Abstract
Objective. To examine drug treatment in nursing home patients at the end of life, and identify predictors of palliative drug therapy. Design. A historical cohort study. Setting. Three urban nursing homes in Norway. Subjects. All patients admitted from January 2008 and deceased before February 2013. Main outcome measures. Drug prescriptions, diagnoses, and demographic data were collected from electronic patient records. Palliative end-of-life drug treatment was defined on the basis of indication, drug, and formulation. Results. 524 patients were included, median (range) age at death 86 (19–104) years, 59% women. On the day of death, 99.4% of the study population had active prescriptions; 74.2% had palliative drugs either alone (26.9%) or concomitantly with curative/preventive drugs (47.3%). Palliative drugs were associated with nursing home, length of stay > 16 months (AOR 2.10, 95% CI 1.12–3.94), age (1.03, 1.005–1.05), and a diagnosis of cancer (2.12, 1.19–3.76). Most initiations of palliative drugs and withdrawals of curative/preventive drugs took place on the day of death. Conclusion. Palliative drug therapy and drug therapy changes are common for nursing home patients on the last day of life. Improvements in end-of-life care in nursing homes imply addressing prognostication and earlier response to palliative needs.
End-of-life care guidelines are centred on cancer patients, while nursing home patients die from various illnesses.
This study shows that palliative drugs were commonly prescribed for nursing home patients during the last days of life.
A diagnosis of cancer and length of stay were associated with palliative drug therapy.
Most initiations of palliative drugs, and most withdrawals of curative/preventive drug therapy, occurred on the day of death.
Introduction
In Norway, 47.5% of deaths occur in nursing homes (NHs), 32.5% in hospitals, and 14.5% at home [Citation1]. About 95% of patients in long-term care will die in the institution [Citation2]. NH patients are prescribed a wider range of medications than any other subpopulation [Citation3,Citation4]. For the dying patient, standing drug treatments must be reconsidered and often discontinued. The last days of life are often characterized by symptoms such as pain, respiratory distress, and anxiety, as well as inability to take oral medications [Citation5]. These symptoms may be palliated by parenterally administered drugs [Citation6].
Whereas palliative literature has a main focus on specialized care for patients with cancer in hospice and hospital, including a range of drug therapy options for the dying [Citation6–8], international consensus on palliative end-of-life (EOL) drug treatment for more heterogeneous NH populations is lacking. Derived from international and Norwegian guidelines [Citation6–8], a shorter drug list has been recommended for use in NHs in Norway, comprising parenteral morphine, benzodiazepines, anticholinergics, and antipsychotics [Citation2,Citation5]. Previous studies on EOL care in NHs have reported on treatment with selected drug groups such as opioids and pulmonary agents [Citation9], and pain relief [Citation4] without a clearly defined palliative drug treatment.
Pharmacological treatment for dying patients is thus an important aspect of EOL care in NHs, of which we have little knowledge. Insight into initiation and discontinuation of drug therapy in this phase may shed light on the quality of EOL care and point to vulnerable patient groups. Our study aimed to examine drug treatment in NH patients at the EOL, and to identify predictors of a clearly defined palliative drug therapy.
Material and methods
Study population
NHs in Norway accommodate around 41 000 beds, corresponding to 18% of the general population 80 years and older. All NHs provide EOL care, but only 42 institutions have specialized palliative care units. Most NH physicians in Norway are part-time engaged general practitioners [Citation1]. The study population comprised all patients in three urban NHs in Norway admitted from January 2008 and deceased before February 2013. The institutions were selected on the basis of using an electronic patient record system optimized for data extraction [Citation10].
Data collection
We collected routinely registered data from the patients’ final NH stay: demographic data (age, gender, date of NH admission and death, long- or short-term stay); diagnoses (ICD-10) [Citation11]; medications (generic name, Anatomical Therapeutic Chemical (ATC) code [Citation12], drug formulation, regular or as-needed schedule, indication, dates of initiation, alteration, or discontinuation). An external IT consultant extracted the data, and replaced ID-numbers with a running number, the key to which remained undisclosed to the research group.
Drug therapy
We defined palliative EOL drug therapy in NHs on the basis of indication, drug, and formulation; (1) any drug prescription with an explicit EOL care indication key word was included: palliative, terminal, death, death rattle, Liverpool Care Pathway, or EOL; (2) we also included prescriptions of specifically recommended injectable palliative EOL drugs for use in NHs [Citation2,Citation5], regardless of missing EOL key words in the indication text (). “Curative/preventive drug therapy”, in contrast, was defined as medication for regular use without an explicit EOL care indication.
Statistical analysis
User rates were established for drugs according to the above categories. We explored predictors of palliative EOL drug therapy by a chi-squared test, and subsequently by binary logistic regression analysis; dependent variable: palliative EOL drug therapy; independent variables: age, gender, length of stay, nursing home, diagnosis of cancer. All variables but age were analysed as categorical. Significance was determined at a level of 5%. IBM SPSS Statistics 20 (SPSS Inc., Chicago, Ill., USA) was used for statistical analyses.
Results
Patient characteristics
The study population comprised 524 deceased patients. Median (range) age at death was 86 (19–104) years, 59.4% were women, 68.1% in long-term care. The most common registered diagnoses were dementia (36.8% of the patients), congestive heart failure (29.6%), and cancer (23.7%) ().
Table II. Patient characteristics (n = 524).
The three NH populations did not differ with regard to gender or number of diagnoses. Compared with the other NHs, more patients at NH C were 86 years and older, or had a diagnosis of infection or cancer, p < 0.01. Patients at NH A had longer stays (p < 0.01), as the EPR data were collected from was used only in the long-term ward.
Patients with cancer more frequently died within two weeks of admission than patients without cancer (41.1% vs. 20.5%, p < 0.01). Patients with dementia more frequently died after stays of longer than 16 months compared with patients without this diagnosis (40.4% vs. 16.0%, p < 0.01).
Drug use on the day of death
On the day of death, 99.4% of the study population were on drug therapy. The most common regular and as-needed drugs are shown in .
Table III. Most common drugs on the date of death (% of patients).
Of the 4736 standing prescriptions (regular and as-needed drugs) on the day of death, palliative EOL drugs comprised 1306 (27.6%) and curative/preventive drugs 2419 (51.1%), while 1011 (21.3%) prescriptions were not classified in either category. Indication was documented for 99.6% of all drugs on the day of death.
Altogether 50.2% of patients were prescribed any drug with a specified EOL care indication. The most common palliative EOL drugs were morphine (71.4% of patients), midazolam (55.0%), glycopyrronium (46.9%), and haloperidol (46.9%) (see ). Palliative EOL drugs were prescribed to 74.2% of the study population, either alone (26.9%) or concomitantly with curative/preventive drugs (47.3%). Curative/preventive drugs were prescribed to 72.5% of patients (alone 25.2%). Some 95.7% of palliative EOL drugs were prescribed as needed. Patients had standing prescriptions of median (25th–75th percentile) three (zero–eight) palliative EOL drugs and three (zero–four) curative/preventive drugs on the date of death. There was a median period of two (zero–seven) days from prescription to death for palliative EOL drugs.
Having prescriptions of palliative EOL drugs at death was associated with length of stay > 16 months (AOR 2.10, 95% CI 1.13–3.95), cancer (2.12, 1.19–3.76), age (1.03, 1.005–1.05), and being at NH B (3.53, 1.99–6.25) or NH C (4.20, 2.36–7.48) ().
Table IV. Associations between palliative EOL drug therapy and patient characteristics.
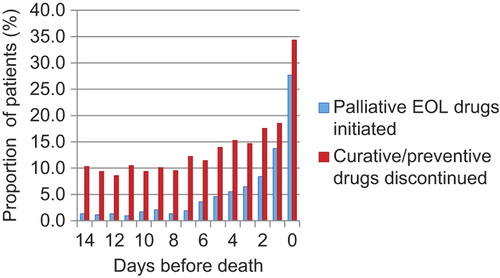
shows that the proportion of patients for whom at least one palliative EOL drug was initiated, or at least one curative/preventive drug was discontinued, increased in the last week before death and peaked on the day of death.
Discussion
Our study shows that palliative EOL drugs were commonly prescribed for NH patients during the last days of life. NH, a diagnosis of cancer, and long stay were associated with palliative EOL drug therapy. Most initiations of palliative EOL drugs, and most withdrawals of curative/preventive drug therapy, occurred on the day of death.
Strengths and weaknesses
The study population comprised patients from all types of wards, and although the diagnostic data are not validated this broad diversity is expected to reflect NH populations in general.
With the exception of short-term care patients from NH A, all patients admitted and deceased in three NHs during the five-year study period were included, limiting selection bias. Only three institutions participated in the study, limiting statistical power and to some extent generalizability.
A complete set of medication data for all patients was collected. The electronic patient record did not include information on whether prescribed medication was actually taken, leading to possible overestimation of drug use. Prescribed medication, on the other hand, may reflect the doctor's treatment decisions more appropriately than given medication. This point is particularly important for palliative drugs, which comprised almost exclusively as-needed drug prescriptions.
Use of indication text secured a comprehensive definition of palliative EOL drugs, while inclusion of specifically recommended palliative EOL drugs ensured that these prescriptions were not missed regardless of missing EOL key words in the indication text. Restricting the latter to injectables, we excluded prescriptions less specific to the dying patient, such as opioid tablets, oral suspensions, and patches. Injectable antipsychotics and benzodia-zepines may on occasion be used to treat neuropsychiatric symptoms in dementia. A median of two days from prescription to death makes it less likely, though, that these prescriptions were issued for their non-palliative indications. Although anticholinergics have other indications, in injectable form, glycopyrronium and scopolamine are seldom used for non-palliative purposes in NHs.
Comparison with other studies
There are few other studies reporting on drug therapy at the EOL in the general NH population, and with considerably smaller sample sizes. Decreased overall treatment intensity has been found in patients perceived as dying, across NH, hospital, and general practice settings in the Netherlands [Citation13]. Patients with dementia dying in American NHs were prescribed unchanged total numbers of drugs, palliative medications replacing other medications [Citation9].
Our study adds to previous knowledge showing that NH patients with the longest duration of stay, or a diagnosis of cancer, were more likely to receive palliative EOL drugs on the day of death than those without these characteristics. More than 80% of long-term care patients have dementia [Citation14], interfering with the communication of suffering, analgesia, and EOL care [Citation15–17]. Accurate survival prediction for patients with advanced dementia is difficult, and may hinder palliative care [Citation18]. Longer NH stays may nevertheless allow time for advance care planning and staff familiarity with the patient, thus facilitating palliation, and perhaps explaining the association found with the longest stays. Patients with cancer often have expected deaths with a typically rapid functional decline, and are at the centre of palliative guidelines [Citation7,Citation19]. Palliative drug therapy for this group was therefore expected. A diagnosis of dementia, heart failure, chronic pulmonary disease, infection, or hip fracture was not associated with initiation of palliative drugs. This may indicate death coming unexpectedly. For respiratory distress in chronic pulmonary disease there may also be a reservation among physicians to prescribe morphine and benzodia-zepines, as they inhibit respiration.
An evidence base for EOL care in non-malignant conditions, which are prevalent in the general NH population, is scarce [Citation20]. We found a high treatment rate with palliative drugs (73.9% overall, 71.9% for morphine), in line with 77% of NH patients with advanced dementia in the Netherlands receiving opioids. Despite extensive prescribing, the Dutch study found that symptoms of pain, shortness of breath, and agitation were prevalent, suggesting that a prescribed drug is no guarantee of satisfactory symptom control [Citation21]. For this, factors such as close symptom assessment as well as appropriate drug dosage and administration are required.
Palliative drug therapy increased and curative/preventive drug treatment decreased in the last week of life, most changes taking place on the day of death. A recent study in long-term care facilities in Canada found that care only changed substantially to palliative in nature during the last hours or days of life, calling for earlier awareness of impending death [Citation22]. Initiation of palliative drugs is not to be expected for all dying patients, nor does it depend only on staff competence. Less palliative drug therapy could also come from less need for it, by having a shorter terminal phase, or less burdensome symptoms. Little is known about the identification and duration of the dying phase in NH patients and for how many it lasts long enough to allow for pharmacological response. Distinct death trajectories have been described for patients with different diseases [Citation23], and timing of palliative care for patients with non-malignant diagnoses has been shown to be particularly challenging [Citation24]. Yet, relatively accurate prediction of survival for these patients in NHs has been shown to be feasible, though only in the last seven days of life [Citation25].
NH A had a lower proportion of patients prescribed palliative medications at death. Differences in prescribing culture between doctors may be one explanation. For the present study we did not collect this variable.
Meaning of the study
Palliative drug prescriptions and drug therapy changes are common for NH patients on the last day of life. Extensive curative/preventive drug therapy and comprehensive changes in drug treatment on the day of death may both point to the known prognostication difficulties in the multimorbidity characterizing NH populations. Improvements of EOL care in NHs must address prognostication and an early response to palliative needs.
Acknowledgements
The authors would like to thank the Municipality of Bergen, and Magne Rekdal at Emetra, for data collection for this study. This study was supported by grants from the Municipality of Bergen, Kavli Research Centre for Ageing and Dementia, and the Foundation for Research in General Practice (PhD grant Kristian Jansen).
Ethical approval
The Regional Committee for Medical and Health Research Ethics (2012/1748), and Norwegian Social Science Data Services (12/30691) approved the study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- Statistics Norway 2014. Available at: http://www.ssb.no (accessed 1 June 2014).
- Husebo BS, Husebo S. Sykehjemmene som arena for terminal omsorg – hvordan gjør vi det i praksis? [Nursing homes as arenas of terminal care – how do we do in practice?]. Tidsskr Nor Laegeforen 2005;125:1352–4.
- Halvorsen KH, Granas AG, Engeland A, Ruths S. Prescribing quality for older people in Norwegian nursing homes and home nursing services using multidose dispensed drugs. Pharmacoepidemiol Drug Saf 2012;21:929–36.
- Chen IC, Liu ML, Twu FC, Yuan CH. Use of medication by nursing home residents nearing end of life: A preliminary report. J Nurs Res 2010;18:199–205.
- Rosland JH, von Hofacker S, Paulsen O. Den døende pasient [The dying patient]. Tidsskr Nor Laegeforen 2006;126: 467–70.
- Norwegian Association for Palliative Medicine. Retningslinjer for symptomlindrende behandling [Guidelines for palliative treatment]; 2007. Available at: http://legeforeningen.no (accessed 1 June 2014).
- Norwegian Directorate of Health. Nasjonalt handlingsprogram med retningslinjer for palliasjon i kreftomsorgen [National program with guidelines for palliation in cancer care]; 2013. Available at: http://www.helsedirektoratet.no (accessed 1 June 2014).
- Hanks G, Cherny NI. Oxford textbook of palliative medicine, 4th ed. Oxford: Oxford University Press; 2009.
- Blass DM, Black BS, Phillips H, Finucane T, Baker A, Loreck D et al. Medication use in nursing home residents with advanced dementia. Int J Geriatr Psychiatry 2008;23:490–6.
- Kruger K. Elektroniske pasientjournaler bør være strukturerte [Electronic medical records should be structured]. Tidsskr Nor Laegeforen 2007;127:2090–3.
- International Classification of Diseases (ICD-10). Available at: http://www.who.int/classifications/icd/en/ (accessed 4 September 2014).
- Anatomical Therapeutic Chemical (ATC) classification system. Available at: http://www.whocc.no/atc_ddd_index (accessed 1 June 2014).
- Veerbeek L, Van Zuylen L, Swart SJ, Jongeneel G, Van Der Maas PJ, Van Der Heide A. Does recognition of the dying phase have an effect on the use of medical interventions? J Palliat Care 2008;24:94–9.
- Selbaek G, Kirkevold O, Engedal K. The prevalence of psychiatric symptoms and behavioural disturbances and the use of psychotropic drugs in Norwegian nursing homes. Int J Geriatr Psychiatry 2007;22:843–9.
- Monroe T, Carter M, Feldt K, Tolley B, Cowan RL. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J Adv Nurs 2012;68:2070–8.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004;164:321–6.
- Li Q, Zheng NT, Temkin-Greener H. Quality of end-of-life care of long-term nursing home residents with and without dementia. J Am Geriatr Soc 2013;61:1066–73.
- Mitchell SL, Kiely DK, Hamel MB, Park PS, Morris JN, Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA 2004;291:2734–40.
- Doyle DW, Woodruff R. The IAHPC manual of palliative care, 3rd ed., 2013. Available at: http://hospicecare.com/resources/publications/manual-of-palliative-care/ (accessed 1 June 2014).
- Luddington L, Cox S, Higginson I, Livesley B. The need for palliative care for patients with non-cancer diseases: A review of the evidence. Int J Palliat Nurs 2001;7:221–6.
- Hendriks SA, Smalbrugge M, Hertogh CM, van der Steen JT. Dying with dementia: Symptoms, treatment, and quality of life in the last week of life. J Pain Symptom Manage 2014; 47:710–20.
- Cable-Williams B, Wilson D. Awareness of impending death for residents of long-term care facilities. Int J Older People Nurs 2014;Jan 17. doi: 10.1111/opn.12045 [Epub ahead of print].
- Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA 2003;289:2387–92.
- Coventry PA, Grande GE, Richards DA, Todd CJ. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: A systematic review. Age Ageing 2005;34:218–27.
- Brandt HE, Ooms ME, Ribbe MW, van der Wal G, Deliens L. Predicted survival vs. actual survival in terminally ill noncancer patients in Dutch nursing homes. J Pain Symptom Manage 2006;32:560–6.