Abstract
Objective. Improving glycaemic control is generally supposed to reduce symptoms experienced by type 2 diabetic patients, but the relationships between glycated haemoglobin (HbA1c), diabetes-related symptoms, and self-rated health (SRH) are unclarified. This study explored the relationships between these aspects of diabetes control. Design. A cross-sectional study one year after diagnosis of type 2 diabetes. Subjects. A population-based sample of 606 type 2 diabetic patients, median age 65.6 years at diagnosis, regularly reviewed in primary care. Main outcome measures. The relationships between HbA1c, diabetes-related symptoms, and SRH. Results. The patients’ median HbA1c was 7.8 (reference interval: 5.4–7.4 % at the time of the study). 270 (45.2%) reported diabetes-related symptoms within the past 14 days. SRH was associated with symptom score (γ = 0.30, p < 0.001) and HbA1c (γ = 0.17, p = 0.038) after correction for covariates. The relation between HbA1c and symptom score was explained by SRH together with other confounders, e.g. hypertension (γ = 0.02, p = 0.40). The relation between the symptom fatigue and SRH was not explained by symptom score and significantly modified the direct association between symptom score and SRH. Conclusions. Symptom relief may not occur even when HbA1c level is at its lowest average level in the natural history of diabetes, and symptoms and SRH are closely linked. Monitoring symptoms in the clinical encounter to extend information on disease severity, as measured e.g. by HbA1c, may help general practitioners and patients to understand the possible impact of treatments and of disease manifestations in order to obtain optimum disease control.
To reduce complications, lowering of HbA1c is a primary objective in diabetes care.
Many patients experience diabetes-related symptoms in spite of acceptable glycaemic control.
These symptoms are closely related to poor SRH while the association with HbA1c is weak.
Patients with type 2 diabetes mellitus (T2DM) are commonly treated in general practice where treatment typically aims to improve glycaemic control in order to prevent complications [Citation1], reduce symptom burden, and improve perceived health [Citation2]. Moreover, the experience of obtaining these goals may improve patients’ motivation for treatment adherence, e.g. lifestyle changes and medication [Citation3,Citation4].
Poor glycaemic control is related to symptoms such as frequent urination, genital itching, and unintended weight loss [Citation5,Citation6]. The association between glycated haemoglobin (HbA1c) levels and specific symptoms is not necessarily close [Citation7,Citation8] except among dysregulated patients, e.g. at the time of diagnosis [Citation5] or in patients with longstanding diabetes [Citation2,Citation6]. Despite the central role of symptom amelioration in treatment, few studies have looked into the relation between HbA1c level and symptoms when HbA1c is supposed to be at its lowest average level in the natural history of diabetes [Citation7,Citation9].
General practitioners (GPs) and patients may evaluate the patient's health differently [Citation10]. The association between the patient's HbA1c level and perceived health is weak [Citation2], or non-existent [Citation1,Citation11]. The patients’ perceived health gauged by a single question, known as perceived health, self-assessed health, or self-rated health (SRH), has been shown to vary with other factors than HbA1c such as symptoms [Citation12,Citation13], sociodemographic factors [Citation14], comorbidities [Citation14–16], and functional ability [Citation12,Citation14]. Recent research has shown that SRH predicts which patients have a higher risk of diabetic complications even after accounting for established risk factors such as HbA1c, but this predictive value may be mediated by presence of symptoms which were not accounted for [Citation16]. Yet the relationships between HbA1c and symptoms, both of which are important treatment targets, and SRH, which is a motivational factor for treatment adherence [Citation2,Citation3], are unclarified. A better insight into these relationships may help GPs to tailor treatments such as to maintain or improve patients’ health, which may include motivating the patient for treatment adherence.
In a population-based sample of patients with T2DM seen in general practice one year after diabetes diagnosis we examined the relationships between HbA1c, symptoms, and SRH primarily to see whether high HbA1c levels are associated with many symptoms and low SRH ratings, and whether many symptoms are associated with low SRH ratings.
Material and methods
This is a cross-sectional study performed one year after diabetes diagnosis in the intervention group patients participating in the Danish randomized trial “Diabetes Care in General Practice” [Citation17].
Study population
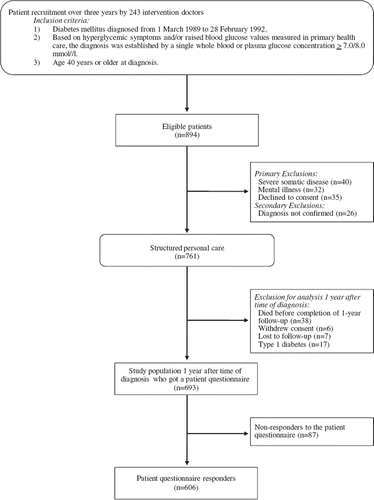
Of 894 eligible newly diagnosed diabetic patients, 693 remained in the study until one year after diagnosis. Of these, 606 completed a patient questionnaire (). At diagnosis, the 87 (12.5%) non-responders’ SRH, sex, age, employment status (working vs. not working), occupation, educational level, HbA1c, blood glucose, and prevalence of diabetic retinopathy, ischaemic heart disease, peripheral vascular disease, and albuminuria did not differ from responders’ but more responders were skilled workers.
The GPs were instructed to give structured personal care, which included individualized goal-setting for important risk factors, supported by prompting of doctors one month before the next expected consultation (the GPs were asked to see patients quarterly and screen them annually for diabetic complications), short clinical guidelines, and feedback on individual patients [Citation17].
The protocol of the study was approved by the ethics committee of Copenhagen and Frederiksberg, and all patients gave informed consent.
Measurements
Immediately after inclusion in the study, the GP recorded the following patient characteristics: body height, sense of touch of cotton wool and pin prick on both feet, pulses on feet, diagnostic fasting plasma glucose, presence of patellar reflexes, amputation of leg or part of it, history of myocardial infarction and stroke causing hospitalization, and drug treatment. A fasting blood sample was drawn. In questionnaires the patients gave information regarding angina pectoris, intermittent claudication, lifestyle, cancer disease, and sociodemographic factors [Citation17].
At the one-year consultation body weight without shoes and outer garments, blood pressure by routine methods after a 10-minute rest in the sitting position, and antidiabetic treatment were recorded. A fasting blood sample was drawn, a freshly voided morning urine sample collected, and the patient was referred to a funduscopy by a practising ophthalmologist. Measurement of HbA1c, fasting blood glucose, and urinary albumin concentration were centralized [Citation17]. Throughout the study, fraction of HbA1c was determined by the same ion-exchange, high-performance liquid chromatography method (HPLC) at Odense University Hospital. Samples from 100 blood donors (age 20–80 years, 33 men, 67 women) were analysed, and the reference interval (mean ± 2SD) was calculated to be 5.4–7.4%. Quality assurance was obtained with commercial control preparations from Bio-Rad. In October–December 1995, the mean (SD) of low (n = 24) and high (n = 29) control samples were 6.7 (0.31)% and 10.4 (0.63)%, respectively, resulting in coefficients of variation (CV = SD 3 100/mean) of 4.6% and 6.0%. This method was later compared with a newer HPLC method (an automated HbA1c analyser, Tosoh G7) which is DCCT-aligned using calibrators from the European Reference Laboratory for Glycated Haemoglobin (ERL). The association between the two methods, which is approximately linear (R2 = 0.9049, n = 484, p < 0.0001), is expressed with the following algorithm: The current method = 0.268 + 1.072 3 the DCCT-aligned method [Citation9]. This indicates that the reference range in this study of 5.4–7.4% may be translated into 4.8–6.7% using a DCCT-aligned method. In the latest Danish clinical guidelines for diabetes the recommended level of HbA1c is < 6.5% which corresponds to approximately < 7.2% with the method used in the present study. Furthermore, at the one-year consultation the GPs handed out a patient questionnaire (no reminders were issued). Due to delay from the study coordinating centre 198 patients received this at a later three-monthly follow-up. Diabetes-related symptoms were recorded with a multiple-response question: “Have you noticed your diabetes within the past 14 days?” The response categories comprised an open-ended category and six symptoms (the most frequent symptoms at diagnosis) considered to be related to hyperglycaemia [Citation5,Citation18]. The patients gave information on their SRH by answering the question: “In general, how would you rate your health at present?” The response categories were “very good”, “good”, “fair”, “poor” and “very poor”.
Statistical analysis
“Very poor” and “poor” SRH were combined in the analyses since very few patients rated their health as very poor. The closest usable HbA1c measured up to 120 days after the patient questionnaire was chosen. If no measurement was found, the closest HbA1c measurement up to 60 days before the questionnaire time was chosen. The responses to the open-ended symptom category were heterogeneous and aggregated into “other symptoms”. A symptom score was constructed by adding together positive answers to the seven categories in the symptom question. A χ2-test or a Kruskal-Wallis test was used for assessment of bivariate association. We used a Mantel-Haenzel χ2 trend test to investigate monotonicity for categorical variables.
We analysed the three associations between HbA1c, symptom score and SRH, conditional on possible confounders (listed in , oral hypoglycaemic agents and insulin were merged in the analyses) with partial γ rank correlation coefficients [Citation19]. A value of the coefficient different from zero, tested with a permutation test [Citation20], indicates that an observed bivariate relationship is not explained by confounding of other variables in the model. A minimal set of variables that is controlled for in each case was determined by backwards elimination (p > 0.10) of insignificant relationships involving possible confounders following an extensive literature search; consequently, such a minimal set is viewed to confound the relationship under study.
Table I. Characteristics of patients with type 2 diabetes one year after diagnosis.
To investigate influences of the individual symptoms beyond those captured in the symptom score, a graphical Rasch model [Citation21] was constructed in which we identified significant item biases and local dependencies between the individual symptoms, and their effect on the relationships between HbA1c, symptom score and SRH by backwards elimination (p > 0.05).
The statistical analyses were performed with SAS software version 9.1 (SAS Institute, Cary, NC) and the statistical software DIGRAM [Citation22].
Results
At diagnosis, the responders’ median fasting plasma-glucose was 13.5 mmol/l and median HbA1c was 9.9%. At one-year follow-up median HbA1c was 7.8% (reference range: 5.4–7.4%, ). Most of the patients had their HbA1c measurement before the patient questionnaire (median distance: −1 day (IQR: −6–0)). HbA1c information was missing in 105 cases because patients received the questionnaire too long after the one-year follow-up for the measurement to be relevant.
Symptoms were common especially among women () and patients with low SRH rating (). No statistically significant association was found between symptom score and HbA1c level ().
Table II. Number of diabetes-related symptoms and their relation to sex, age, and haemoglobin A1c in patients with type 2 diabetes one year after diagnosis.
Table III. Relation between type 2 diabetic patients’ self-rated health one year after diagnosis and sex, age, haemoglobin A1c, and symptoms.
In contrast, SRH deteriorated with increasing HbA1c levels (see ). No statistically significant relation was found between age, sex, and SRH. The number of symptoms and type of symptoms are strongly related to SRH.
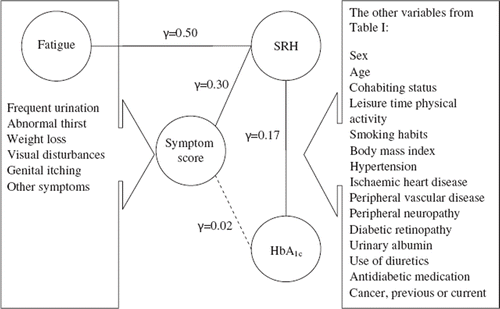
The Rasch analysis controlling for direct relations between symptoms in the symptom score and other variables showed a direct relation between fatigue and SRH (γ = 0.50, p < 0.001). No direct association between a single symptom and sex, age, or HbA1c was found. The multivariate analysis (not shown) on the associations between SRH, HbA1c, and symptom score was therefore adjusted for fatigue together with other covariates. Even after adjusting for fatigue, the reporting of many symptoms was directly associated with decreasing SRH ratings (γ = 0.30, p < 0.001), additionally controlling for confounding by some of the other possible confounders (HbA1c and sex). Decreasing SRH was associated with increasing HbA1c levels (γ = 0.17, p = 0.038) controlling for confounding by symptom score, antidiabetic medication, urinary albumin, and fatigue. The relation between HbA1c and symptom score was explained by other variables (SRH and hypertension, γ = 0.02, p = 0.40). This shows that an association between HbA1c and symptom burden is explained by a mutual association with SRH and additional clinical factors such as hypertension. For example, if SRH and hypertension is known, HbA1c does not add information on symptom burden. illustrates the relation between symptoms, SRH, and HbA1c.
Discussion
Principal findings
Despite average HbA1c being only 0.4% above the upper end of the normal range (5.4–7.4%), almost half of the patients reported typical hyperglycaemic symptoms. SRH was found to worsen markedly with increasing number of diabetes-related symptoms, and the symptom of fatigue was a major contributor to this relation. A similar, but weaker, relationship was found between SRH and HbA1c. A weak association between HbA1c and symptom score was explained through mutual relationships with SRH and hypertension.
Study strengths and weaknesses
The major study strength is the population-based sample of patients with T2DM treated in general practice and examined at a well-defined time in the natural history of the disease.
This study was limited by the use of self-reported questionnaire data, because patients may have overestimated actual behaviour to provide a socially desirable response [Citation23,Citation24]. Non-response to the patient questionnaire and missing HbA1c values are unlikely to have biased our results: non-responders and responders were similar in variables of interest at diagnosis, and 105 of 123 missing HbA1c values can be assumed to be missing completely at random [Citation25] because of the questionnaire delay. Finally, the study is cross-sectional and any causal interpretation should be made with caution.
Relation to other studies
Our results confirm previous studies that found a close relation between patients’ reports of diabetes-related, especially hyperglycaemic, symptoms and relatively poor SRH ratings [Citation2,Citation26] or poor health-related quality of life [Citation27]. The symptom of fatigue made a major contribution to the association between symptom score and SRH, and this has previously been demonstrated in two large population-based studies [Citation13,Citation28]. Not all patients who reported fatigue had low SRH. Paying attention to these patients may still be important as fatigue may be a stronger predictor of mortality than SRH and other potential predictors [Citation29].
In our study we found no association between HbA1c and symptom score in the multivariate analyses, which tallies with the results from two other cross-sectional studies [Citation7,Citation8]: one study examined patients before entry in the UK Prospective Diabetes Study and the other the effect of insulin treatment in poorly regulated patients. Other cross-sectional studies have found that an augmented HbA1c level is accompanied by higher symptom scores in patients with a longer duration of diabetes [Citation2,Citation6] and in dysregulated patients [Citation5,Citation27]. Patients with different HbA1c levels may, however, report identical types of symptoms, but perceived symptom burden and length of occurrence may differ. Conversely patients with identical HbA1c levels may notice both different types and number of symptoms, because the threshold for symptom perception generally tends to be individual [Citation30,Citation31].
Our finding of a moderate decrease in SRH rating with increasing HbA1c level is in accordance with the results of one cross-sectional study [Citation2], while four other studies found no association at all [Citation1,Citation11,Citation27,Citation32]. Evidence suggests that patients with diabetic complications rate their health worse than patients without complications [Citation15,Citation33]; however, in our study only the presence of hypertension mediated the association between SRH and HbA1c, although approximately half of the patients had at least one complication [Citation17]. The weak relation between HbA1c levels and SRH may indicate that patients with hypertension may improve in SRH when HbA1c levels decrease, but not as much as patients without this comorbidity [Citation34]. Recent research suggests that even though many patients with diabetes receive antihypertensive treatment this treatment could still be improved [Citation35].
Implications for clinicians and future research
Many patients obviously do not experience symptom relief, and this experience seems to influence their SRH negatively. This could be because patients give priority to symptom control over prevention of complications [Citation36], which may happen through optimizing glycaemic control. We suggest that both symptoms and SRH carry important information concerning the patient's perception of his/her own health which cannot be explained fully by the patient's objective health status. This additional information may help GPs and patients to discuss the possible impact of treatment and disease manifestations and thus obtain optimum disease control. This seems to be important since SRH in patients with T2DM provided additional information beyond that gained from clinical history and risk factors [Citation16]. Future studies should focus on how diabetic patients’ SRH may be improved through intervention targeting those with poor SRH ratings.
Acknowledgements
The authors would like to thank the participating GPs and patients and Kirsti Malterud, Hanne Hollnagel, Thomas Drivsholm, Willy Karlslund, Lise Bergsøe, and Annie Keldebæk.
Major funding
The Danish Medical Research Council, the Danish Research Foundation for General Practice, the Health Insurance Foundation, the Danish Ministry of Health, Novo Nordisk Farmaka Denmark, and the Pharmacy Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Weinberger M, Kirkman MS, Samsa GP, Cowper PA, Shortliffe EA, Simel DL . The relationship between glycemic control and health-related quality of life in patients with non-insulin-dependent diabetes mellitus. Med Care 1994;32:1173–81.
- Van der Does FE, De Neeling JN, Snoek FJ, Kostense PJ, Grootenhuis PA, Bouter LM . Symptoms and well-being in relation to glycemic control in type II diabetes. Diabetes Care 1996;19:204–10.
- Kelleher D. Coming to terms with diabetes: Coping strategies and non-compliance. Andersson R, Bury M. Living with chronic illness. London: Unwin Hyman; 1988. 137–155.
- Adolfsson ET, Smide B, Rosenblad A, Wikblad K. Does patient education facilitate diabetic patients’ possibilities to reach national treatment targets? A national survey in Swedish primary healthcare. Scand J Prim Health Care 2009;27: 91–6.
- Drivsholm T, Olivarius NdF, Nielsen AB, Siersma V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia 2005;48:210–4.
- Konen JC, Curtis LG, Summerson JH. Symptoms and complications of adult diabetic patients in a family practice. Arch Fam Med 1996;5:135–45.
- Bulpitt CJ, Palmer AJ, Battersby C, Fletcher AE. Association of symptoms of type 2 diabetic patients with severity of disease, obesity, and blood pressure. Diabetes Care 1998; 21:111–15.
- Johansen J, Claudi T, Holtedahl K. Insulin treatment for poorly regulated diabetic patients in general practice: Better regulation and symptom relief? Scand J Prim Health Care 1999;17:244–9.
- Olivarius NF, Siersma V, Hansen LJ, Drivsholm T, Horder M. Changes in levels of haemoglobin A1c during the first 6 years after diagnosis of clinical type 2 diabetes. Scand J Clin Lab Invest 2009;69:851–7.
- Geest TA, Engberg M, Lauritzen T. Discordance between self-evaluated health and doctor-evaluated health in relation to general health promotion. Scand J Prim Health Care 2004;22:146–51.
- Petterson T, Lee P, Hollis S, Young B, Newton P, Dornan T. Well-being and treatment satisfaction in older people with diabetes. Diabetes Care 1998;21:930–5.
- Jylha M, Leskinen E, Alanen E, Leskinen A-L, Heikkinen E. Self-rated health and associated factors among men of different ages. J Gerontology 1986;41:710–17.
- Molarius A, Janson S. Self-rated health, chronic diseases, and symptoms among middle-aged and elderly men and women. J Clin Epidemiol 2002;55:364–70.
- Fylkesnes K, Forde OH. Determinants and dimensions involved in self-evaluation of health. Soc Sci Med 1992;35: 271–9.
- Klein BE, Klein R, Moss SE. Self-rated health and diabetes of long duration. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 1998;21:236–40.
- Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care 2008;31:795–7.
- Olivarius NdF, Beck-Nielsen H, Andreasen AH, Horder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ 2001; 323:970–5.
- Ruige JB, de Neeling JN, Kostense PJ, Bouter LM, Heine RJ. Performance of an NIDDM screening questionnaire based on symptoms and risk factors. Diabetes Care 1997;20:491–6.
- Agresti A. Analysis of ordinal categorical data. New York: Wiley; 1984.
- Kreiner S. Analysis of multidimensional contingency tables by exact conditional tests: Techniques and strategies. Scand J Stat 1987;14:97–112.
- Kreiner S, Christensen KB. Validity and objectivity in health related scales: Analysis by graphical loglinear Rasch models. Davier MV, Carstensen CH. Multivariate and mixture distribution Rasch models: Extensions and applications. Berlin: Springer; 2007.
- Klein JP, Keiding N, Kreiner S. Graphical models for panel studies, illustrated on data from the Framingham Heart Study. Stat Med 1995;14:1265–90.
- Little P, Margetts B. Dietary and exercise assessment in general practice. Fam Pract 1996;13:477–82.
- Eccles M, Ford GA, Duggan S, Steen N. Are postal questionnaire surveys of reported activity valid? An exploration using general practitioner management of hypertension in older people. Br J Gen Pract 1999;49:35–8.
- Rubin DB. Inference and missing data. Biometrika 1976;63: 581–90.
- Van der Does FE, De Neeling JN, Snoek FJ, Grootenhuis PA, Kostense PJ, Bouter LM, . Randomized study of two different target levels of glycemic control within the acceptable range in type 2 diabetes. Effects on well-being at 1 year. Diabetes Care 1998;21:2085–93.
- Kleefstra N, Ubink-Veltmaat LJ, Houweling ST, Groenier KH, Meyboom-de Jong B, Bilo HJ. Cross-sectional relationship between glycaemic control, hyperglycaemic symptoms and quality of life in type 2 diabetes (ZODIAC-2). Neth J Med 2005;63:215–21.
- Norrelund N, Hollnagel H. [Fatigue among 40-year-olds]. Ugeskr Laeger 1979;141:1425–29.
- Avlund K, Schultz-Larsen K, Davidsen M. Tiredness in daily activities at age 70 as a predictor of mortality during the next 10 years. J Clin Epidemiol 1998;51:323–33.
- Cox DJ, Gonder-Frederick L, Pohl S, Pennebaker JW. Reliability of symptom-blood glucose relationships among insulin-dependent adult diabetics. Psychosom Med 1983;45:357–60.
- Freund A, Johnson SB, Rosenbloom A, Alexander B, Hansen CA. Subjective symptoms, blood glucose estimation, and blood. glucose concentrations in adolescents with diabetes. Diabetes Care 1986;9:236–43.
- Blaum CS, Velez L, Hiss RG, Halter JB. Characteristics related to poor glycemic control in NIDDM patients in community practice. Diabetes Care 1997;20:7–11.
- Anon. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). UK Prospective Diabetes Study Group. Diabetes Care 1999;22: 1125–36.
- Van der Does FE. Influence of glycaemic control on well-being and cardiovascular risk indicators in type 2 diabetes. Amsterdam: Free University Amsterdam, the Netherlands, 1997.
- Juul L, Sandbaek A, Foldspang A, Frydenberg M, Borch-Johnsen K, Lauritzen T. Adherence to guidelines in people with screen-detected type 2 diabetes, ADDITION, Denmark. Scand J Prim Health Care 2009;27:223–31.
- Murphy E, Kinmonth AL. No symptoms, no problem? Patients’ understandings of non-insulin dependent diabetes. Fam Pract 1995;12:184–92.