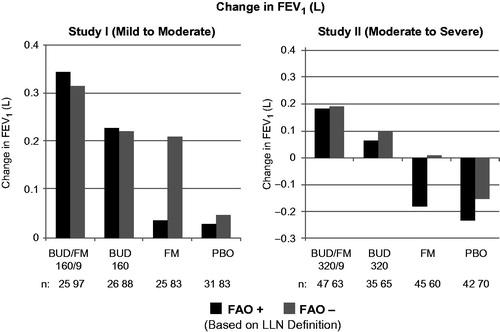
The pulmonary function and composite asthma control variables in show the effects of treatment by FAO category for studies I and II. See Supplementary material for changes in FVC outcomes (Figure E2). Lung function, as assessed by changes from baseline in predose FEV1 after 12 weeks of therapy was lower for FAO+ versus FAO− patients receiving placebo in both studies (). Slight overall improvements in FEV1 were observed for placebo-treated patients with mild-to-moderate asthma (study I), irrespective of FAO status, whereas patients with moderate-to-severe asthma (study II) experienced a sharp decrease in FEV1. This difference is explained by the fact that there is no change in background therapy moving from the run-in to the placebo treatment period for study I (i.e. as needed SABAs throughout); whereas, there is a step-down from low-dose budesonide run-in to placebo for study II. While LABA monotherapy is not an endorsed treatment step for asthma, it is notable that FAO− patients showed substantial improvements in lung function with formoterol, approaching those seen with budesonide in study I (mild-to-moderate patients). In contrast, it is notable that in FAO+ patients, lung function was essentially non-responsive to formoterol monotherapy in both studies (). Both FAO+ and FAO− patients showed nearly equivalent improvement in lung function with budesonide monotherapy in both studies; the magnitude of improvement was smaller for study II, in which patients had received low-dose budesonide as part of their run-in. Budesonide/formoterol combination therapy produced the largest lung function improvements in FAO+ and FAO− patients in both studies, with a consistently greater-than-additive effect observed with combination treatment relative to budesonide and formoterol alone in FAO+ patients that generally was not observed in FAO− patients.
Figure 2. Adjusted* mean changes from baseline in % of asthma control days by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; LLN, lower limit of normal; PBO, placebo.
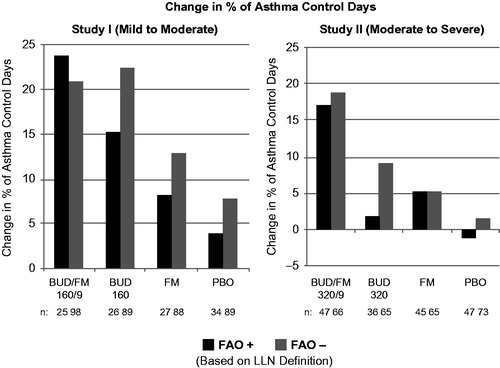
Figure 3. Adjusted* mean changes from baseline in predose FEV1 by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; FEV1, forced expiratory volume in 1 s; LLN, lower limit of normal; PBO, placebo.
As expected, improvement in the percentage of asthma control days was lowest and withdrawals due to predefined asthma events were more common in the placebo group relative to the other treatment groups in both FAO+ and FAO− patients in both studies. In FAO+ patients, the greatest improvements in asthma control days were observed in the budesonide/formoterol group compared with either monocomponent or placebo (). Among FAO− patients, improvements in asthma control days were greatest with budesonide/formoterol in those classified as moderate-to-severe (study II) and were fairly similar, albeit slightly lower, with budesonide/formoterol versus budesonide in mild-to-moderate patients (study I). Withdrawals due to predefined asthma events were more common in FAO+ versus FAO− patients treated with placebo or formoterol monotherapy (). Withdrawals due to predefined asthma events decreased with increasing intensity of treatment for FAO+ patients in both studies, with the lowest rates observed for budesonide/formoterol. This relationship was also true for FAO− patients classified as moderate-to-severe (study II) but not for those classified as mild-to-moderate (study I), for whom budesonide led to slightly fewer withdrawals for a predefined asthma event than combination therapy. FAO+ patients had consistently better treatment outcomes with budesonide/formoterol pMDI versus all other treatments in both studies.
Treatment outcomes, based on rescue medication use and percentage of awakening-free nights, are shown in . Mild-to-moderate (study I) FAO+ patients displayed higher rescue medication use during placebo treatment but contrastingly larger positive treatment responses to all active therapies compared to FAO− patients. In moderate-to-severe patients (study II), the treatment responses to budesonide/formoterol and formoterol alone for rescue medication use were fairly similar between FAO+ and FAO− patients, but responses to budesonide alone were lower in FAO+ than FAO− patients. In both studies, reductions in rescue medication use were greatest with budesonide/formoterol pMDI, irrespective of FAO category. Awakening-free nights invariably improved with all active therapies compared with placebo, irrespective of disease severity or FAO status, with modestly larger effects for the budesonide-containing arms compared with formoterol.
Table 3. Treatment outcomes (adjusteda mean changes from baseline in rescue medication use and the percentage of awakening-free nights) by FAO category (LLN definition).
Treatment outcomes using the <0.7 definition for FAO were generally similar to those using the LLN definition (Supplementary Tables E3 and E4).
Discussion
The present report evaluates whether FAO defines a clinically distinct asthma population according to both baseline clinical characteristics and treatment responses, as assessed in clinically stable patients with mild-moderate or moderate-severe asthma. FAO was determined during their screening evaluation for participation in two randomized controlled trials of pharmacotherapy. In this descriptive post hoc analysis of data from two randomized, double-blinded, placebo-controlled, 12-week studies, FAO defined by a screening post-bronchodilator FEV1/FVC ratio below the LLN was relatively common, with a prevalence of 24% in patients previously classified as having mild-to-moderate asthma (study I) and 40% in those classified as having moderate-to-severe asthma (study II). In both studies, FAO+ patients were more likely to be male and have a longer asthma duration compared with FAO− patients, consistent with a previous report from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimen study [Citation2]. In the present analysis, baseline lung function was lower and rescue medication use higher in FAO+ versus FAO− patients, consistent with a greater degree of impairment. These findings are consistent with results from previous analyses showing associations between the presence of FAO and physician-identified severe asthma [Citation2] and lower FEV1 % predicted values [Citation11].
Asthma symptom scores in the present analysis were similar in FAO+ and FAO− patients. This finding may have resulted from the narrow range and relative imprecision of the Likert-based symptom scoring used (0 = none to 3 = severe) and belies the differences observed in baseline rescue medication use. In addition, the correlation between asthma symptomatology and lung function is generally weak [Citation15].
Interestingly, formoterol alone had essentially no effect on treatment-averaged FEV1 and FVC in FAO+ patients, differentiating them physiologically both from FAO− patients, who did demonstrate physiologic improvements with formoterol, and from patients with moderate to very severe chronic obstructive pulmonary disease who generally respond well to formoterol [Citation16]. FAO+ patients remained responsive to budesonide for most end points, highlighting the importance of anti-inflammatory treatment in this population. As lung function outcomes for formoterol were similar to placebo in FAO+ patients, one would expect the efficacy of the combination of budesonide and formoterol to be driven by budesonide alone. Interestingly, however, the fixed combination of budesonide and formoterol resulted in a greater effect on lung function than the monocomponents in both FAO+ and FAO− patients, and these effects were more than additive in FAO+ patients. This notion is consistent with results of in vitro studies showing cooperative, additive and synergistic anti-inflammatory and bronchoprotective effects between formoterol and budesonide [Citation17–21].
Following patients over time also gives insight into the effect of FAO status on future risk of adverse outcomes, best seen in the placebo arms and in the differential responsiveness to active treatments. Prospective evaluation of asthma control days and withdrawals due to asthma events in the placebo arm suggest that FAO+ patients are at increased future risk relative to FAO− patients. A stepwise improvement in these composite asthma control metrics was observed in FAO+ patients moving from formoterol to budesonide to budesonide/formoterol. This response to budesonide/formoterol was similar for FAO+ and FAO− patients with respect to these metrics.
There are several limitations to this analysis. The post hoc nature of the analysis and relatively small FAO+ subgroups preclude formal statistics; therefore, these data must be considered hypothesis-generating only. Therefore, any conclusions rest on the magnitude and consistency of the effects between the two studies. Differences in run-in treatment between the two studies (placebo in study I and budesonide in study II) limit direct comparison of baseline variables assessed during the run-in period and changes in efficacy outcomes between the two studies.
Other potential limitations involve the FEV1/FVC assessment, which was based on a single screening visit assessment; FAO status was not defined after treatment that could have optimized lung function. Additionally, the albuterol dose used to determine screening post-bronchodilator lung function (two to four puffs) and the timing of spirometry (15−30 min after bronchodilator) could vary. Therefore, it is possible that some patients may have been misclassified with respect to FAO status because of day-to-day variability in FEV1 or less than maximal bronchodilation based on a single spirometry test. Furthermore, it is possible that patients originally classified as FAO+ at screening could have switched to FAO− during the 12-week study period. However, since both trials were placebo-controlled and evaluated responses to three different active medications, post-randomization classification of FAO would not have been possible without biasing the impact of FAO status on treatment outcomes.
The 12-week duration of the studies also may have been too short to attain an optimal effect of ICS treatment on efficacy outcomes. The exclusion of races other than Caucasian, African–American and Mexican–American based on the LLN equations used limits generalization of the findings to some populations.
In conclusion, stratification of asthma patients according to post-bronchodilator FEV1/FVC and a LLN cutoff as determined at the onset of two randomized, controlled clinical trials identified clinically distinct populations based on demography, baseline control and subsequent responsiveness to asthma controllers. FAO+ patients were more likely male with longer asthma duration and were more impaired based on pulmonary function and rescue medication use. Budesonide and budesonide/formoterol combination therapy showed efficacy in both FAO+ and FAO− patients in both studies, whereas FAO+ patients were distinctly non-responsive to formoterol monotherapy treatment. The most positive treatment outcomes generally were observed with the fixed combination of budesonide and formoterol, irrespective of FAO category, with a greater than additive effect of budesonide/formoterol treatment observed in FAO+ patients. The findings from this analysis support treatment of patients with FAO+ asthma based on current asthma guidelines [Citation1,Citation22], although more aggressive asthma management may be necessary in this population.
Declaration of interest
The authors thank Scientific Connexions, who provided medical writing support funded by AstraZeneca LP (Wilmington, DE). This work was funded by AstraZeneca, LP. D.P.T. has the following conflicts of interest to declare: AstraZeneca (Consultancy, Honoraria, Speaker’s Bureau); Novartis (Consultancy, Honoraria, Speaker’s Bureau); Sunovion (Consultancy, Honoraria, Research Grant to Institution); Theravance (Consultancy); GlaxoSmithKline (Research Grant to Institution); Pearl (Consultancy; Research Grant to Institution); Boehringer Ingelheim (Speaker’s Bureau); Forest (Speaker’s Bureau). B.E.C. has the following conflicts of interest to declare: AstraZeneca (Consultancy, Speaker’s Bureau); Genentech (Consultancy, Speaker’s Bureau); GlaxoSmithKline (Consultancy, Speaker’s Bureau); SRxA (Consultancy); Novartis (Consultancy, Speaker’s Bureau); Sunivion (Consultancy, Speaker’s Bureau); Merck-Schering (Consultancy, Speaker’s Bureau); Bausch+Lomb (Speaker’s Bureau); Dey (Consultancy). F.T. has the following conflicts of interest to declare: AstraZeneca (Employment, Stock Options). J.G.Z. has the following conflicts of interest to declare: AstraZeneca (Former Employment, Stock Options).
Supplementary Material
Download PDF (285.1 KB)Acknowledgements
The authors thank Tom Uryniak, MS, from AstraZeneca LP for helpful comments and statistical oversight.
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2012 update. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_March13.pdf [last accessed 19 Nov 2013]
- Lee JH, Haselkorn T, Borish L, Rasouliyan L, Chipps BE, Wenzel SE. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study. Chest 2007;132:1882–1889
- Guerra S, Martinez FD. Epidemiology of the origins of airflow limitation in asthma. Proc Am Thorac Soc 2009;6:707–711
- Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 2011;364:2006–2015
- Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 2012;185:356–362
- Bergeron C, Al-Ramil W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc 2009;6:301–305
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2013 update. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf [last accessed 19 Nov 2013]
- Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008;63:1046–1051
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–187
- Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present, and future. Eur Respir J 2010;36:12–19
- Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Antó JM, Künzli N, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax 2008;63:1040–1045
- Corren J, Korenblat PE, Miller CJ, O’Brien CD, Mezzanotte WS. Twelve-week, randomized, placebo-controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered-dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clin Ther 2007;29:823–843
- Noonan M, Rosenwasser LJ, Martin P, O’Brien CD, O’Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered-dose inhaler in adults and adolescents with moderate to severe asthma: a randomized clinical trial. Drugs 2006;66:2235–2254
- American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136
- Teeter JG, Bleeker EG. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest 1998;113:272–277
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, Silkoff PE. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 2009;69:549–565
- Persdotter S, Lindahl M, Malm-Erjeflt M, von Wachenfeldt K, Korn SH, Stevens T, Miller-Larsson A. Cooperative inhibitory effects of budesonide and formoterol on eosinophil superoxide production stimulated by bronchial epithelial cell conditioned medium. Int Arch Allergy Immunol 2007;143:201–210
- Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Additive anti-inflammatory effect of formoterol and budesonide on human lung fibroblasts. Thorax 2002;57:237–241
- Cooper PR, Kurten RC, Zhang J, Nicholls DJ, Dainty IA, Panettieri RA. Formoterol and salmeterol induce a similar degree of β2-adrenoceptor tolerance in human small airways but via different mechanisms. Br J Pharmacol 2011;163:521–532
- Horvath G, Mendes ES, Schmid N, Schmid A, Conner GE, Fregien NL, Salathe M, Wanner A. Rapid nongenomic actions of inhaled corticosteroids on long-acting β2-agonist transport in the airway. Pulmon Pharmacol Ther 2011;24:654–659
- Skevaki CL, Christodoulou I, Spyridaki IS, Tiniakou I, Georgiou V, Xepapadaki P, Kafetzis DA, Papadopoulos NG. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin Exp Allergy 2009;39:1700–1710
- National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma – Full Report 2007. NIH Publication Number 08-4051, 2007

