Abstract
Objective. To determine the influence of ischaemic cardiovascular (CV) risk on prescription of non-steroidal anti-inflammatory drugs (NSAIDs) by general practitioners (GPs) in patients with musculoskeletal complaints. Design. Cohort study. Setting. A healthcare database containing the electronic GP medical records of over one million patients throughout the Netherlands. Patients. A total of 474 201 adults consulting their GP with a new musculoskeletal complaint between 2000 and 2010. Patients were considered at high CV risk if they had a history of myocardial infarction, angina pectoris, stroke, transient ischaemic attack, or peripheral arterial disease, and at low CV risk if they had no CV risk factors. Main outcome measures. Frequency of prescription of non-selective (ns)NSAIDs and selective cyclooxygenase-2 inhibitors (coxibs). Results. Overall, 24.4% of patients were prescribed an nsNSAID and 1.4% a coxib. Of the 41,483 patients with a high CV risk, 19.9% received an nsNSAID and 2.2% a coxib. These patients were more likely to be prescribed a coxib than patients with a low CV risk (OR 1.9, 95% CI 1.8–2.0). Prescription of nsNSAIDs decreased over time in all risk groups and was lower in patients with a high CV risk than in patients with a low CV risk (OR 0.8, 95% CI 0.7–0.8). Conclusion. Overall, patients with a high CV risk were less likely to be prescribed an NSAID for musculoskeletal complaints than patients with a low CV risk. Nevertheless, one in five high CV risk patients received an NSAID, indicating that there is still room for improvement.
International guidelines recommend avoiding the prescription of NSAIDs in patients at high ischaemic cardiovascular risk. In this study, we found that:
NSAIDs are prescribed in one in five patients with a high cardiovascular risk.
Prescription of coxibs is higher in patients with a high cardiovascular risk than in those with a low cardiovascular risk.
NSAID prescription decreased over time in all risk groups, but it appears that general practitioners do not fully consider the cardiovascular risks associated with NSAID use, indicating that there is room for improvement.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in the treatment of musculoskeletal (MSK) complaints because of their analgesic and anti-inflammatory properties. International and national guidelines on various MSK complaints, such as back pain, shoulder pain, and osteoarthritis, recommend prescribing NSAIDs, either as a first-choice analgesic or as a second choice if paracetamol fails to provide sufficient pain relief [Citation1–6]. The use of NSAIDs is known to be associated with peptic ulcer disease and its complications, most notably upper gastrointestinal (UGI) bleeding, obstruction, and perforation [Citation7,Citation8]. The need to limit these UGI complications led to the development of selective cyclooxygenase-2 inhibitors (coxibs), which are associated with a significantly lower incidence of UGI complications when compared with traditional, non-selective NSAIDs (nsNSAIDs) [Citation9–12].
However, shortly after the introduction of coxibs, concerns were raised regarding their cardiovascular (CV) safety profile. In September 2004, rofecoxib was withdrawn from world markets after a randomized controlled trial showed the incidence of stroke, myocardial infarction, or sudden cardiac death in patients taking rofecoxib was twice that of patients taking a placebo [Citation13]. An increased risk of ischaemic CV events was also observed in studies of other coxibs, leading the European Medicines Agency to contraindicate the use of any coxib in patients with established ischaemic heart disease, stroke or peripheral arterial disease in 2005 [Citation14]. Since then, there is increasing evidence that the risk of ischaemic CV events is increased not only by the use of coxibs but also by the use of nsNSAIDs, with the possible exception of naproxen [Citation15–18]. Recent guidelines and consensus therefore recommend avoiding the prescription of NSAIDs in general in patients at high CV risk [Citation19–21].
In this population-based cohort study, we aimed to examine the association between ischaemic CV risk and the prescription of NSAIDs in patients with MSK complaints. In addition, we aimed to determine the influence of demographic factors, prior NSAID prescription, the type of MSK complaint presented and the presence of UGI risk factors and renal insufficiency on NSAID prescription in this group of patients.
Material and methods
Setting
A cohort study was conducted in the Integrated Primary Care Information (IPCI) database. This primary health care database contains the electronic patient records of over one million patients registered with GPs throughout the Netherlands. In the Netherlands, all 16.8 million citizens are registered with a GP, who forms the first point of care and acts as a gatekeeper in a two-way exchange of information with secondary care. The electronic medical record of each patient can therefore be assumed to contain all relevant medical information, including medical findings and diagnoses from secondary care. Further details of the database have been described elsewhere [Citation22,Citation23].
Study cohort
The study population comprised all patients ≥ 18 years of age newly diagnosed with a MSK complaint between 1 January 2000 and 31 December 2010. Diagnoses were considered new if the patient had not been diagnosed with the same MSK complaint in the six months prior to consultation. Only patients with at least 12 months of valid database history prior to study entry were included. Diagnoses of MSK complaints were identified based on International Classification for Primary Care (ICPC) coding [Citation24]. If the patient consulted his/her GP again with the same complaint within six months of initial diagnosis, this consultation was considered part of the same MSK complaint episode. For each patient, only the first newly diagnosed complaint episode was included. The date of first consultation was considered the index date.
Cardiovascular risk, upper gastrointestinal risk, and renal insufficiency
In defining CV risk, UGI risk, and renal insufficiency we aimed to conform to Dutch prescription guidelines as much as possible. For cardiovascular risk, no Dutch guideline is currently available, but a national consensus report was published in 2009 containing prescription recommendations [Citation21]. In line with this report, patients were considered at high CV risk if they had a history of myocardial infarction, angina pectoris, stroke, transient ischaemic attack, or peripheral arterial disease prior to the index date. They were considered at moderate CV risk if they did not have one of the risk factors described above but did have a history of diabetes, hypertension, or hyperlipidaemia. Patients without any of these CV risk factors were considered at low CV risk. In addition, risk factors for the occurrence of UGI complications were identified. Based on the most recent Dutch guideline on the prescription of NSAIDs [Citation25], patients were considered at high UGI risk if they had a history of upper gastrointestinal bleeding or ulceration, were aged over 70 years or had two or more of the following risk factors: age 60–70 years, history of heart failure, diabetes, or severe rheumatoid arthritis, use of antithrombotics, corticosteroids, or selective serotonin reuptake inhibitors. They were considered at moderate UGI risk if only one of the latter risk factors was present. In the absence of any of these risk factors patients were considered to have a low UGI risk. Finally, we identified each patient's most recent available laboratory measurement of glomerular filtration rate (GFR) prior to the index date. If this GFR was < 30 mL/min, patients were considered to have significant renal insufficiency [Citation26].
The history of the diseases and conditions described above were assessed based on ICPC coding and free text search strings. In the case of diabetes and hyperlipidaemia, the use of respectively antidiabetic and lipid-modifying drugs, identified based on ATC classification code [Citation27], was taken into account in addition to ICPC coding as proxy. If patients had a history of rheumatoid arthritis based on an ICPC code L88, this was defined as being severe if they also had a prescription in the year prior to the index date of specific antirheumatic agents, immunosuppressants, hydroxychloroquine, sulfasalazine, or cyclophosphamide.
NSAID prescription
For all included patients, the first NSAID prescription issued during the complaint episode was identified based on ATC classification code [Citation27]. Only NSAID prescriptions issued on the day of a consultation for the MSK complaint were included. It has been suggested that the use of naproxen is less likely to increase cardiovascular risk than the use of other nsNSAIDs, and that the prescription of naproxen may be warranted in patients at a high CV risk [Citation19,Citation20,Citation28]. In addition, there are indications that the risk of CV disease increases with NSAID use in a dose-dependent manner [Citation29]. To examine whether GPs take these possibilities into account, a sensitivity analysis was conducted excluding naproxen and excluding all low-dosed nsNSAID and low-dosed coxib prescriptions, which was defined as a prescribed daily dosage (PDD) smaller than half the defined daily dosage (DDD).
Statistics
Baseline characteristics of the moderate and high CV risk groups were compared with those of the low CV risk group using a chi-squared test for dichotomous variables and independent t-test for age as a continuous variable. Univariate analyses of potential predictors of NSAID prescription such as age, gender, CV risk, and UGI risk were conducted and unadjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated by performing logistic regression analyses. For the predictor CV risk group, the same univariate logistic regression analyses were performed stratified per UGI risk group. For this stratified analysis, we also conducted multivariate analyses to present ORs adjusted for other predictors of nsNSAID and coxib prescription. Finally, the influence of CV risk on coxib and nsNSAID prescription was studied stratified per time period, and ORs adjusted for the year of the MSK complaint episode within each time period were calculated, again using multivariate logistic regression analysis. All analyses were performed using SPSS version 20 (SPSS, Chicago, IL).
Study approval
The study was approved by the Board of Directors of the IPCI database.
Results
Study cohort
Between 2000 and 2010, 804 261 adult patients aged over 18 years contributed data to the IPCI database. These patients were comparable to the general population of the Netherlands with regard to age and gender (mean age 40 years, 52% female versus 41 years, 51% female in the Dutch general population) [Citation30]. Of these, 474 201 patients (59%) presented with a new MSK complaint and were included in the cohort. Baseline characteristics of all included patients are described in . This table also shows the baseline characteristics per CV risk group. When comparing patients with a moderate or high CV risk with those with a low CV risk, statistically significant differences were found for almost all characteristics with the exception of two symptomatic diagnoses of the MSK system.
Table I. Baseline characteristics in the study population.
Predictors of nsNSAID and coxib prescription
In total, 115 713 (24.7%) of all MSK complaint episodes were treated with an nsNSAID and 6456 (1.4%) were treated with a coxib (). The most frequently prescribed nsNSAIDs were diclofenac, naproxen, and ibuprofen (respectively 58%, 13%, and 12% of all nsNSAIDs prescribed) and the most frequently prescribed coxibs were rofecoxib and etoricoxib (49% and 33% of all coxibs prescribed, results not shown in table).
Table II. Predictors of prescription of nsNSAIDs and coxibs.
Age, gender, and NSAID prescription in the six months prior to the index date were all predictive of nsNSAID and coxib prescription. The frequency of nsNSAID and coxib prescription also varied depending on the type of MSK complaint diagnosed. The prescription of coxibs was particularly high in patients suffering from arthritis. When corrected for age and gender, the odds of receiving a coxib were still tenfold in patients with arthritis when compared with those with complaints after trauma (adjusted OR 9.8; 95% CI 8.4–11.5, not shown in table). The individual CV risk factors were all associated with a higher chance of coxib prescription and a lower chance of nsNSAID prescription. Similarly, patients in the moderate and high CV risk group were significantly more likely to receive a coxib than patients in the low CV risk group. The pattern for prescription of nsNSAIDs was less clear, as they were prescribed somewhat more frequently to patients with a moderate CV risk when compared with those with a low CV risk, but less frequently to those with a high CV risk than those with a low CV risk. UGI risk was also a strong predictor of coxib prescription, whereas a high UGI risk was associated with a lower chance of nsNSAID prescription. Patients with renal insufficiency were less likely to be prescribed an nsNSAID and more likely to be prescribed a coxib than patients without renal insufficiency.
Influence of UGI risk
shows the odds of coxib and nsNSAID prescription versus no NSAID prescription in patients with a high or moderate CV risk versus patients with a low CV risk, stratified per UGI risk group. Within each UGI risk group, differences in prescription of nsNSAIDs and coxibs were found when comparing patients with a high or moderate CV risk with patients with a low CV risk. Notably for coxib prescription the direction of this difference varied depending on the UGI risk group. When adjusted for age, gender, previous NSAID prescription, the type of MSK complaint diagnosed, and the presence of renal insufficiency, these differences diminished in magnitude but the same pattern still remained.
Table III. Prescription of coxibs and nsNSAIDs versus no NSAID in moderate and high CV risk patients versus low CV risk patients per UGI risk group.
Prescription of nsNSAIDs and coxibs over time
shows the prescription of nsNSAIDs and coxibs over time. The prescription of coxibs initially increased over time in both high and low CV risk patients, until 2004, after which a sharp decrease is observed. During the peak year of 2004, the odds of prescription of coxibs in high CV risk patients was around three times higher than in 2000 and in 2005 (OR 2.9, 95% CI 2.2–3.7 and OR 3.4, 95% CI 2.5–4.6 for 2004 versus respectively 2000 and 2005, not shown in figure).
Figure 1. Percentage of patients with a high CV risk and with a low CV risk prescribed an nsNSAID or a coxib per year.
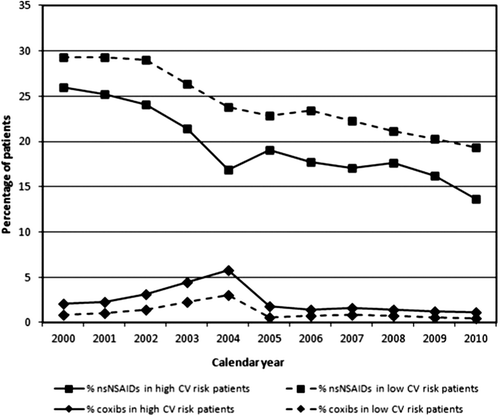
The odds of coxib prescription were significantly higher in patients at high CV risk than in patients at low CV risk (), not only between 2000 and 2004 but also between 2005 and 2010. The odds of nsNSAID prescription remained significantly lower in patients at high CV risk than in patients at low CV risk in both time periods. In a sensitivity analysis in which prescriptions of naproxen and prescriptions with a PDD smaller than half the DDD were excluded, the odds of prescription of an nsNSAID or coxib versus no NSAID, in patients with a high CV risk versus patients with a low CV risk, were almost the same as that of all nsNSAIDs or coxibs versus no NSAIDs in both time periods (OR 0.8, 95% CI 0.8–0.8 and OR 0.7, 95% CI 0.7–0.8, for nsNSAID versus no NSAID in 2000–2004 and 2005–2010 respectively; OR 2.0, 95% CI 1.8–2.2 and OR 1.9, 95% 1.7–2.2 for coxib versus no NSAID in 2000–2004 and 2005–2010 respectively, not shown in table).
Table IV. Prescription of coxibs and nsNSAIDs versus no NSAID in moderate and high CV risk patients versus low CV risk patients per time period.
Discussion
Statement of principal findings
In this study, we examined the prescription of NSAIDs in the treatment of MSK complaints by GPs over the course of the last decade, in which evidence emerged regarding the CV risks of these drugs. We found that one-quarter of all patients presenting with a MSK complaint were treated with an NSAID. Prescription varied widely depending on the type of MSK complaint diagnosed. Coxibs gained in popularity during the first five years of marketing in the Netherlands, with prescription among high CV risk patients in 2004 almost three times higher than in 2000. After rofecoxib was removed from the market, a decrease in coxib prescription was observed. The decrease in coxib prescription observed after 2004 occurred not only in patients with a high CV risk, but equally in patients with a low CV risk, even after their use was contraindicated in these patients by the European Medicines Agency in 2005 [Citation14]. Conversely, nsNSAIDS were prescribed less frequently in patients with a high CV risk than in patients with a low CV risk throughout the study period. These observed differences in prescription between CV risk groups can be partly explained by the overlap between CV risk and UGI risk. When stratified for UGI risk, the odds of both nsNSAID and coxib prescription decreased with increasing CV risk in patients at moderate or high UGI risk. Interestingly, however, for coxibs the opposite pattern was observed for patients with a low UGI risk. When corrected for other predictors, within the low UGI group coxibs were still prescribed more frequently in those with a high CV risk than in those with a low CV risk, suggesting that other factors play a role in GPs’ decision to prescribe these drugs.
Strengths and weaknesses of the study
The strength of this study is that it was conducted in a database containing a large number of patients reflecting the Dutch general population. Nonetheless, some limitations should be considered when reviewing the results. First, only patients presenting with an ICPC-coded MSK complaint were included in the cohort. As some GPs may apply the ICPC coding more diligently than others, this may have led to an underestimation or overestimation of NSAID treatment, if the prescribing behaviour of GPs is in any way related to their tendency to apply the ICPC coding. Second, we did not have any information on over-the-counter (OTC) use of analgesics. Although coxibs are not available without prescription in the Netherlands, nsNSAIDs are freely available. While this is important, our objective in this study was to determine the association between the cardiovascular risk profile of a patient and NSAID prescription by the GP.
Strengths and weaknesses in relation to other studies
Various studies examining changes in NSAID prescription in primary care over the past decade have been published [Citation31–34]. However, few large-scale studies have focused specifically on the influence of CV risk on the prescription of NSAIDs by GPs, which was the aim of the present study. One prior study did investigate this in the primary care population as we did, but it only reported on the years 2000 to 2004, before evidence emerged of the CV risk of NSAIDs [Citation35]. Other studies which have reported on CV risk and the use of NSAIDs both before and after rofecoxib withdrawal in 2004 [Citation36,Citation37] were not population-based. In addition, in these studies the CV risk profile of the patients was determined based on surrogate pharmacy markers. The strength of our population-based study lies in the fact that we were able to identify all relevant risk factors by conducting free text searches, assessing ICPC-codes and prescriptions of medication, using GP medical records which form a complete record of each patient's medical data.
Meaning of the study
Although international guidelines have provided recommendations on NSAID prescription in patients with CV risk factors [Citation19,Citation20], as of yet no national Dutch guideline has been published specifically on this topic. The most recent Dutch guideline specifically on NSAID prescription was published in 2003 [Citation25], at which point in time little was known about the CV risks associated with NSAID use. Over time, prescription of NSAIDs has decreased in all risk groups, which might relate to awareness of GPs regarding risks associated with NSAIDs. Nonetheless, overall one in five patients with a high CV risk presenting with a new MSK complaint received an NSAID. It appears that GPs do not fully consider the CV risks associated with NSAID use when prescribing NSAIDs in these patients, indicating that there is still room for improvement.
Funding
The study was supported by a grant from the Dutch Arthritis Foundation. The funding body had no involvement in the design of the study, data collection, management, analysis, interpretation of data, drafting of the report, or decision to submit the report for publication.
Declaration of interest
Vera Valkhoff conducted research for AstraZeneca in the past as an employee of the Erasmus MC University Medical Center. Miriam Sturkenboom coordinates a research group that occasionally performs research for pharmaceutical industries. None of the grants was related to the submitted work. The other authors declare no other relationships or activities that could appear to have influenced the submitted work. The authors alone are responsible for the content and writing of the paper.
References
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15:S192–300.
- Chavannes AW, Mens JMA, Koes BW, Lubbers WJ, Ostelo R, Spinnewijn WEM, et al. NHG-standaard aspecifieke lagerugpijn [NHG guideline non-specific low back pain]. Huisarts Wet. 2005;48:113–23.
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014 Jan 24 [Epub ahead of print].
- Van Tulder M, Becker A, Bekkering T, Breen A, del Real MT, Hutchinson A, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15:S169–91.
- Winters JC, Van der Windt DAWM, Spinnewijn WEM, De Jongh AC, Van der Heijden GJMG, Buis PAJ, et al. NHG-standaard schouderklachten [NHG guideline shoulder complaints]. Huisarts Wet. 2008;51:555–65.
- Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:377–88.
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of non-steroidal anti-inflammatory drugs: A meta-analysis. Ann Intern Med. 1991;115:787–96.
- Hernandez-Diaz S, Rodriguez LA. Association between non-steroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: An overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–99.
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–28.
- Rostom A, Muir K, Dube C, Jolicoeur E, Boucher M, Joyce J, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: A Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28.
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: Randomised controlled trial. Lancet. 2004;364:665–74.
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs non-steroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–55.
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102.
- European Medicines Agency. Press release: European Medicines Agency concludes action on COX-2 inhibitors. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/01/WC500059088.pdf (accessed 21 Nov 2013).
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis?Meta-analysis of randomised trials. BMJ. 2006;332:1302–8.
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and non-selective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44.
- Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta- analysis. BMJ. 2011;342:c7086.
- Varas-Lorenzo C, Riera-Guardia N, Calingaert B, Castellsague J, Pariente A, Scotti L, et al. Stroke risk and NSAIDs: A systematic review of observational studies. Pharmacoepidemiol Drug Saf. 2011;20:1225–36.
- Chan FK, Abraham NS, Scheiman JM, Laine L. Management of patients on non-steroidal anti-inflammatory drugs: A clinical practice recommendation from the First International Working Party on Gastrointestinal and Cardiovascular Effects of Non-steroidal Anti-inflammatory Drugs and Anti-platelet Agents. Am J Gastroenterol. 2008;103:2908–18.
- Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–38.
- Warlé-Van Herwaarden MF, Kramers C, Sturkenboom MC, Van den Bemt PMLA, De Smet PAGM. Targeting outpatient drug safety: Recommendations of the Dutch Harm- Wrestling Task Force. Drug Saf. 2012;35:245–59.
- Van der Lei J, Duisterhout JS, Westerhof HP, van der Does E, Cromme PV, Boon WM. The introduction of computer-based patient records in The Netherlands. Ann Intern Med. 1993; 119:1036–41.
- Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, Sturkenboom MC, et al. Postmarketing surveillance based on electronic patient records: The IPCI project. Methods Inf Med. 1999;38:339–44.
- Lamberts H WM, Hofmans-Okkens IM. International primary care classifications: The effect of fifteen years of evolution. Fam Pract. 1992;9:330–39.
- Richtlijn NSAID-gebruik en preventive van maagschade [Guideline NSAID use and prevention of gastric damage]. Utrecht: Dutch Institute for Health Care improvement CBO; 2003.
- De Grauw WJC, Kaasjager HAH, Bilo HJG, Faber EF, Flikweer S, Gaillard CAJM, et al. Landelijke transmurale afspraak chronische nierschade [National transmural consensus chronic kidney disease]. Huisarts Wet. 2009;52:586–97.
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. Available at: http://www.whocc.no/ (accessed 21 Nov 2013).
- McGettigan P, Henry D. Cardiovascular risk with non- steroidal anti-inflammatory drugs: Systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098.
- Singh BK, Haque SE, Pillai KK. Assessment of nonsteroidal anti-inflammatory drug-induced cardiotoxicity. Expert Opin Drug Metab Toxicol. 2014;10:143–56.
- Bevolking; kerncijfers [Population; key statistics]. Central Bureau of Statistics; 2012. Available at: http://statline. cbs.nl/StatWeb/publication/?VW = T&DM = SLNL&PA = 37296ned&D1=a&D2=0,10,20,30,40,50,(l-1)-l &HD = 130215-1329&HDR = G1&STB = T (accessed 3 March 2014).
- Brattwall M, Turan I, Jakobsson J. Musculoskeletal pain: Prescription of NSAID and weak opioid by primary health care physicians in Sweden 2004–2008: A retrospective patient record review. J Pain Res. 2010;3:131–5.
- Bedson J, Belcher J, Martino OI, Ndlovu M, Rathod T, Walters K, et al. The effectiveness of national guidance in changing analgesic prescribing in primary care from 2002 to 2009: An observational database study. Eur J Pain. 2013; 17:434–43.
- Alacqua M, Trifiro G, Cavagna L, Caporali R, Montecucco CM, Moretti S, et al. Prescribing pattern of drugs in the treatment of osteoarthritis in Italian general practice: The effect of rofecoxib withdrawal. Arthritis Rheum. 2008;59:568–74.
- Valkhoff VE, van Soest EM, Masclee GM, de Bie S, Mazzaglia G, Molokhia M, et al. Prescription of nonselective NSAIDs, coxibs and gastroprotective agents in the era of rofecoxib withdrawal: A 617 400-patient study. Aliment Pharmacol Ther. 2012;36:790–9.
- Mosis G, Stijnen T, Castellsague J, Dieleman JP, van der Lei J, Stricker BH, et al. Channeling and prevalence of cardiovascular contraindications in users of cyclooxygenase 2 selective non-steroidal antiinflammatory drugs. Arthritis Rheum. 2006;55:537–42.
- Usher C, Bennett K, Teeling M, Feely J. Characterizing new users of NSAIDs before and after rofecoxib withdrawal. Br J Clin Pharmacol. 2006;63:494–97.
- Theibaud P, Patel BV, Nichol MB. Impact of rofecoxib withdrawal on cyclooxygenase-2 utilization among patients with and without cardiovascular risk. Value Health. 2006; 9:361–8.

