Abstract
Objective. In hypertensive primary care patients below 65 years of age, (i) to describe the occurrence of undiagnosed obstructive sleep apnoea (OSA), and (ii) to identify the determinants of moderate/severe OSA. Design. Cross-sectional. Setting. Four primary care health centres in Sweden. Patients. 411 consecutive patients (52% women), mean age 57.9 years (SD 5.9 years), with diagnosed and treated hypertension (BP >140/90). Main outcome measures. Occurrence of OSA as measured by the apnoea hypopnoea index (AHI). Results. Mild (AHI 5–14.9/h) and moderate/severe (AHI > 15/h) OSA were seen among 29% and 30% of the patients, respectively. Comparing those without OSA with those with mild or moderate/severe OSA, no differences were found in blood pressure, pharmacological treatment (anti-hypertensive, anti-depressive, and hypnotics), sleep, insomnia symptoms, daytime sleepiness, or depressive symptoms. Obesity (BMI > 30 kg/m2) was seen in 30% and 68% of the patients with mild and moderate/severe OSA, respectively. Male gender, BMI > 30 kg/m2, snoring, witnessed apnoeas, and sleep duration >8 hours were determinants of obstructive sleep apnoea. Conclusion. Previously undiagnosed OSA is common among patients with hypertension in primary care. Obesity, snoring, witnessed apnoeas, long sleep duration, and male gender were the best predictors of OSA, even in the absence of daytime sleepiness and depressive symptoms.
Current awareness:
Obstructive sleep apnoea has been linked to hypertension in sleep clinic populations, but there is a lack of knowledge regarding the occurrence in Swedish hypertensive primary care patients.
Main statements:
Undiagnosed mild and moderate/severe obstructive sleep apnoea was seen among 29% and 30% of patients, respectively.
Comparing subjects with mild or moderate/severe obstructive sleep apnoea with those without, no differences were found in blood pressure, self-rated sleep duration, insomnia, daytime sleepiness, or depressive symptoms.
Male gender, BMI > 30 kg/m00B2, snoring, witnessed apnoeas, and sleep duration >8 hours were determinants of moderate/severe obstructive sleep apnoea in hypertensive primary care patients.
Background
Hypertension (HT) is a common but treatable risk factor for cardiovascular disease (CVD) and ischaemic heart disease (IHD). The prevalence of HT has been estimated to be approximately 30% in the United States and 40% in European countries [Citation1]. Studies show that obstructive sleep apnoea (OSA) occurs in up to one-quarter of men and women in the general population [Citation2–5] when defined as at least five respiratory pauses (i.e. apnoeas and hypopnoeas) per hour of sleep. It is linked to HT, CVD, IHD, and mortality [Citation6,Citation7]. A proposed mechanism is the increased cardiovascular stress (i.e. sympathetic activation) caused by the respiratory disturbance [Citation6]. Another link is the shared prevalence of obesity [Citation8]. Obesity is significantly less prevalent in Sweden than in the United States, where most studies of OSA and HT have been conducted. However, recent Swedish data indicate an increasing obesity problem [Citation9], which might increase the occurrence of OSA. Depression and sleep complaints are other prevalent problems in primary care. Both are associated with OSA in patients referred to sleep clinics and in the general population with poor health perceptions [Citation10].
Two previous Swedish studies [Citation11,Citation12] have investigated the prevalence of OSA in hypertensive patients. Sjöström et al. [Citation11] found that 37% of middle-aged men had mild OSA and Hedner et al. [Citation12] found a prevalence of 83% having mild OSA in patients with both HT and diabetes. However, metabolic syndrome is one of the best predictors for OSA [Citation13], which might affect the prevalence. Furthermore, none of these studies investigated the association of OSA with sleep complaints, depression, and self-rated health, all plausible predictors of undiagnosed OSA in a primary care setting. The aim of the present study was to (i) describe the occurrence of undiagnosed obstructive sleep apnoea (OSA), and (ii) identify the determinants of moderate/severe OSA in hypertensive primary care patients below 65 years of age.
Material and methods
Design and selection criteria
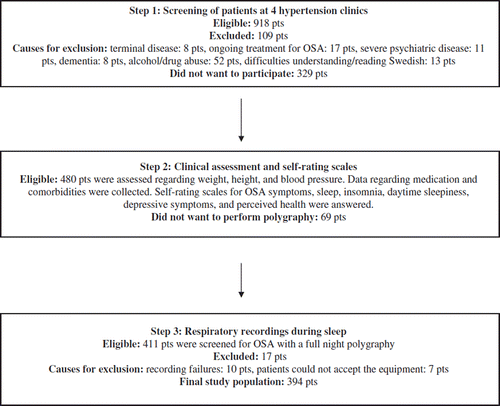
A cross-sectional design was used. After ethical approval (M29-07), all 918 eligible patients 18–65 years of age with diagnosed hypertension (140/90 mmHg) at four primary care centres in Sweden were screened. Exclusion criteria were terminal disease, ongoing treatment for OSA, severe psychiatric disease, dementia, alcohol/drug abuse, or difficulties reading and understanding the Swedish language.
Clinical variables
Data regarding weight, height, blood pressure, subjective sleep (i.e. sleep duration, estimated sleep need), medication, and comorbidities were collected during a clinical examination. Diagnosis of diabetes mellitus was based on history, current treatment (oral therapy or insulin), or repeated fasting blood glucose values ≥7mmol/l. IHD was defined as a history of angina pectoris and/or myocardial infarction and/or coronary angioplasty and/or coronary bypass surgery. Respiratory disease was defined as a history of asthma or chronic obstructive pulmonary disease, or on current treatment (β2 agonists and/or inhaled corticosteroids).
Self-rating scales
The Berlin Sleep Apnea Questionnaire (BSAQ) (11 items), focusing on occurrence of OSA symptoms/characteristics, was used to measure the risk of having OSA [Citation14]. The Minimal Insomnia Symptoms Scale (three items) was used to measure difficulties initiating sleep, difficulties maintaining sleep, and difficulties with non-restorative sleep [Citation15]. The patients grade their difficulties on a scale ranging from no problems (0), to very great problems (4). The Epworth sleepiness scale was used to measure excessive daytime sleepiness (EDS) [Citation16]. The total score of the eight items ranged from 0 to 24 points, with a cut-off of >10 indicating EDS. The Hospital Anxiety and Depression scale (HAD) (14 items) was used to measure depressive symptoms [Citation17]. The total score of the seven depression items range from 0 to 21; the higher the score the more depressive symptoms. A cut-off of > 7 was used to indicate depressive symptoms. The first question concerning current health status from the SF-36 was used to measure perceived health [Citation18]. The participants ranked their health as (1) excellent, (2) very good, (3) good, (4) fair, or (5) poor.
Recordings of sleep disordered breathing
Full-night respiratory recordings with monitoring of nasal airflow, pulse oximetry, respiratory movements, and body position were performed in the patients’ homes using polygraphic equipment [Citation19] (Embletta, ResMed AB, Trollhättan, Sweden). Apnoeas and hypopnoeas were manually scored. An apnoea was scored if the nasal pressure signal amplitude dropped ≥90% for > 10 seconds and 90% of the event met amplitude reduction criteria. A hypopnoea was scored if the nasal pressure signal amplitude dropped ≥30%, oxygen-saturation dropped ≥4% for > 10 seconds and 90% of the events met amplitude reduction criteria. Sleep time was estimated from sleep-log and trace patterns. Recordings were scored by the second author blinded to the other data. The total number of apnoeas and hypopnoeas was divided by the estimated sleep time giving the apnoea–hypopnoea index (AHI). An oxygen-desaturation index (ODI) was calculated in the same manner based on desaturations of > 4%. Patients were defined as having mild OSA or moderate/severe OSA if they had AHI 5–14.9/h or > 15/h, respectively.
Statistical analysis
Categorical data were described with percentages and numbers, whereas continuous data were described with means and 95% confidence interval. Normally distributed variables were analysed with a t-test, ANOVA, or Pearson correlations. A Mann–Whitney test, Kruskal–Wallis test, chi-squared test, or Spearman rank correlations were used on non-normally distributed or dichotomous variables. To examine variables independently associated with AHI >15, logistic regression analysis was used. Based on bivariate correlations, variables with an association of p <0.20 with AHI > 15 were entered as predictors into a multiple logistic regression. The significance level was set to p <0.05. All statistical analyses were performed with the PASW statistics 18 (IBM Inc., USA).
Results
Study population
Of the 918 patients, 12% (50 men/59 women) were excluded and 28% (170 men/159 women) chose not to participate. Of the 480 patients who participated in the clinical examination, 411 had a respiratory recording although 17 were lost due to technical problems (). Thus, the final study population consisted of 394 patients. Population characteristics, comorbidities, and medications are given in .
Table I. Characteristics, medication, and comorbidities across the severity groups for obstructive sleep apnoea in hypertensive primary care patients below 65 years of age (n = 394).
Occurrence of OSA
It total, 59% of the 394 patients had OSA (AHI > 5). Mild and moderate/severe OSA occurred among 29% and 30% of the patients, respectively. Average ODI was four and 16 times as high in the groups with mild and moderate/severe OSA (7.9 and 32.0, respectively) compared with those without OSA (2.0) (see ). Neither systolic or diastolic blood pressure or medication differed between the two groups of patients with OSA, or compared with patients without OSA. BMI was significantly associated with AHI. Obesity (BMI > 30 kg/m2) was seen in 30% and 68% of the patients with mild and moderate/severe OSA, respectively.
Diabetes was significantly more prevalent among patients with at least mild OSA. Neither history of IHD nor respiratory disease was associated with severity of OSA (see ).
Differences among OSA groups and determinants of OSA
No differences were found regarding estimated sleep need, sleep sufficiency index, insomnia, EDS, or depressive symptoms with regard to different degrees of OSA (). The choice of variables included in the regression analysis was based on their bivariate relationship to OSA (AHI >15). In the final analysis only male gender, BMI >30 kg/m2, snoring, reports of witnessed apnoeas, and sleep duration >8 hours were significant determinants of OSA (AHI ≥15). Specifically, men had 1.7 times the odds of having moderate/severe OSA; BMI >30 kg/m2 presented 4.0 times the odds; snorers had 3.9 times the odds; witnessed apnoeic events 3.2 times the odds; and sleep duration >8 hours had 3.6 times the odds of OSA in this sample (). Age, daytime sleepiness, diabetes, hypercholesterolemia, perceived health, or depressive symptoms were not significant indicators of OSA in this sample.
Table II. Self-rated sleep, daytime sleepiness, depressive symptoms, and perceived health across the severity groups for obstructive sleep apnoea in hypertensive primary care patients below 65 years of age (n =394).
Table III. Multivariate regression model for apnoea–hypopnoea index >15 in hypertensive primary care patients below 65 years of age (n =394).
Discussion
The major findings of this study were that more than one-half of a cohort of hypertensive primary care patients had objective evidence of OSA. But no relationship between OSA and severity of blood pressure elevation, or specific comorbid conditions was seen. Diabetes, depressive symptoms, insomnia, and EDS were common, but not associated with OSA. Male gender, BMI >30 kg/m2, snoring, witnessed apnoeas, and sleep duration >8 hours were significant correlates of OSA.
OSA in this sample was more common than that found in community-based Scandinavian and European general populations [Citation3–5] despite our focus on the occurrence of undiagnosed OSA (e.g. patients with treated OSA were excluded). This could be explained by our selection of hypertensive patients [Citation8]. Sjöström et al. [Citation11] found in a stratified sample of hypertensive men that 37% had AHI >10. In a community-based case-control study, Hedner et al. [Citation12] found AHI > 10 in 83% of middle-aged patients with both HT and diabetes. Comparing our data with those from these studies is difficult, since they used different cut-off scores for AHI and Hedner's subjects also had metabolic syndrome, one of the best predictors for OSA [Citation13].
Prior studies [Citation20–22] have suggested that OSA is commonly associated with HT. We found no association between OSA severity and level of blood pressure elevation or pharmacological treatment, in contrast to previous studies where a dose–response relationship was found between night-time blood pressure and increasing AHI [Citation23]. This may be due to the fact that our patients were already treated for HT, mostly successfully. EDS and depressive symptoms, commonly assumed as predictors of OSA [Citation13], were not associated with OSA although we used validated questionnaires to assess them [Citation16,Citation17]. The fact that we did not enrol patients from a sleep clinic might explain the lower occurrence of sleeping difficulties, EDS, and depressive symptoms found compared with other samples [Citation13]. These findings shed light on the difficulties that GPs have in identifying patients with OSA in primary care who are in need of weight reduction or treatment of OSA [Citation24,Citation25]. However, we identified that hypertensive men who are obese (i.e. BMI > 30 kg/m2), snore, and report witnessed apnoeas, as well as long sleep duration (i.e. >8 hours) can be suspected to have OSA. Obesity is common in sleep clinic patients [Citation8,Citation13], and intensive interventions based on low-calorie diets to reduce weight have been shown to decrease AHI [Citation26], but long-term results are lacking. Questionnaires, such as the BSAQ [Citation14,Citation27], focusing on weight gain/obesity, snoring, witnessed apnoeas, and EDS may, together with simple two-channel recording devices [Citation28], be suitable in routine screening in primary care settings. Importantly, results from the BSAQ and the two channel devices are not comparable to validated diagnostic tools, such as polygraphy or polysomnography, and need to be more thoroughly evaluated regarding sensitivity and specificity. Large, well- designed studies are therefore needed to explore symptom profiles, clinical characteristics, and different suitable screening procedures for hypertensive patients with undiagnosed OSA of different severity levels in primary care settings. Such studies can help to identify patients who would benefit from referral to a sleep clinic.
We found that desaturations were highly prevalent (i.e. mean ODI of 32.0) in those with AHI > 15. Hypoxia is an important contributor to CVD in patients with OSA (i.e. by sympathetic activation and increased levels of catecholamines, causing inflammation, arterial stiffness, and atherosclerosis) [Citation6]. Initiation of continuous positive airway pressure (CPAP) can eliminate apnoeas and desaturations, and reduce cardiovascular morbidity and mortality [Citation29], particularly in patients with severe OSA and daytime symptoms. Blood pressure reductions after CPAP have been found, especially in sleepy patients with frequent desaturations, but also when HT is severe, untreated, or refractory [Citation30]. However, the effect of CPAP in hypertensive primary care patients with OSA, especially those with mild and moderate OSA, is unknown.
In conclusion, undiagnosed OSA (AHI > 15) is common in hypertensive primary care patients. Snoring male patients with elevated BMI, long sleep duration, and witnessed apnoeas should be investigated for OSA. Future studies should focus on randomized controlled trials evaluating long-term effects of CPAP in hypertensive primary care patients with OSA. Stratifying men and women with different levels of OSA will strengthen the evidence base for treatment. Potential confounding factors for CVD, such as obesity, should also be controlled.
Limitations of this study include the cross- sectional design, and the lack of a normotensive control group matched for age, gender, and BMI, as well as the relatively low participation rate. Furthermore, no objective data regarding sleep were collected, which means that the occurrence of OSA might have been even higher since there are difficulties in estimating sleep onset and wake-up time with polygraphy [Citation28].
Acknowledgement
This study was supported by the Swedish Heart Lung Foundation, Grant 20090547.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, . Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003;289:2363–9.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5.
- Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163:685–9.
- Hrubos-Strøm H, Randby A, Namtvedt SK, Kristiansen HA, Einvik G, Benth J, . Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J Sleep Res. 2011;20(1 Pt 2):162–70.
- Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men: An epidemiological study. J Clin Epidemiol 1988;41:571–6.
- Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med 2010;31:203–20.
- Lavie P. Mortality in sleep apnoea syndrome: A review of the evidence Eur Respir Rev 2007;16:203–10.
- Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension 2003;42:1067–74.
- Asplund R. Obesity in elderly people with nocturia: Cause or consequence? Can J Urol 2007;14:3424–8.
- Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psych 2003;64:1195–1200.
- Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: A population based study. Thorax 2002; 57:602–7.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: A population-based case-control study. Eur Respir J 2006;27:564–70.
- Drager LF, Genta PG, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, . Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol 2010;105:1135–9.
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–91.
- Broman JE, Smedje H, Mallon L, Hetta J. The Minimal Insomnia Symptom Scale (MISS): A brief measure of sleeping difficulties. Ups J Med Sci 2008;113:131–42.
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14:540–5.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: Conceptual framework and item selection. Med Care 1992;30:473–83.
- Collop NA, McDowell Anderson, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, . Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007;3:737–7.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–84.
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, . Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000;288:1829–36.
- Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med 1998; 157:111–15.
- Baguet JP, Barone-Rochette G, Pépin JL. Hypertension and obstructive sleep apnoea syndrome: Current perspectives. J Hum Hypertens 2009;23:431–43.
- Wikstrand I, Torgerson J, Bengtsson Boström KB. Very low calorie diet (VLCD) followed by a randomized trial of corset treatment for obesity in primary care. Scand J Prim Health Care 2010;28:89–94.
- Kramer NR, Cook TE, Carlisle CC, Corwin RW, Millman RP. The role of the primary care physician in recognizing obstructive sleep apnea. Arch Intern Med 1999;159:965–8.
- Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rössner S, . Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: Prospective observational follow-up study. BMJ 2011;342:d3017.
- Netzer NC, Hoegel JJ, Loube D, Netzer CM, Hay B, Alvarez-Sala R, . Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003;124:1406–14.
- Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–76.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005;365:1046–53.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, . Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised parallel trial. Lancet 2002;359: 204–10.