Abstract
Objective. To validate A Quick Test of Cognitive Speed (AQT) as an instrument in diagnostic dementia evaluations against final clinical diagnosis and compare AQT with the Mini-Mental State Examination (MMSE) and Clock Drawing Test (CDT) in primary care. Design. Primary health care cohort survey. Setting. Four primary health care centres and a geriatric memory clinic in Sweden. Patients. 81 patients (age 65 and above) were included: 52 with cognitive symptoms and 29 presumed cognitively healthy. None of the patients had a previous documented dementia diagnosis. All patients performed MMSE, CDT, and AQT at the primary health care clinic and were referred for extensive neuropsychological testing at a memory clinic. AQT was validated against final clinical diagnosis determined by a geriatric specialist and a neuropsychologist. Main outcome measures. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratios, correlation data, and receiver operating characteristic (ROC). Results. For MMSE, sensitivity and specificity was 0.587 and 0.909; CDT 0.261 and 0.879; and AQT 0.783 and 0.667, respectively. For the combination of MMSE and CDT, sensitivity and specificity was 0.696 and 0.788, for MMSE and AQT 0.913 and 0.636. The ROC curve for AQT showed an area under curve (AUC) of 0.773. Conclusion. Our results suggest AQT is a usable test for dementia assessments in primary care. Sensitivity for AQT is superior to CDT, equivalent to MMSE, and comparable to the combination MMSE and CDT. MMSE in combination with AQT improves sensitivity. Because AQT is user-friendly and quickly administered, it could be applicable for primary care settings.
Accurate diagnostic instruments are needed in primary care for dementia.
Our data show that A Quick Test of Cognitive Speed (AQT) is usable for diagnostic dementia evaluations in primary care.
AQT is equivalent to the Mini-Mental State Examination (MMSE) and superior to the Clock Drawing Test (CDT) in diagnosing dementia.
Introduction
The global prevalence of dementia for people 65 years and older is 5–10% and increasing [Citation1]. The indirect and direct costs associated with dementia patients are substantial [Citation2]. Primary health care plays an important role in early detection of cognitive dysfunction [Citation3,Citation4]. However, dementia is frequently underestimated and diagnosed late in the disease process [Citation5,Citation6]. The recognition of patients with cognitive impairment in primary care is difficult [Citation7,Citation8]. Objective test measurements have been shown to be more reliable than a patient's subjective memory complaints [Citation9]. Early detection of dementia may facilitate future health care planning for patients and their family care networks [Citation10]. Good knowledge among general practitioners and adequate diagnostic tools suited for dementia evaluations in primary care is important [Citation10].
The Mini-Mental State Examination (MMSE) is the most widely used diagnostic test for dementia, but its accuracy has been questioned [Citation11,Citation12]. The test results are influenced by age, education, and ethnicity [Citation13,Citation14]. The MMSE's diagnostic accuracy is improved if MMSE is used in conjunction with the Clock Drawing Test (CDT) [Citation9,Citation15]. Both MMSE and CDT are limited in their ability to identify patients with early dementia and mild cognitive impairment (MCI) [Citation12,Citation16].
A Quick Test of Cognitive Speed (AQT) measures perception speed and overall cognitive speed [Citation17]. Preliminary data indicate that reductions in perceptual and cognitive speed precede reductions in cognitive linguistic abilities in mild-to-moderate Alzheimer's disease (AD) [Citation18]. Because AQT is user-friendly and quickly administered, it may be appropriate for use in primary care [Citation19]. Furthermore, the results are affected minimally by age (on average 1 second per decade) and not at all by gender or education [Citation19]. Studies from hospital memory clinics have been promising, but data from primary care clinics are scarce.
The aim of this study was to validate AQT as a cognitive instrument in the diagnostic evaluation of dementia against final clinical diagnosis and to compare the AQT results with the MMSE and CDT in primary care.
Material and methods
This study was conducted according to provisions of the Helsinki Declaration and the Ethical Review Board, Linköping University, Sweden (DNR M137-07). Written informed consent was collected from all study participants.
Study cohort
A total of 81 participants (65 years old or older and living at home) were recruited from four primary health care centres in Sweden between December 2007 and May 2009 (). Of these patients, 52 exhibited possible cognitive impairment and 29 were presumed cognitively healthy and visiting primary care for some other medical problem. The four primary care centres served a total of 49 800 people; 11 200 were 65 years or older. All 81 patients were asked to participate in the study during an appointment with a general practitioner. One patient declined. The dementia evaluation was initiated by the patients, their relatives, or staff at the primary care centre. Exclusion criteria consisted of cerebral infection, brain tumour, ongoing verified psychiatric illness at study start, and stroke or head trauma within the last four weeks before inclusion. However, patients with a previous psychiatric diagnosis but in a clinically stable condition (determined by the general practitioner) and unmodified antidepressant medication for the last six months were included. The 29 presumed cognitively healthy patients were asked to complete a short questionnaire regarding self-estimated memory. These patients identified themselves as cognitively well functioning; the general practitioner agreed with their self-assessment. All 81 patients underwent physical examination by a general practitioner, routine lab examinations, and ECG evaluation. The 52 patients with cognitive symptoms also had a CT brain scan performed as a part of the clinical workup.
Table I. Descriptive data shown for the total study group (SG) and subcategorized for patients with cognitive symptoms (CS) and presumed cognitively healthy patients with other medical problems (CH) as primary reason for admission.
Instruments
All 81 patients performed the MMSE, CDT, and AQT during the same appointment with an occupational therapist in primary care. Completing the tests with instructions took on average 30 minutes per patient; the time to finish the AQT was between 5 and 10 minutes. AQT measures perception speed in three parts (Pearson Education, Inc., TX, USA). Parts one and two measure single dimensions (e.g. form or colour naming). Part three measures overall cognitive speed with dual dimensions (e.g. form and colour naming) using 40 visual stimuli [Citation17]. The visual stimuli are geometric figures – circles, squares, rectangles, or triangles, coloured red, black, yellow, or blue. For the single dimension, the patients are asked to name the colour and then the shape as quickly as possible. For the dual dimension, the patients are asked to name the colour and the form for all consecutive 40 figures. The AQT score considered here consists of the number of seconds it takes to complete part three. MMSE assesses orientation in time and place, attention, memory (delayed word recall), language (various verbal tasks), and visual construction, with a maximum score of 30 points. The Clock Drawing Test (CDT) measures visuo-spatial and executive function [Citation16]. The patients were instructed to draw the face of a clock on a blank piece of paper with all the numbers on it and to set the time to 10 past 11.
The patients were referred to a university memory clinic for more extensive testing in a standardized order by a neuropsychologist using the memory test Alzheimer's Disease Assessment Scale-cognitive (ADAS-cog) [Citation20], the letter Verbal Fluency Test (VTF) [Citation21], immediate and delayed Story Recall, TMT A and B [Citation22], language test (WAISS-III in Swedish translation), and examination. In addition, the patients were examined by a geriatric specialist. AQT was validated against the final clinical diagnosis (ICD-10) using the results of the neuropsychological tests, the lab results, the CT scan, and the consensus conclusions provided by the neuropsychologist and a geriatric specialist. Two patients from the presumed cognitively healthy group were excluded from the calculations since the geriatric specialist and the neuropsychologist's evaluation, used as the standard for final diagnosis, concluded that a prior unrecognized MCI existed (). The final study group from which data were analysed therefore consisted of a total of 79 patients. The results of the AQT, MMSE, and CDT were not accessible during the diagnostic procedure; they were used only in the comparative analysis.
Table II. Data showing final diagnosis, cognitive impairment, and no cognitive impairment, for patients with cognitive symptoms and presumed cognitively healthy patients as primary reason for admission.
Measurements
Data were collected and calculated using the statistical program SPSS for Windows 19:0 (SAS Institute, Cary, NC, USA). In the descriptive calculations, chi-square was used for gender, comorbidity, and medication; independent t-test was used for age, education, and the test variables ( and ). For AQT, the dual dimension with a time cut-off between cognitively healthy and cognitive impairment (standard 70 s) was used in the calculations [Citation17]. Results on MMSE ≤ 26/30 were considered as cognitive impairment as well as every value below a full five points in the CDT according to Shulman [Citation23]. Sensitivity, specificity, predictive values, and likelihood ratios were calculated and compared for AQT, MMSE, and CDT as well as for the combinations MMSE and CDT, AQT and MMSE, AQT and CDT, and the three tests altogether. In the combination calculations, one positive result in any of the tests was enough to register the combination result as positive for cognitive impairment. Correlation data between MMSE and AQT were analysed parametrically with Pearson's correlation and non-parametrically with Spearman's rank correlation. Using a receiver operator characteristic (ROC) curve, the area under the curve (AUC) for MMSE, CDT, and AQT was analysed and possible cut-off times in AQT and their effect on sensitivity were ranked [Citation24].
Table III. Patient data categorized by final clinical diagnosis: Listed as total study group (SG), after exclusion of two individuals, patients with cognitive impairment (CI), and patients with no cognitive impairment (NCI).
Results
Using the criteria defined above, 33 of 81 patients were diagnosed as having no objective cognitive impairment (see and ) and 46 patients received a diagnosis of cognitive impairment: MCI 16 (35%), AD 12 (26%), mixed dementia six (13%), vascular dementia five (11%), Lewy bodies dementia one (2%), Parkinson's disease with dementia one (2%), dementia of uncertain origin two (4%), and three patients (7%) had comorbidity with depressive disorder. Two patients from the cognitively healthy group were excluded. Hence, the results are based on calculations from the final study group of 79 people.
Sensitivity and specificity values were as follows: for MMSE, 0.587 and 0.909; for CDT, 0.261 and 0.879; and for AQT, 0.783 and 0.667. The positive predictive value for MMSE was 0.900 (90%), for CDT 0.750 (75%), and for AQT 0.766 (77%). The negative predictive value for MMSE was 0.612 (61%), for CDT 0.460 (46%), and for AQT 0.688 (69%). Sensitivity and specificity values for the combinations of tests were as follows: for MMSE and CDT, 0.696 and 0.788; for MMSE and AQT, 0.913 and 0.636; and for AQT and CDT, 0.783 and 0.576. When MMSE, CDT, and AQT were combined, sensitivity was 0.913 and specificity was 0.545.
The positive likelihood ratios were as follows: MMSE: 6.45; AQT: 2.35; and CDT: 2.15. The negative likelihood ratios were as follows: MMSE: 0.45; AQT: 0.33; and CDT: 0.84.
Analysis of correlation data between MMSE and AQT gave a significant negative Pearson's correlation: r = –0.246 (p = 0.029) and Spearman's rank correlation: rs = –0.359 (p = 0.001). A plot of the data suggested a non-linear relation.
ROC curve analysis of AQT at cut-off 70 s showed an AUC of 0.773, for MMSE of 0.849, and for CDT of 0.574 (). Plotting coordinates for sensitivity and 1-specificity visualized the cut-off time of 70 s as reasonable, with an optimal cut-off estimated to be between 65 and 70 s ().
Figure 1. Receiver operating characteristic (ROC) curve. Null hypothesis: true area = 0.5. Area under curve (AUC): AQT = 0.773; MMSE = 0.849; and CDT = 0.574.
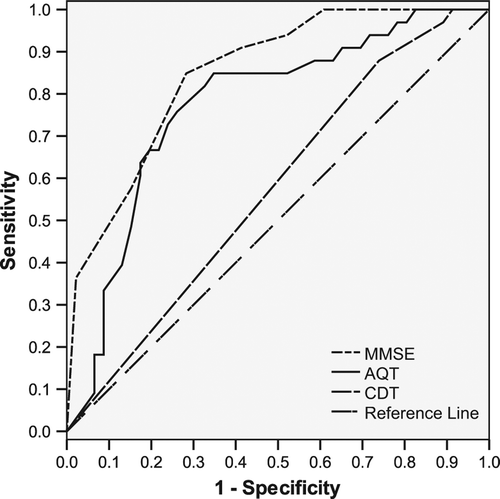
Table IV. Different AQT cut-off times analysed by receiver operating characteristics (ROC).
Discussion
Our results show that AQT is a usable test for diagnostic dementia evaluations in primary care. Sensitivity and negative predictive values were better for AQT than MMSE and CDT. In this study, MMSE had a slightly higher likelihood ratio, specificity, and positive predictive value compared with AQT. The AUC for AQT was similar to MMSE and above the recommended level of 0.7 for cognitive tests overall. AUC values around 0.8 are regarded as good. Our study confirms the previous recommended cut-off time of 70 s [Citation17]. The range between 63 s and 70 s has been regarded as slower than normal and > 70 s atypical; our results are consistent with these findings (unpublished data Palmqvist et al.). There is a significant negative correlation between MMSE and AQT, but correlative data are weak and the plot of the data suggests a non-linear relation. This is probably due to the different cognitive domains reflected in the two tests. MMSE in conjunction with CDT is generally recommended for dementia diagnostics performed in primary care [Citation12,Citation15]. Our results support a possible new combination – MMSE and AQT – that produces better overall sensitivity and reasonable specificity. Since the three tests together did not achieve an obvious improvement in the overall results, we regard AQT as a possible alternative to CDT and a complement to MMSE. AQT has previously been proven to sensitively screen for patients with AD and Lewy bodies dementia in a hospital setting [Citation18,Citation25]. Our results show the test to be sensitive to dementia in a primary care population as well. AQT requires minimal administration training, a desirable quality for a primary care instrument. There are other tests available that measure reading time, such as the Stroop test, that use inhibition of executive functions [Citation26]. AQT was designed to avoid the frontal lobe function, as AQT's main purpose is to detect dementia, not to subcategorize. Since these are the first AQT data collected in primary care, we have validated only the dual dimension to have access to comparable results [Citation25]. The relevance of errors in AQT is uncertain and there are no current recommendations available with regard to error interpretation. Error scores in the Stroop Test have been shown to be unrelated to dementia type and severity [Citation26]. MCI patients have been shown to have impairment in other cognitive domains besides episodic memory [Citation27]. The combination of dementia batteries with several domains and AQT would be interesting for further studies. We do not recommend general dementia screening programmes of the elderly in primary care [Citation28–30]. The results of the predictive values presented here should be interpreted with respect to a population with some suspicion of cognitive impairment.
The study has several limitations. The sample of 81 patients is small and there are some population differences between the groups. A more even distribution between gender, age, education, and comorbidity would have been preferable. There are notably a statistically higher proportion of patients on antidepressant medication at inclusion in the group with cognitive symptoms compared with the group presumed cognitively healthy. However, the intention was to realize the study as close to the clinical primary care work setting as possible. Consequently, it was difficult to extend and regulate the inclusion criteria even more without a subsequently protracted inclusion time. Two patients from the presumed cognitively healthy group were excluded from the calculations. A more objective memory inclusion test for the patients in the presumed cognitively healthy group might have been one way to avoid this. The exclusion criteria may influence the specificity of the test and probably contributed to the low number of patients with depression as a final diagnosis. Patients living in a nursing home were not included. This might be part of the explanation for the proportional distribution between MCI and dementia as a final diagnosis. A follow-up after one year to confirm the final diagnoses would have improved the quality of the study, but the diagnoses have been set according to standards and a thorough investigation by a specialist. The patients participated in several tests during the same appointment. To limit possible bias from fatigue, the extended neuropsychological tests were performed during another appointment. This methodology may lead to inaccurate results if there were any fluctuations in cognitive capability. However, all the tests took place within four weeks, making significant deterioration in the cognitive capacity less likely.
Our data do not answer the question of whether AQT can detect cognitive decline over time. To what extent AQT can be used to distinguish between MCI and patients with a more advanced dementia disease, or differentiate between subtypes of dementia and affective disorders, remains to be investigated. Since decline in visual speed processing might appear earlier than memory problems in MCI patients, AQT can enhance the possibility of accurately diagnosing early dementia. In conclusion, AQT seems to be a usable instrument for diagnostic evaluation of dementia in a primary care setting. In addition, AQT might be able to complement MMSE and be an alternative to CDT as a primary diagnostic tool.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by Swedish Brain Power, ALF Grants and research funding from the County Council of Östergötland.
References
- Ferri CP, Prince M, Brayne C, . Global prevalence of dementia: A Delphi consensus study. Lancet 2005;366: 2112–17.
- Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: Estimates for 2009.Alzheimers Dement2010; 6:98–103.
- Olafsdottir M, Marcusson J. Diagnosis of dementia at the primary care level. Acta Neurol Scand Suppl 1996;165: 58–62.
- Luck T, Riedel-Heller SG, Kaduszkiewicz H, . Mild cognitive impairment in general practice: Age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe). Dement Geriatr Cogn Disord 2007;24:307–16.
- Lopponen M, Raiha I, Isoaho R, Vahlberg T, Kivela SL. Diagnosing cognitive impairment and dementia in primary health care: A more active approach is needed. Age Ageing 2003; 32:606–12.
- Stoppe G, Haak S, Knoblauch A, Maeck L. Diagnosis of dementia in primary care: A representative survey of family physicians and neuropsychiatrists in Germany. Dement Geriatr Cogn Disord 2007;23:207–14.
- Ravdin LD, Mattis PJ, Lachs MS. Assessment of cognition in primary care: Neuropsychological evaluation of the geriatric patient. Geriatrics 2004;59:37–40.
- Waldorff FB, Rishoj S, Waldemar G. Identification and diagnostic evaluation of possible dementia in general practice: A prospective study. Scand J Prim Health Care 2005;23: 221–6.
- Harvan JR, Cotter V. An evaluation of dementia screening in the primary care setting. J Am Acad Nurse Pract 2006;18: 351–60.
- Schoenmakers B, Buntinx F, Delepeleire J. What is the role of the general practitioner towards the family caregiver of a community-dwelling demented relative? A systematic literature review. Scand J Prim Health Care 2009;27:31–40.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
- Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43:411–31.
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–91.
- Scazufca M, Almeida OP, Vallada HP, Tasse WA, Menezes PR. Limitations of the Mini-Mental State Examination for screening dementia in a community with low socioeconomic status: Results from the Sao Paulo Ageing & Health Study. Eur Arch Psychiatry Clin Neurosci 2009;259:8–15.
- Kirby M, Denihan A, Bruce I, Coakley D, Lawlor BA. The clock drawing test in primary care: Sensitivity in dementia detection and specificity against normal and depressed elderly. Int J Geriatr Psychiatry 2001;16:935–40.
- Ehreke L, Luppa M, Luck T, . Is the clock drawing test appropriate for screening for mild cognitive impairment? Results of the German study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Dement Geriatr Cogn Disord 2009;28:365–72.
- Nielsen NP, Wiig EH, Warkentin S, Minthon L. Clinical utility of color-form naming in Alzheimer's disease: Preliminary evidence. Percept Mot Skills 2004;99:1201–4.
- Ross-Swain D, Wiig EH. Reductions in “Ross Information Processing Test-Geriatric” information processing and “A Quick Test of Cognitive Speed” processing speed in Alzheimer's disease: Which lead and which follow?Int J Rehabil Res 2008;31:81–4.
- Nielsen NP, Wiig EH. Alzheimer's Quick Test cognitive screening criteria for West African speakers of Krio. Age Ageing 2006;35:503–7.
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–64.
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: A meta-analysis. Neuropsychologia 2004;42:1212–22.
- Ashendorf L, Jefferson AL, O’Connor MK, Chaisson C, Green RC, Stern RA. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol 2008;23:129–37.
- Shulman KI. Clock-drawing: Is it the ideal cognitive screening test?Int J Geriatr Psychiatry 2000;15:548–61.
- Zweig MH, Campbell G. Receiverioperating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–77.
- Andersson M, Wiig EH, Minthon L, Londos E. A Quick Test for Cognitive Speed: A measure of cognitive speed in dementia with Lewy bodies. Am J Alzheimers Dis Other Demen 2007;22:313–8.
- Koss E, Ober BA, Delis DC, Friedland RP. The Stroop color–word test: Indicator of dementia severity. Int J Neurosci 1984;24:53–61.
- Matsuda O, Saito M. Multiple cognitive deficits in patients during the mild cognitive impairment stage of Alzheimer's disease: How are cognitive domains other than episodic memory impaired?Int Psychogeriatr 2009;21:970–6.
- Doraiswamy PM, Steffens DC, Pitchumoni S, Tabrizi S. Early recognition of Alzheimer's disease: What is consensual? What is controversial? What is practical?J Clin Psychiatry 1998;59:6–18.
- Small GW, Rabins PV, Barry PP, . Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA 1997;278:1363–71.
- Lavery LL, Lu SY, Chang CC, Saxton J, Ganguli M. Cognitive assessment of older primary care patients with and without memory complaints. J Gen Intern Med 2007;22:949–54.